Published online Jun 18, 2023. doi: 10.13105/wjma.v11.i5.218
Peer-review started: December 24, 2022
First decision: February 28, 2023
Revised: March 27, 2023
Accepted: May 6, 2023
Article in press: May 6, 2023
Published online: June 18, 2023
Processing time: 173 Days and 19.1 Hours
The definition of diabetic foot syndrome (DFS) varies depending on the location and resources. Few classifications are available according to the indication. DF ulcers and vitamin D deficiency are common diseases among patients with diabetes. Previous literature has shown an association between DF ulcer (DFU) and vitamin D deficiency. However, the available meta-0analysis was limited by substantial bias.
To investigate the association between DFUs and vitamin D levels.
We searched PubMed, MEDLINE, and Cochrane Library, EBSCO, and Google Scholar for studies comparing vitamin D levels and DF. The keywords DFU, DFS, diabetic septic foot, vitamin D level, 25-hydroxy vitamin D, vitamin D status, and vitamin D deficiency were used. The search engine was set for articles published during the period from inception to October 2022. A predetermined table was used to collect the study information.
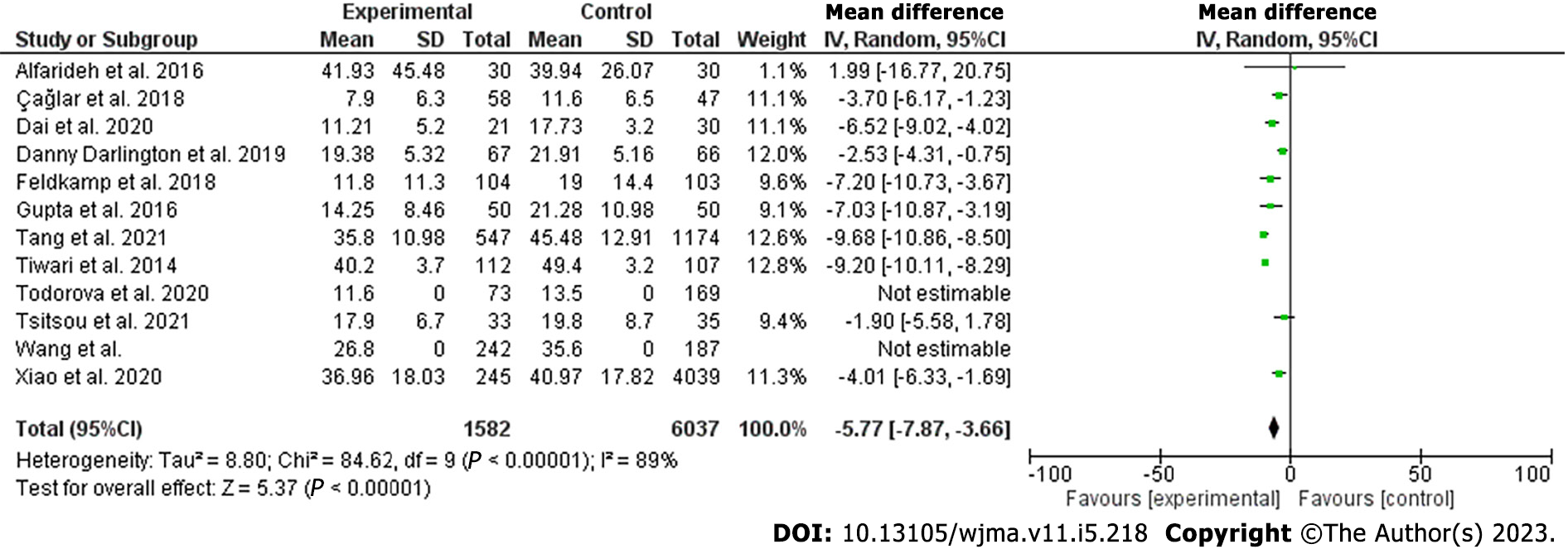
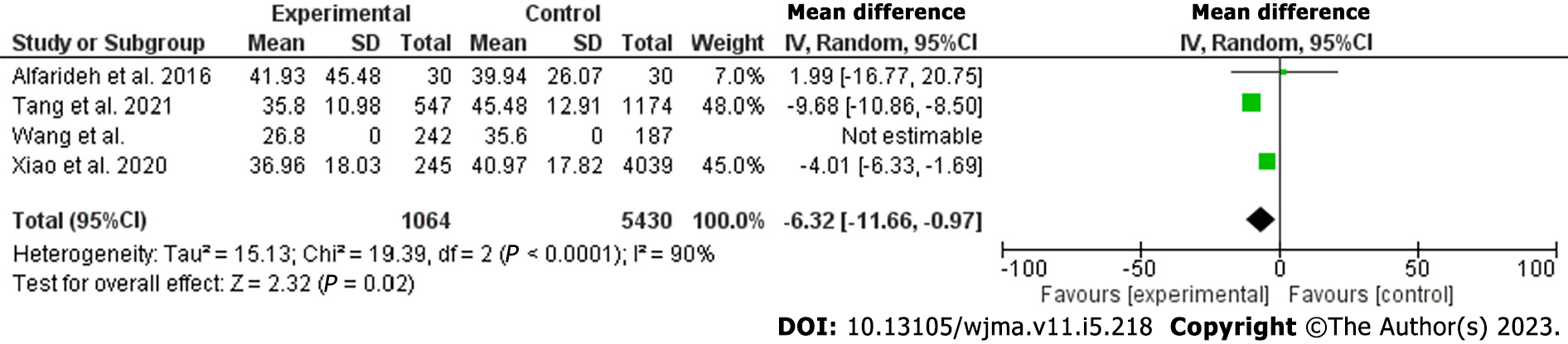
Vitamin D level was lower among patients with DFU compared to their counterparts [odds ratio (OR): -5.77; 95% confidence interval (CI): -7.87 to -3.66; χ2 was 84.62, mean difference, 9; I2 for heterogeneity, 89%; P < 0.001 and P for overall effect < 0.001]. The results remained robust for hospitalized patients (OR: -6.32 95%CI: -11.66 to -0.97; χ2 was 19.39; mean difference, 2; I2 for heterogeneity, 90%; P = 0.02).
Vitamin D was lower among outpatients and hospitalized patients with DFUs. Further larger randomized controlled trials are needed.
Core Tip: This is the first study to assess the relationship between diabetic foot ulcer and vitamin D deficiency, avoiding the bias of the two published meta-analyses.
- Citation: Mirghani HO. Vitamin D deficiency among outpatients and hospitalized patients with diabetic foot ulcers: A systematic review and meta-analysis. World J Meta-Anal 2023; 11(5): 218-227
- URL: https://www.wjgnet.com/2308-3840/full/v11/i5/218.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i5.218
Diabetes mellitus (DM) is an epidemic globally. DM is a morbid disease with many complications including microvascular and microvascular disease. Diabetic foot syndrome (DFS) is defined as peripheral neuropathy, limited joint mobility, peripheral arterial disease, immunopathy, ulceration, and Charcot arthropathy[1]. The combination of FS elements provides an environment for unrecognized injury, foot infection, and possible amputation[2]. DFS is characterized by peripheral arterial disease, but the symptoms are masked by the accompanying peripheral neuropathy. The pathology varies from pre-ulcerative callouses, ulceration, and necrosis developing at the site of high pressure (deformities of the toes and feet). Patient education and feet inspection are mandatory because repetitive trauma might pass unnoticed due to the loss of pain sensation[3]. DFS is a common complication of diabetes with a great economic burden; DTS substantially affects the patient's quality of life and leads to premature death. In addition, patients with DFS are prone to psychiatric disease[4].
There are nearly 40 classifications for DFS, with wide variation depending on the availability of resources and geographical variations. It is recommended to use classification in light of specific indications. Few classifications have been validated for use; the site, ischemia, neuropathy, bacterial infection, area, and depth (SINBAD) is six questions with yes or no answers with a maximum of six points. SINBAD score is better for communication between clinicians[5]. While, the Infectious Diseases Society of America/International Working Group on Diabetic Foot, and wound depth, ischemia, and foot infection scoring are better for infection and perfusion respectively[6,7]. The spectrum of DFS varies from minor erythema to tissue necrosis and lower limb deformity and amputation [8]. The mortality of DFS is comparable to breast and lung cancer. Five-year mortality for minor and major amputations, Charcot, and DF ulcer (DFU) were 56.6%, 46.2%, 30.5%, 29%, respectively. The pooled mortality from breast, all cancer, and lung cancer were 9%, 30%, and 80% respectively[9].
The lifetime of developing FUs among patients with diabetes varies between 19% and 34% with nearly two-thirds of recurrence in 5 years, and 1 in 5 patients with moderate to severe FUs resulting in amputation. The majority of lower extremities amputations are preceded by FUs and three amputations occur every minute due to diabetes. Patients with FUs had a 2.5 times mortality rate compared to their counterparts[10,11].
25-hydroxyvitamin D (25(OH)D) is present in almost all immune cells and is a major immunomodulatory hormone. In addition, the vitamin is a potent endothelial membrane stabilizer[12]. Due to its anti-inflammatory effects, the active form of vitamin D plays an important role in inflammatory diseases including rheumatic disorders, and a growing piece of evidence is present regarding its effects on infectious diseases[13]. Vitamin D deficiency is common; larger studies suggest that in Europe, 40% and 13% of the population are vitamin D-deficient and severely deficient, respectively[14]. Vitamin D deficiency is associated with vascular diseases including DM, hypertension, and dyslipidemias[15].
The small number of included studies, including studies published by the same authors and including poster presentations[16,17], limits the previous meta-analysis on vitamin D deficiency and diabetic septic foot. Therefore, this meta-analysis investigated vitamin D levels among patients with the diabetic septic foot.
The studies were eligible if they compared the level of vitamin D among patients with DFU and their counterparts without DFUs and they are randomized controlled trials or case-control studies, prospective and retrospective cohorts, and cross-sectional studies. Case reports, case series, and animal and experimental studies were excluded.
The primary outcome was the level of vitamin D among patients with DFUs.
Vitamin D measurement varied between the included studies. References 18, 19, 21, and 23 used the enzyme-linked immunosorbent assay; references 20, 22, and 25 used radioimmunoassays; references 24, 26, and 28 used the electrochemiluminescence immunoassay; reference 27 used liquid chromatography-tandem mass spectrometry; and reference 29 used the chemiluminescence assay.
All of the studies used outpatients except 18, 24, 28, and 29, in which hospitalized patients were included.
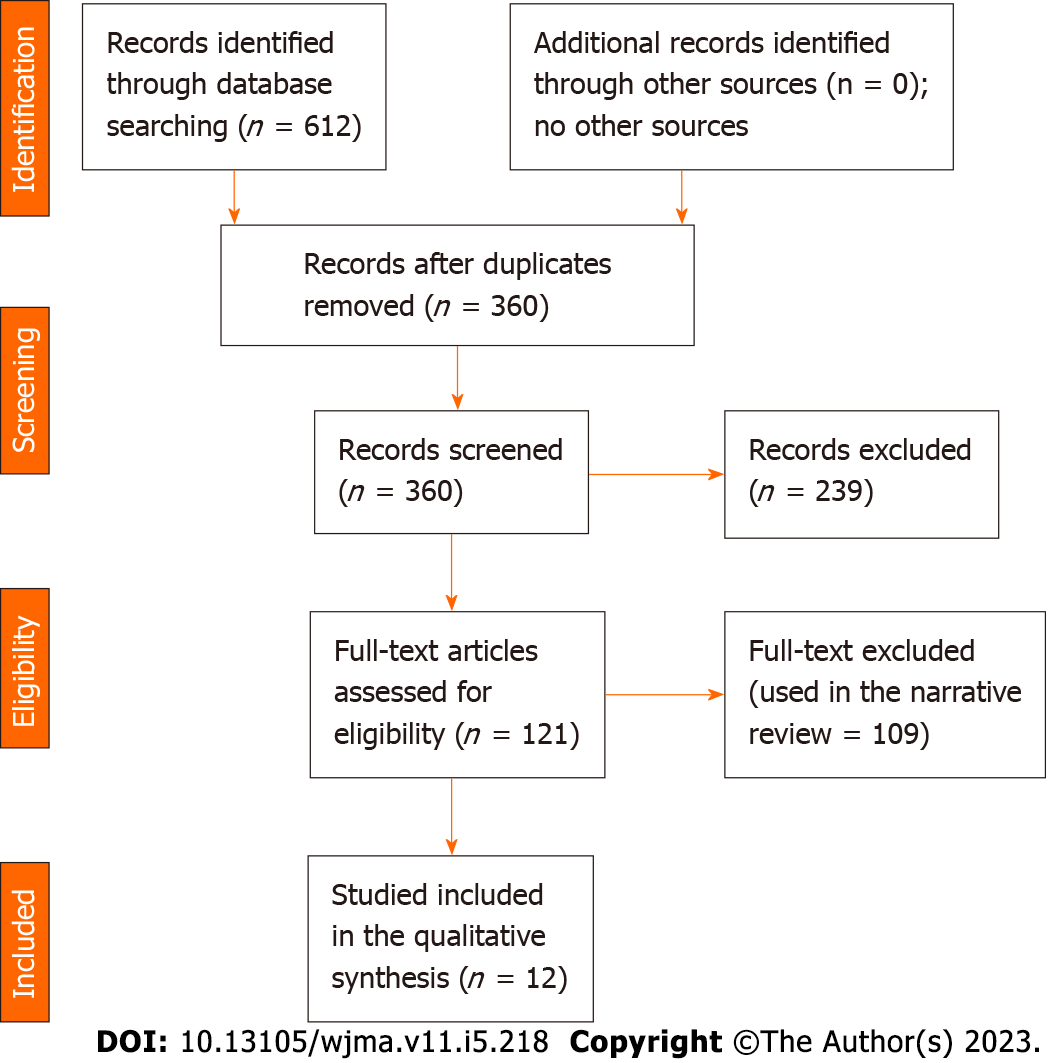
The researcher searched PubMed, MEDLINE, and Cochrane Library, EBSCO, and Google Scholar using the keywords DFU, DFS, diabetic septic foot, vitamin D level, 25-hydroxy vitamin D, vitamin D status, and vitamin D deficiency. The search engine was set for articles published during the period from inception to October 2022. A predetermined table was used to collect study information including author name, year of publication, country, age, sex, patient’s number in the control and interventional groups, duration of diabetes, hemoglobin A1c (HbA1c) in the intervention and control groups, vitamin D level among patients with FUs and control groups (Figure 1 and Tables 1-3).
| Ref. | Study type | Country | Duration | Diabetes | Control | Results |
| Afarideh et al[18], 2016 | Cross-sectional, 30, and 30 | Iran | - | 41.93 ± 45.48 | 39.94 ± 26.07 | Non-significant, 0.487 |
| Çağlar et al[19], 2018 | Prospective, 58 interventions and 47 controls | Turkey | 12 mo | 7.9 ± 6.3 | 11.6 ± 6.5 | Lower among diabetes, < 0.001 |
| Dai et al[20], 2020 | Prospective, 21, and 30 | China | 9 mo | 11.21 ± 5.20 | 17.73 ± 3.20 | Lower among diabetes, < 0.001 |
| Danny Darlington et al[21], 2019 | Cross-sectional, 67, and 66 | India | - | 19.38 ± 5.32 | 21.91 ± 5.16 | No significant difference, 0.306 |
| Feldkamp et al[22], 2018 | Cross-sectional, 104, and 103 | Germany | - | 11.8 ± 11.3 | 19 ± 14.4 | Lower among diabetes, < 0.001 |
| Gupta et al[23], 2016 | Retrospective, 50, and 50 | India | - | 14.25 ± 8.46 | 21.28 ± 10.98 | Lower among diabetes, < 0.001 |
| Tang et al[24], 2021 | Prospective, 547, and 1174 | China | 8 yr | 35.8 ± 10.98 | 45.48 ± 12.91 | Lower among diabetes, < 0.001 |
| Tiwari et al[25], 2014 | Cross-sectional, 112 cases, 107 controls | India | - | 40.2 ± 3.7 | 49.4 ± 3.2 | Lower among diabetes, 0.06 |
| Todorova et al[26], 2020 | Cross-sectional, 73, and 169 | Bulgaria | - | 11.6 | 13.5 | Lower among diabetes, 0.001 |
| Tsitsou et al[27], 2021 | Cross-sectional, 33, and 35 | Greece | - | 17.9 ± 6.7 | 19.8 ± 8.7 | Non-significant, 0.329 |
| Wang et al[28], 2022 | Retrospective, 242, 187 | China | 34 mo | 26.89 | 35.64 | Lower among diabetes, < 0.001 |
| Xiao et al[29], 2020 | Cross-sectional, 245, and 4039 | China | - | 36.96 ± 18.03 | 40.97 ± 17.82 | Lower among diabetes, 0.001 |
| Ref. | Study type | Country | Age | Sex | DM duration | HbA1c |
| Afarideh et al[18], 2016 | Cross-sectional, 30, and 30 | Iran | Matched | Matched | Matched | Matched |
| Çağlar et al[19], 2018 | Prospective, 58 interventions and 47 controls | Turkey | Controls younger | Matched | Controls newly diagnosed | Matched |
| Dai et al[20], 2020 | Prospective, 21, and 30 | China | Matched | Matched | Matched | Matched |
| Danny Darlington et al[21], 2019 | Cross-sectional, 67, and 66 | India | Matched | Matched | Matched | Poor glycemic among foot ulcer |
| Feldkamp et al[22], 2018 | Cross-sectional, 104, and 103 | Germany | Matched | Matched | Matched | Matched |
| Gupta et al[23], 2016 | Retrospective, 50, and 50 | India | Control was younger | Males high among DM | Lon among diabetes | Poor glycemic among foot ulcer |
| Tang et al[24], 2021 | Prospective, 547, and 1174 | China | Control was younger | Higher females in control | Lon among diabetes | Matched |
| Tiwari et al[25], 2014 | Cross-sectional, 112 cases, 107 controls | India | Matched | Matched | Matched | Matched |
| Todorova et al[26], 2020 | Cross-sectional, 73, and 169 | Bulgaria | Control was younger | Matched | Matched | NA |
| Tsitsou et al[27], 2021 | Cross-sectional, 33, and 35 | Greece | Matched | Matched | Matched | Matched |
| Wang et al[28], 2022 | Retrospective, 242, 187 | China | Control was younger | Males higher among DM | Lon among diabetes | NA |
| Xiao et al[29], 2020 | Cross-sectional, 245, and 4039 | China | Matched | Females more | Matched | Poor glycemic among foot ulcer |
| Ref. | Country | Selection bias | Comparability bias | Outcome | Total score |
| Afarideh et al[18], 2016 | Iran | 4 | 2 | 2 | 8 |
| Çağlar et al[19], 2018 | Turkey | 4 | 2 | 2 | 8 |
| Dai et al[20], 2020 | China | 4 | 2 | 2 | 8 |
| Danny Darlington et al[21], 2019 | 4 | 1 | 2 | 7 | |
| Feldkamp et al[22], 2018 | India | 4 | 2 | 2 | 8 |
| Gupta et al[23], 2016 | Germany | 4 | 2 | 2 | 8 |
| Tang et al[24], 2021 | India | 4 | 2 | 2 | 8 |
| Tiwari et al[25], 2014 | China | 4 | 1 | 2 | 7 |
| Todorova et al[26], 2020 | India | 4 | 2 | 2 | 8 |
| Tsitsou et al[27], 2021 | Bulgaria | 4 | 1 | 2 | 7 |
| Wang et al[28], 2022 | Greece | 4 | 2 | 2 | 8 |
| Xiao et al[29], 2020 | China | 4 | 1 | 2 | 7 |
The RevMan (version 5.4) system for meta-analysis was used, and the data were all continuous. We pooled data from 12 studies to compare vitamin D levels among patients with and without diabetic septic foot; a subanalysis was done to compare vitamin D among hospitalized patients. Random effect was used because significant heterogeneity was observed. Funnel plots were used to assess lateralization. P < 0.05 was considered statistically significant.
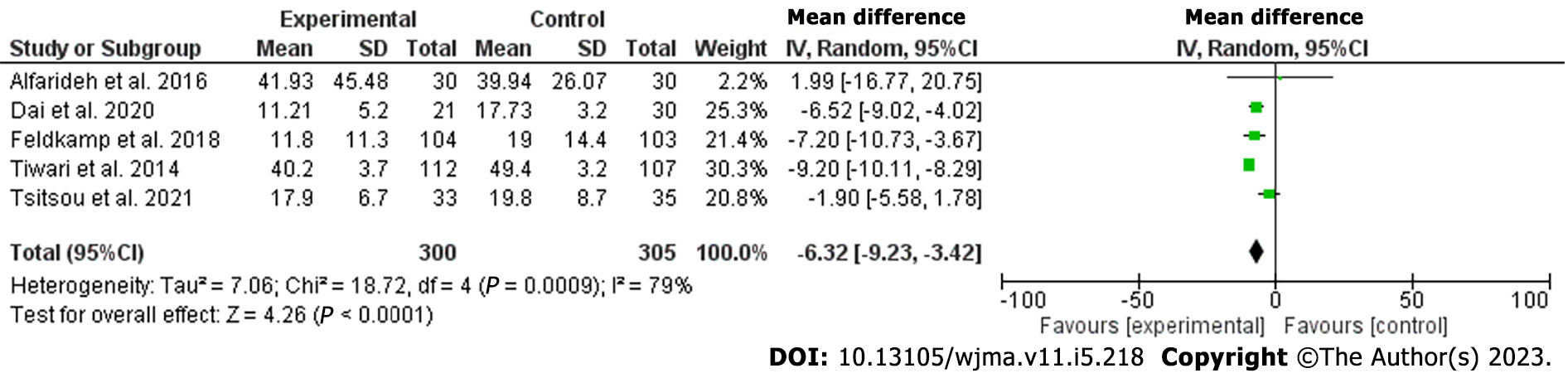
The current meta-analysis included 12 studies including 7619 patients. The included studies were seven cross-sectional, three prospective, and two retrospective studies; nine were published in Asia and three were from Europe[18-29]. The included studies were of good quality as assessed by the Newcastle Ottawa Scale[30]. Vitamin D was lower among patients with DFUs [odds ratio (OR): -5.77, 95% confidence interval (CI): -7.87 to -3.66; χ2 was 84.62; mean difference, 9; I2 for heterogeneity, 89%; P < 0.001, and P for overall effect < 0.001] (Figure 2). Vitamin D level was low when a subanalysis was conducted including only hospitalized patients with diabetes septic foot (OR: -6.32; 95%CI: -11.66 to -0.97; χ2 was 19.39; mean difference, 2; I2 for heterogeneity, 90%; P = 0.02) (Figure 3). Vitamin D level was lower among patients with DFUs after including studies that controlled for age, sex, duration of diabetes, and HbA1c (OR: -6.32; 95%CI: -923 to -3.42; χ2 was 18.72; mean difference, 4; I2 for heterogeneity, 79%; P < 0.001) (Figure 4).
In the present meta-analysis, vitamin D levels were lower among patients with DFUs compared to their counterparts without FUs (OR: -5.77; 95%CI: -7.87 to -3.66). There were no differences between hospitalized patients and outpatients. The results remained robust when including studies that controlled for age, sex, duration of diabetes, and HbA1c. The quality of the included studies was good[30]. The current findings were in line with a narrative review including three studies[31]. The present findings were similar to the first meta-analysis published by Dai and colleagues in 2019. Dai et al[16] found an association between vitamin D levels and DFUs. However, Kota et al[32] included studies published by the same authors and some were poster presentations. Yammine et al[33] found similar results. Importantly, Yammine and colleagues included poster presentations, studies published by the same authors, and studies that assessed Charcot’s joints[34]. In addition, the previous meta-analysis included Zubair et al[35] study in which vitamin D median was reported and not the mean ± standard deviation. A recently published meta-analysis reported similar findings to our results. However, the substantial heterogeneity including posters, research by the same authors, and different primary outcomes limited their results[17]. The main strength of this meta-analysis is the subanalysis on vitamin D among hospitalized patients. Although a single measurement is not enough during stress, the results remain robust even among admitted patients[36].
Vitamin D has been considered a magic bullet and cures many chronic disorders. However, the results were obtained from observational studies. The findings of lower FUs among patients with higher vitamin D may not prove causality. Other confounders might explain the lower vitamin D levels among patients with DFUs including a healthier diet, good exposure to sunlight, and physical activity[37,38]. In addition, vitamin D improves glycemic control among patients with diabetes[39,40]. Thus, high vitamin D may indirectly protect against DFUs by improving glycemic control.
Osteoblasts (bone formation) and osteoclasts (bone resorption) orchestrate bone remodeling. Osteoclasts genesis activation is through receptor activator of tumor necrosis factor (RANK-osteoprotegerin), ultimately leading to osteolysis and destruction of bone tissue. This pathway is of great therapeutic and clinical implications. Medications that influence different levels of RANK-osteoprotegerin are bisphosphonates, calcitonin, and denosumab. Denosumab is encouraging for the treatment of Charcot diabetic foot. However, bisphosphonates have been evaluated recently due to the adverse events. Calcitonin efficacy is limited[41,42].
In this review, some of the included studies were not matched for age, duration of diabetes, duration of diabetes, or HbA1c. The young age of control subjects, their good glycemic control, and the short duration of diabetes might increase their risk of DFUs.
Although, the association between low vitamin D levels and diabetic septic foot was documented. However, the effect of vitamin D therapy on DFUs is unclear. In addition, it is not clear if the relationship is correlated or causal[43]. A double-blinded randomized controlled trial showed that high-dose vitamin D supplementation (170 μg/d) was superior to low doses (20 μg/d) on diabetic ulcer healing[44]. A recent review showed that vitamin D improved diabetic septic foot healing, an effect mediated by the remodeling and proliferation of cells involved. In addition, vitamin D suppresses proinflammatory responses, enhances antimicrobial peptides, and enhances anti-inflammatory effects[45]. The review by Papaioannou and colleagues, which included 34 studies[46], supported the above findings. A randomized controlled trial published in Asia showed that vitamin D supplementation reduced ulcer length, width, and depth[47]. A recent review of the literature concluded that vitamin D supplementation might slow the progression of neural damage. In addition to the adjuvant role in neuropathic pain and cardiovascular autonomic neuropathy among patients with type 2 diabetes[48].
The current meta-analysis strength is that we included observational studies excluding poster presentations, studies published by the same authors, and studies that used the median of vitamin D. The limitation of this study was the substantial heterogeneity.
Vitamin D levels were lower among patients with DFUs compared to their counterparts without ulcers. A low level was observed among hospitalized patients. Randomized control trials investigating the association of vitamin D and DFs and assessing the role of vitamin D supplementation are needed.
Vitamin D deficiency is associated with various disorders ranging from glycemic control to cancer and suicide. Diabetic foot syndrome (DFS) is a common disorder with high morbidity and mortality. The association of DF ulcers (DFUs) with vitamin D deficiency was documented. However, the available meta-analyses were limited by bias and few included studies.
Diabetes mellitus (DM) is approaching an epidemic, the disease is associated with vascular and neuropathic complications. Most people with diabetes are not approaching the recommended targets for cardiovascular risk factors with increasing FUs. DFUs are a preventable disease and vitamin D deficiency is promising. Despite the association of vitamin D deficiency and DM and its complications. However, a cause and effect were not confirmed. In addition, vitamin D supplementation is not without complications and vitamin D is readily synthesized by sun exposure. We included vitamin D supplementation to address this issue.
To assess vitamin D levels among patients with diabetic septic foot and the role of vitamin D supplementation in the treatment of DFS.
We searched four databases and included studies other than case reports, perspectives, opinions, and editorials. The studies were included if they assessed the relationship between diabetic foot ulcers and vitamin D levels. The most recent RevMan system was used for data analysis.
Evidence from observational studies confirmed the association between vitamin D deficiency and diabetic foot ulcers, both among outpatients and hospitalized patients, the associations remained robot after controlling for demographic factors, the duration since the diagnosis of type 2 diabetes, and glycated hemoglobin (odds ratio: -6.32, 95% confidence interval: -923 to -3.42).
Vitamin D deficiency was associated with DFUs, and vitamin D supplementation was effective in slowing the progress. Various therapies along the RANK-osteoprotegerin pathway are promising.
The question of vitamin D and the optimal effective dose is elucidated. In addition, future therapies along the RANK-osteoprotegerin might address this dangerous diabetes complication.
The author would like to acknowledge the Saudi Digital Library for the free access of the databases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Saudi Arabia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: de Melo FF, Brazil; Greco T, Italy S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yu HG
| 1. | Lavery LA, Oz OK, Bhavan K, Wukich DK. Diabetic Foot Syndrome in the Twenty-First Century. Clin Podiatr Med Surg. 2019;36:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Rümenapf G, Morbach S, Rother U, Uhl C, Görtz H, Böckler D, Behrendt CA, Hochlenert D, Engels G, Sigl M; Kommission PAVK und Diabetisches Fußsyndrom der DGG e. V. [Diabetic foot syndrome-Part 1 : Definition, pathophysiology, diagnostics and classification]. Chirurg. 2021;92:81-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Morbach S, Lobmann R, Eckhard M, Müller E, Reike H, Risse A, Rümenapf G, Spraul M. Diabetic Foot Syndrome. Exp Clin Endocrinol Diabetes. 2021;129:S82-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Navarro-Flores E, Cauli O. Quality of Life in Individuals with Diabetic Foot Syndrome. Endocr Metab Immune Disord Drug Targets. 2020;20:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Ince P, Abbas ZG, Lutale JK, Basit A, Ali SM, Chohan F, Morbach S, Möllenberg J, Game FL, Jeffcoate WJ. Use of the SINBAD classification system and score in comparing outcome of foot ulcer management on three continents. Diabetes Care. 2008;31:964-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Mills JL Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, Andros G; Society for Vascular Surgery Lower Extremity Guidelines Committee. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI). J Vasc Surg. 2014;59:220-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 796] [Cited by in RCA: 1112] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 7. | Monteiro-Soares M, Boyko EJ, Jeffcoate W, Mills JL, Russell D, Morbach S, Game F. Diabetic foot ulcer classifications: A critical review. Diabetes Metab Res Rev. 2020;36 Suppl 1:e3272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 8. | Pérez-Panero AJ, Ruiz-Muñoz M, Cuesta-Vargas AI, Gónzalez-Sánchez M. Prevention, assessment, diagnosis and management of diabetic foot based on clinical practice guidelines: A systematic review. Medicine (Baltimore). 2019;98:e16877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Martins-Mendes D, Monteiro-Soares M, Boyko EJ, Ribeiro M, Barata P, Lima J, Soares R. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28:632-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021;17:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 196] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 11. | Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020;13:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 499] [Article Influence: 99.8] [Reference Citation Analysis (2)] |
| 12. | Charoenngam N, Holick MF. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 559] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 13. | Ao T, Kikuta J, Ishii M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 210] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 14. | Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, Martucci G, Pilz S, Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 780] [Cited by in RCA: 775] [Article Influence: 155.0] [Reference Citation Analysis (0)] |
| 15. | Gouni-Berthold I, Berthold HK. Vitamin D and Vascular Disease. Curr Vasc Pharmacol. 2021;19:250-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Dai J, Jiang C, Chen H, Chai Y. Vitamin D and diabetic foot ulcer: a systematic review and meta-analysis. Nutr Diabetes. 2019;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Lin J, Mo X, Yang Y, Tang C, Chen J. Association between vitamin D deficiency and diabetic foot ulcer wound in diabetic subjects: A meta-analysis. Int Wound J. 2023;20:55-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Afarideh M, Ghanbari P, Noshad S, Ghajar A, Nakhjavani M, Esteghamati A. Raised serum 25-hydroxyvitamin D levels in patients with active diabetic foot ulcers. Br J Nutr. 2016;115:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Çağlar S, Çağlar A, Pilten S, Albay C, Beytemur O, Sarı H. Osteoprotegerin and 25-hydroxy vitamin D levels in patients with diabetic foot. Eklem Hastalik Cerrahisi. 2018;29:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Dai J, Yu M, Chen H, Chai Y. Association Between Serum 25-OH-Vitamin D and Diabetic Foot Ulcer in Patients With Type 2 Diabetes. Front Nutr. 2020;7:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Danny Darlington CJ, Suresh Kumar S, Jagdish S, Sridhar MG. Evaluation of Serum Vitamin D Levels in Diabetic Foot Infections: A Cross-Sectional Study in a Tertiary Care Center in South India. Iran J Med Sci. 2019;44:474-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Feldkamp J, Jungheim K, Schott M, Jacobs B, Roden M. Correction: Severe Vitamin D3 Deficiency in the Majority of Patients with Diabetic Foot Ulcers. Horm Metab Res. 2018;50:e9. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Gupta B, Singh SK. Invitro study of role of vitamin d on macrophages dysfunction in patients with diabetic foot infection. Int J Adv Res. 2016;4:1633-1637. [DOI] [Full Text] |
| 24. | Tang W, Chen L, Ma W, Chen D, Wang C, Gao Y, Ran X. Association between vitamin D status and diabetic foot in patients with type 2 diabetes mellitus. J Diabetes Investig. 2022;13:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Tiwari S, Pratyush DD, Gupta SK, Singh SK. Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr. 2014;112:1938-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Todorova AS, Jude EB, Dimova RB, Chakarova NY, Serdarova MS, Grozeva GG, Tsarkova PV, Tankova TI. Vitamin D Status in a Bulgarian Population With Type 2 Diabetes and Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2022;21:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Tsitsou S, Dimosthenopoulos C, Eleftheriadou I, Andrianesis V, Tentolouris N. Evaluation of Vitamin D Levels in Patients With Diabetic Foot Ulcers. Int J Low Extrem Wounds. 2023;22:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Wang F, Zhou L, Zhu D, Yang C. A Retrospective Analysis of the Relationship Between 25-OH-Vitamin D and Diabetic Foot Ulcer. Diabetes Metab Syndr Obes. 2022;15:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Xiao Y, Wei L, Xiong X, Yang M, Sun L. Association Between Vitamin D Status and Diabetic Complications in Patients With Type 2 Diabetes Mellitus: A Cross-Sectional Study in Hunan China. Front Endocrinol (Lausanne). 2020;11:564738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 770] [Cited by in RCA: 1634] [Article Influence: 148.5] [Reference Citation Analysis (0)] |
| 31. | Macido A. Diabetic Foot Ulcers and Vitamin D Status: A Literature Review. SAGE Open Nurs. 2018;4:2377960818789027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Kota SK, Meher LK, Jammula S, Modi KD. Inflammatory markers in diabetic foot and impact of vitamin D deficiency. Endocr Abstr. 2013;32:P384. [DOI] [Full Text] |
| 33. | Yammine K, Hayek F, Assi C. Is there an association between vitamin D and diabetic foot disease? A meta-analysis. Wound Repair Regen. 2020;28:90-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Greenhagen RM, Frykberg RG, Wukich DK. Serum vitamin D and diabetic foot complications. Diabet Foot Ankle. 2019;10:1579631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Zubair M, Malik A, Meerza D, Ahmad J. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr. 2013;7:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Quraishi SA, Camargo CA Jr. Vitamin D in acute stress and critical illness. Curr Opin Clin Nutr Metab Care. 2012;15:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Gaudet M, Plesa M, Mogas A, Jalaleddine N, Hamid Q, Al Heialy S. Recent advances in vitamin D implications in chronic respiratory diseases. Respir Res. 2022;23:252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 38. | Pop TL, Sîrbe C, Benţa G, Mititelu A, Grama A. The Role of Vitamin D and Vitamin D Binding Protein in Chronic Liver Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 39. | Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 40. | Lee CJ, Iyer G, Liu Y, Kalyani RR, Bamba N, Ligon CB, Varma S, Mathioudakis N. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: A systematic review and meta-analysis of intervention studies. J Diabetes Complications. 2017;31:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Shofler D, Hamedani E, Seun J, Sathananthan A, Katsaros E, Liggan L, Kang S, Pham C. Investigating the Use of Denosumab in the Treatment of Acute Charcot Neuroarthropathy. J Foot Ankle Surg. 2021;60:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Greco T, Mascio A, Comisi C, Polichetti C, Caravelli S, Mosca M, Mondanelli N, Troiano E, Maccauro G, Perisano C. RANKL-RANK-OPG Pathway in Charcot Diabetic Foot: Pathophysiology and Clinical-Therapeutic Implications. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Smith K, Hewlings S. Correlation between vitamin D levels and hard-to-heal wounds: a systematic review. J Wound Care. 2021;30:S4-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Halschou-Jensen PM, Sauer J, Bouchelouche P, Fabrin J, Brorson S, Ohrt-Nissen S. Improved Healing of Diabetic Foot Ulcers After High-dose Vitamin D: A Randomized Double-blinded Clinical Trial. Int J Low Extrem Wounds. 2021;15347346211020268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Kurian SJ, Miraj SS, Benson R, Munisamy M, Saravu K, Rodrigues GS, Rao M. Vitamin D Supplementation in Diabetic Foot Ulcers: A Current Perspective. Curr Diabetes Rev. 2021;17:512-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Papaioannou I, Pantazidou G, Kokkalis Z, Georgopoulos N, Jelastopulu E. Vitamin D Deficiency in Elderly With Diabetes Mellitus Type 2: A Review. Cureus. 2021;13:e12506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, Asemi Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 121] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 48. | Putz Z, Tordai D, Hajdú N, Vági OE, Kempler M, Békeffy M, Körei AE, Istenes I, Horváth V, Stoian AP, Rizzo M, Papanas N, Kempler P. Vitamin D in the Prevention and Treatment of Diabetic Neuropathy. Clin Ther. 2022;44:813-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |