Published online Feb 3, 2023. doi: 10.13105/wjma.v11.i2.47
Peer-review started: October 20, 2022
First decision: November 14, 2022
Revised: November 30, 2022
Accepted: January 17, 2023
Article in press: January 17, 2023
Published online: February 3, 2023
Processing time: 104 Days and 13.4 Hours
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and causes one third of cancer related deaths world-wide. Approximately one third of patients with HCC are eligible for curative treatments that include hepatic resection, liver transplantation or imaging guided tumor ablation. Recurrence rates after primary therapy depends on tumor biology and pre-treatment tumor burden with early recurrence rates ranging from 30%-80% following surgical resection and ablation. HCC recurs in over ten percent following liver trans
Core Tip: Tumor recurrence is frequent following potentially curative modalities for hepatocellular carcinoma. Patients should undergo surveillance imaging following curative treatments and once diagnosed, are potentially eligible for repeat hepatic resection, ablation, trans-arterial embolic therapies, or systemic therapies.
- Citation: Frager SZ, Cooper W, Saenger Y, Schwartz JM. Treatment of recurrent hepatocellular carcinoma following liver resection, ablation or liver transplantation. World J Meta-Anal 2023; 11(2): 47-54
- URL: https://www.wjgnet.com/2308-3840/full/v11/i2/47.htm
- DOI: https://dx.doi.org/10.13105/wjma.v11.i2.47
Hepatocellular carcinoma (HCC) incidence has been increasing over the last three decades[1] but fortunately may have potentially begun to plateau in the United States[2]. HCC is the most common form of primary liver cancer[3] and the sixth most common cancer overall and has a high case fatality rate[4]. Based on the Scientific Registry of Transplant Recipients (SRTR) 2020 data[5], HCC composes 10.9% of new liver transplant waiting list registrations, a rate that has doubled over the past decade.
The treatment algorithm and prognostic staging for primary HCC after initial diagnosis has been clearly defined by the Barcelona Clinic Liver Cancer (BCLC) staging system[6]. The BCLC system characterizes patients according to tumor size, tumor number, severity of liver disease using the Child Turcotte Pugh classification, and the Eastern Cooperative Oncology Group performance status[7]. Imaging guided tumor ablation, liver resection, or orthotopic liver transplantation (OLT) are considered curative options for very early and early-stage HCC with a post treatment median overall survival between 6-10 years[8]. The Milan Criteria has been used for over twenty-five years to risk stratify HCC patient eligibility for OLT in the setting of HCC. The Milan criteria is defined as a single lesion greater than or equal to 2 cm and less than or equal to 5 cm, or 2 to 3 Lesions, each greater than or equal to 1 cm and less than or equal to 3 cm in the absence of extrahepatic metastases or main portal vein invasion[9]. Patients with intermediate stage HCC are treated with trans-arterial modalities including chemoembolization and Yttrium-90 (90Y) radio-embolization.
Efforts to expand criteria for primary resection and liver transplantation have evolved. For example, Yin et al[10] in 2014, data has shown that resection of HCC outside Milan criteria might lead to better outcomes compared to trans-arterial chemoembolization (TACE) in the appropriate clinical setting. In addition, successful reduction of tumor burden to within the Milan criteria has resulted in successful transplant outcomes[11].
Over the last decade, the systemic therapeutic options for HCC have advanced dramatically[3]. The improvement of imaging modalities and vascular techniques have also allowed for earlier diagnosis and more selective locoregional therapies for both ablation and chemo-embolic options.
Nevertheless, HCC recurs 50%-70% of patients after primary hepatic resection and in 8%-17% of patients after liver transplantation[12-15] with early recurrence (< 24 mo) portending worsening survival[16]. There is long-term data showing a 34.3% chance of recurrence after 10 year survival with 10% of the overall cohort surviving with locoregional therapy alone[17]. Given the high overall rate of HCC recurrence, this article will review the available options for patients specifically regarding HCC recurrence following curative modalities such as hepatectomy/resection, tumor ablation, and liver transplantation.
Hepatic resection is considered the primary treatment modality for patients with BCLC stage 0-A HCC without evidence of portal hypertension or hepatic decompensation (ascites, varices, hepatic encephalopathy). The Model for End Stage Liver Disease score (MELD) and CPT score[18,19] have been used for risk stratification. Data by Bismuth et al[20] showed 5%-15% of patients presenting with HCC will be eligible for hepatic resection. Post resection HCC recurrence rates are 19%, 54%, and 70% for 1, 3, and 5 years, respectively. As with pre-treatment HCC diagnosis, tumor recurrence is defined radiographically using the Liver Reporting and Data System (LI-RADS)[21] or modified Response Evaluation Criteria in Solid Tumors (mRECIST)[22]. When imaging is indeterminate, a liver biopsy can be performed for tissue sampling, however tumor biopsy is typically not performed to establish a diagnosis of HCC recurrence.
In patients with early-stage HCC and compensated liver disease, all Eastern and Western societies[15,23,24] recommend hepatectomy as the first-line therapy with 5-year survival rates ranging from 60%-80%. However, recurrence can occur in up to 80% of patients despite resection[23]. Tumor recurrence can be characterized as early and late based on the time to recurrence from initial resection with a cut-off of 2 years[25]. Intrahepatic metastasis is associated with early recurrence of HCC. Late recurrence of HCC is often not related to the primary tumor and likely reflects the underlying malignant predisposition of the background liver parenchyma. There is no specific treatment guidance for repeat hepatectomy for HCC recurrence and practices are based on local expertise and expert opinion. In general, patients with a single localized recurrent tumor without portal hypertension and normal liver synthetic function are good candidates for repeat hepatectomy.
There is heterogeneity in the surgical trials advocating for repeat hepatectomy stemming from diverse inclusion criteria. The data has been collected in the Eastern Hemisphere with only one large Western study presented by Roayaie et al[26] (2011). The 5-year survival rate was 67% in this study. In this cohort, a higher five-year overall survival (OS) (66.8% vs 55.5% respectively, P = 0.006) was seen in patients undergoing anatomic resection (AR) vs non-anatomic resection (NAR). However, there was no significant difference in peri-operative morbidity or mortality rates between anatomic vs AR or NAR. A large Eastern series by Zou et al[27] showed a 1, 3, and 5-year overall all survival rates of 96.9, 74.8, and 47.8%, respectively. Post-operative complication rates range from 0-6% (ascites, bile leak, liver failure)[28].
There is scant data comparing repeat laparoscopic resection vs open hepatectomy. In a study by Cai et al[29], 2019, there was a similar 90-d mortality between these groups although other metrics (blood loss, hospital length of stay) were better in the laparoscopic cohort. The selection of surgical techniques is based on both patient and tumor characteristics and is an evolving area of interest in surgical literature.
Locoregional therapies are available for patients with unresectable recurrent HCC or for a patient with worsening portal hypertension/Liver function following primary hepatic resection. Radiofrequency or microwave ablation has been utilized for recurrent tumors < 3 cm in diameter although caution must be used to avoid collateral structural damage. A meta-analysis of 18 prior studies showed ablation for recurrent HCC has a post-ablation recurrence rate of 79% with a complication rate of near 2.9% although this may be an inaccurate value given much of the data was not reported[30]. There are very few good studies comparing the outcomes for post recurrence ablation vs hepatic resection alone[31].
Trans-arterial chemoembolization (TACE) is a non-curative modality that can be used for tumor control for patients who are not candidate for repeat hepatic resection. Three-year survival post-TACE is 29% for primary HCC[32]. In a review from Erridge et al[33], the 5-year survival was 15.5% in patients who underwent TACE for recurrent HCC. Other studies have shown that the outcome could be worse, and that palliation is the end goal for this therapy modality[34].
In the United States, patients who are eligible for liver transplantation following HCC recurrence benefit from early evaluation and placement on the transplant waiting list without a 6-mo waiting period[15]. Studies by Hu et al[35], 2012, and Kostakis et al[36], 2019, and have evaluated salvage liver trans
A burgeoning area of interest and study is adjuvant immunotherapy post following hepatic resection[37]. The NIVOLVE trial tested adjuvant nivolumab with median recurrence free survival of 26.3 mo[38]. This compares quite favorably with the median recurrence free survival of 8.5 mo observed with sorafenib in the STORM trial[39]. Based on data in the metastatic setting, the addition of a vascular endothelial growth factor/vascular endothelial growth factor receptor inhibitor (such as bevacizumab) to an immune checkpoint inhibitor backbone could further improve outcomes. Multiple trials of adjuvant immunotherapy are ongoing including IMBRAVE 050 (atezolizumab+ bevacizumab), KEYNOTE 937 (pembrolizumab), and Checkmate 9DX (nivolumab) for which results are anticipated.
When recurrence is not amenable to surgical resection or local regional therapy, systemic therapy is often the only option. The current standard of care is to use the combination of the immune checkpoint inhibitor atezolizumab with bevacizumab, a regimen that showed significant survival benefit relative to sorafenib, the prior standard therapy[40]. For patients with prior episodes of bleeding or mucosal inflammation precluding use of bevacizumab, single agent anti-PD1 therapy may offer benefit. The combination of ipilimumab (anti-CTLA-4) and nivolumab is now FDA approved based on trial data showed a 30% response rate in all treatment arms[41]. Multiple novel immunotherapy combinations are being studied including combinations with anti-Lag3 antibodies, a therapeutic that has yielded survival benefit in melanoma[42].
Based on the BCLC staging system, HCC ablation is offered for patients who are not candidates for surgical resection or are ineligible for liver transplantation based on medical or psychosocial barriers. HCC recurrence after RFA occurs can occur in up to 15% of patients undergoing this treatment modality[43]. While not fully understood, the reason is thought to be due to micro-tumor spread via arterial-portal shunts related to thermal and mechanical damage caused during the RFA procedure. Patients with recurrence can be treated by repeating RFA alone or combining RFA and TACE or initiation of systemic therapy based on the extent of tumor recurrence.
There is strong rationale for combining local therapy with immunotherapy in the setting of recurrent HCC, both because the probability of long-term cure with repeat local treatment is lower than with initial therapy, and because local treatment can release tumor antigens and favorably alter the tumor immune micro-environment. Multiple studies are ongoing examining these combinations including trials combining ablation, RFA, brachytherapy and/or TACE with pembrolizumab, tislezumab, atezoliczumab and bevacizumab, and others[44]. One recent retrospective study examined 31 patients who underwent concurrent TACE and nivolumab and found that they achieved a significantly longer median survival (8.8 mo) than patients treated with TACE alone (3.7 mo) with some patients achieving prolonged survival greater than 20 mo[45].
Liver transplantation can be curative among select patients with hepatocellular carcinoma who are ineligible for hepatic resection. Initial studies by Mazzaferro et al[9] showed excellent long term recurrent free survival. These criteria are known as the Milan criteria.
HCC recurs at a rate of 10%-15% following liver transplantation among patients who meet the Milan criteria prior to liver transplantation with higher recurrence rates in patients who exceed the Milan criteria[12]. HCC typically recurs in the lungs, bones, and in the liver. Other sites of recurrence include the adrenal glands and the central nervous system.
Data regarding pre transplant risk factors for HCC recurrence including pre-transplant Alpha fetoprotein (AFP) and a short duration between listing for transplantation and the transplant surgery has influenced the United States transplant regulatory agency, United Network of Organ Sharing to incorporate AFP criteria and as well as a six-month waiting period prior to transplant eligibility.
There are several prognostic systems that help to predict HCC recurrence following liver transplantation (LT). Markers of tumor biology such as pre transplant AFP, explant tumor differentiation and the presence of microvascular tumor invasion are incorporated into most of these models. One such model, metroticket 2.0 includes AFP and tumor morphology can be used to predict post-transplant outcomes[46]. Another readily applied model is the Risk Estimation of Tumor Recurrence After Transplant (RETREAT) predictive model that included AFP, tumor size and microvascular invasion[47].
HCC typically recurs in the first 3 years following LT and the tumor biology of recurrent HCC is influence by the immunosuppressed state[48]. It is unresolved whether reduced calcineurin inhibitor and addition or substitution of calcineurin inhibitors with m-TOR inhibitors leads to reduced HCC recurrence[49].
Patients typically undergo surveillance imaging for up to five years following LT with contrast enhanced computed tomography (CT) or magnetic resonance imaging every six to 12 months as well as non-contrast chest CT and AFP testing to identify HCC recurrence as early as possible. Evidence to support this practice is lacking, and it may be appropriate to target patients at highest risk with more frequent surveillance.
There is also no data to support chemoprevention with systemic chemotherapy in this context. In addition, it is unclear whether pre transplant immunotherapy reduces rates of HCC recurrence.
Patients with HCC recurrence have significantly lower survival than patients who do not recur. Most recurrences occur in extrahepatic locations, and patients who are eligible for surgical intervention are more likely to have improved outcomes[50].
Patients with widely metastatic recurrent HCC following LT are eligible for systemic therapy as described above including the use of sorafenib, regorafenib, lenvantinib and cabozantinib. These agents are often difficult to tolerate given significant drug interactions with immunosuppressive agents.
Treatment with immunotherapeutic agents that target programmed cell death protein 1 (PD1)/ programmed cell death ligand 1 (PD-L1) mechanistically enhance immune response against malignant cells. These agents can activate the immune cascade with resultant graft loss due to rejection.
While there are multiple reports of safe use of immunotherapy prior to transplant, use of immunotherapy after transplant is much riskier. A literature review identified 28 patients who had immunotherapy after transplantation[51]. Early mortality occurred in 6 patients and 9 patients experienced allograft rejection that was frequently severe. Rejection was more likely to occur earlier after transplantation. Median overall survival was 7.3 mo. If used at all, these agents should be used with extreme caution, perhaps with higher levels of immunosuppression or in the context of a clinical trial at a specialized high volume transplant center.
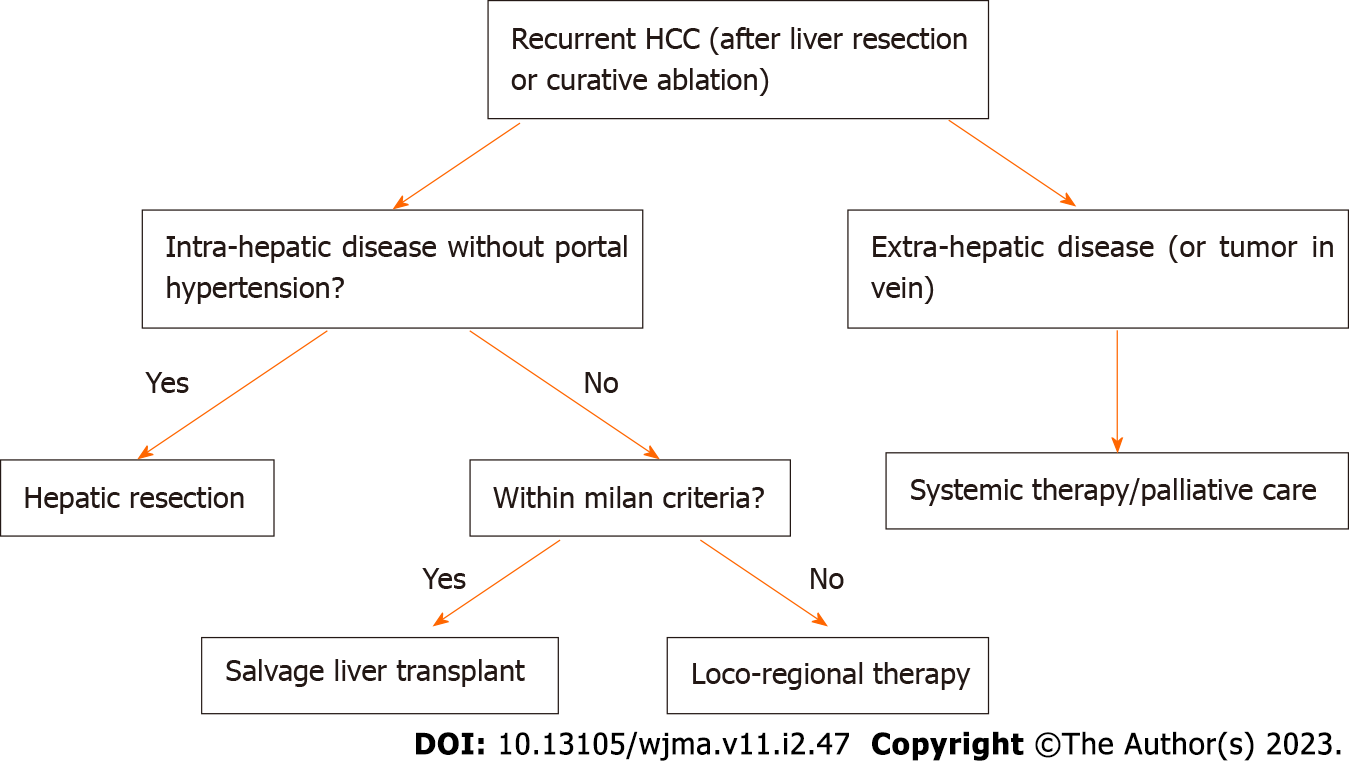
HCC recurrence is common after initial therapy and early vs late recurrence may impact overall survival. The increase is treatment options for primary HCC over the last decade has allowed the field to evolve and extrapolate these modalities for use in HCC recurrence. In general, the overall therapeutic approach to HCC recurrence is similar to primary HCC despite specific anatomical and immune related constraints which may occur after liver transplantation or hepatic resection. Advancement in systemic chemo/immune therapies both in the adjuvant and neoadjuvant phase has allowed for additional survival in cases of unresectable HCC recurrence. New frontiers in locoregional therapies have also allowed for better HCC tumor recurrence control. The authors recommend using the treatment algorithm based on Figure 1. These treatment approach incorporates all of the aforementioned treatment modalities and gives the clinician a data driven and simplified approach to HCC recurrence.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Luo Y, China; Soldera J, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis. 2015;19:223-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 653] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 2. | Han J, Wang B, Liu W, Wang S, Chen R, Chen M, Fu Z. Declining disease burden of HCC in the United States, 1992-2017: A population-based analysis. Hepatology. 2022;76:576-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3887] [Article Influence: 971.8] [Reference Citation Analysis (3)] |
| 4. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64696] [Article Influence: 16174.0] [Reference Citation Analysis (177)] |
| 5. | Kwong A, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA, Noreen SM, Foutz J, Miller E, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2018 Annual Data Report: Liver. Am J Transplant. 2020;20 Suppl s1:193-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 314] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 6. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2618] [Article Influence: 872.7] [Reference Citation Analysis (59)] |
| 7. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7038] [Cited by in RCA: 8007] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 8. | Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1276] [Article Influence: 141.8] [Reference Citation Analysis (2)] |
| 9. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5313] [Article Influence: 183.2] [Reference Citation Analysis (0)] |
| 10. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 272] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 11. | Yao FY, Mehta N, Flemming J, Dodge J, Hameed B, Fix O, Hirose R, Fidelman N, Kerlan RK Jr, Roberts JP. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 370] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 12. | Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261:947-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 659] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 13. | Fernandez-Sevilla E, Allard MA, Selten J, Golse N, Vibert E, Sa Cunha A, Cherqui D, Castaing D, Adam R. Recurrence of hepatocellular carcinoma after liver transplantation: Is there a place for resection? Liver Transpl. 2017;23:440-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Chagas AL, Felga GEG, Diniz MA, Silva RF, Mattos AA, Silva RCMA, Boin IFSF, Garcia JHP, Lima AS, Coelho JCU, Bittencourt PL, Alves VAF, D'Albuquerque LAC, Carrilho FJ; Brazilian HCC Study Group. Hepatocellular carcinoma recurrence after liver transplantation in a Brazilian multicenter study: clinical profile and prognostic factors of survival. Eur J Gastroenterol Hepatol. 2019;31:1148-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 16. | Toso C, Cader S, Mentha-Dugerdil A, Meeberg G, Majno P, Morard I, Giostra E, Berney T, Morel P, Mentha G, Kneteman NM. Factors predicting survival after post-transplant hepatocellular carcinoma recurrence. J Hepatobiliary Pancreat Sci. 2013;20:342-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Sempokuya T, Wong LL. Ten-year survival and recurrence of hepatocellular cancer. Hepatoma Res. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D'Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3678] [Article Influence: 153.3] [Reference Citation Analysis (0)] |
| 19. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5738] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 20. | Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 633] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 21. | Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 785] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 22. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 23. | Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res. 2019;49:1109-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 398] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 24. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 25. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1061] [Cited by in RCA: 1234] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 26. | Roayaie S, Bassi D, Tarchi P, Labow D, Schwartz M. Second hepatic resection for recurrent hepatocellular cancer: a Western experience. J Hepatol. 2011;55:346-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Zou Q, Li J, Wu D, Yan Z, Wan X, Wang K, Shi L, Lau WY, Wu M, Shen F. Nomograms for Pre-operative and Post-operative Prediction of Long-Term Survival of Patients Who Underwent Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma. Ann Surg Oncol. 2016;23:2618-2626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol. 2013;22:e23-e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Cai W, Liu Z, Xiao Y, Zhang W, Tang D, Cheng B, Li Q. Comparison of clinical outcomes of laparoscopic versus open surgery for recurrent hepatocellular carcinoma: a meta-analysis. Surg Endosc. 2019;33:3550-3557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Thomasset SC, Dennison AR, Garcea G. Ablation for recurrent hepatocellular carcinoma: a systematic review of clinical efficacy and prognostic factors. World J Surg. 2015;39:1150-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Kawano Y, Sasaki A, Kai S, Endo Y, Iwaki K, Uchida H, Shibata K, Ohta M, Kitano S. Prognosis of patients with intrahepatic recurrence after hepatic resection for hepatocellular carcinoma: a retrospective study. Eur J Surg Oncol. 2009;35:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, Rodés J, Bruix J; Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2611] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 33. | Erridge S, Pucher PH, Markar SR, Malietzis G, Athanasiou T, Darzi A, Sodergren MH, Jiao LR. Meta-analysis of determinants of survival following treatment of recurrent hepatocellular carcinoma. Br J Surg. 2017;104:1433-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 34. | Takayasu K, Arii S, Ikai I, Kudo M, Matsuyama Y, Kojiro M, Makuuchi M; Liver Cancer Study Group of Japan. Overall survival after transarterial lipiodol infusion chemotherapy with or without embolization for unresectable hepatocellular carcinoma: propensity score analysis. AJR Am J Roentgenol. 2010;194:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Hu Z, Wang W, Li Z, Ye S, Zheng SS. Recipient outcomes of salvage liver transplantation versus primary liver transplantation: a systematic review and meta-analysis. Liver Transpl. 2012;18:1316-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Kostakis ID, Machairas N, Prodromidou A, Stamopoulos P, Garoufalia Z, Fouzas I, Sotiropoulos GC. Comparison Between Salvage Liver Transplantation and Repeat Liver Resection for Recurrent Hepatocellular Carcinoma: A Systematic Review and Meta-analysis. Transplant Proc. 2019;51:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Girardi DM, Pacífico JPM, Guedes de Amorim FPL, Dos Santos Fernandes G, Teixeira MC, Pereira AAL. Immunotherapy and Targeted Therapy for Hepatocellular Carcinoma: A Literature Review and Treatment Perspectives. Pharmaceuticals (Basel). 2020;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Kudo M UK, Nakahira S, Nishida N, Ida H, Minami Y. Adjuvant nivolumab for hepatocellular carcinoma (HCC) after surgical resection (SR) or radiofrequency ablation (RFA) (NIVOLVE): a phase 2 prospective multicenter single-arm trial and exploratory biomarker analysis. Journal of Clinical Oncology. 2021;39 (suppl 15):4070. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM; STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 788] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 40. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4707] [Article Influence: 941.4] [Reference Citation Analysis (2)] |
| 41. | Wong JSL, Kwok GGW, Tang V, Li BCW, Leung R, Chiu J, Ma KW, She WH, Tsang J, Lo CM, Cheung TT, Yau T. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 42. | Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas HJ, Lao CD, De Menezes JJ, Dalle S, Arance A, Grob JJ, Srivastava S, Abaskharoun M, Hamilton M, Keidel S, Simonsen KL, Sobiesk AM, Li B, Hodi FS, Long GV; RELATIVITY-047 Investigators. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N Engl J Med. 2022;386:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 1159] [Article Influence: 386.3] [Reference Citation Analysis (0)] |
| 43. | Kang TW, Lim HK, Cha DI. Aggressive tumor recurrence after radiofrequency ablation for hepatocellular carcinoma. Clin Mol Hepatol. 2017;23:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Hatzidakis A, Müller L, Krokidis M, Kloeckner R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 45. | Marinelli B, Kim E, D'Alessio A, Cedillo M, Sinha I, Debnath N, Kudo M, Nishida N, Saeed A, Hildebrand H, Kaseb AO, Abugabal YI, Pillai A, Huang YH, Khan U, Muzaffar M, Naqash AR, Patel R, Fischman A, Bishay V, Bettinger D, Sung M, Ang C, Schwartz M, Pinato DJ, Marron T. Integrated use of PD-1 inhibition and transarterial chemoembolization for hepatocellular carcinoma: evaluation of safety and efficacy in a retrospective, propensity score-matched study. J Immunother Cancer. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 46. | Mazzaferro V, Sposito C, Zhou J, Pinna AD, De Carlis L, Fan J, Cescon M, Di Sandro S, Yi-Feng H, Lauterio A, Bongini M, Cucchetti A. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154:128-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 47. | Mehta N, Heimbach J, Harnois DM, Sapisochin G, Dodge JL, Lee D, Burns JM, Sanchez W, Greig PD, Grant DR, Roberts JP, Yao FY. Validation of a Risk Estimation of Tumor Recurrence After Transplant (RETREAT) Score for Hepatocellular Carcinoma Recurrence After Liver Transplant. JAMA Oncol. 2017;3:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 291] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 48. | Escartin A, Sapisochin G, Bilbao I, Vilallonga R, Bueno J, Castells L, Dopazo C, Castro E, Caralt M, Balsells J. Recurrence of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2007;39:2308-2310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten TM, Schmidt J, Settmacher U, Heise M, Rossi G, Cillo U, Kneteman N, Adam R, van Hoek B, Bachellier P, Wolf P, Rostaing L, Bechstein WO, Rizell M, Powell J, Hidalgo E, Gugenheim J, Wolters H, Brockmann J, Roy A, Mutzbauer I, Schlitt A, Beckebaum S, Graeb C, Nadalin S, Valente U, Turrión VS, Jamieson N, Scholz T, Colledan M, Fändrich F, Becker T, Söderdahl G, Chazouillères O, Mäkisalo H, Pageaux GP, Steininger R, Soliman T, de Jong KP, Pirenne J, Margreiter R, Pratschke J, Pinna AD, Hauss J, Schreiber S, Strasser S, Klempnauer J, Troisi RI, Bhoori S, Lerut J, Bilbao I, Klein CG, Königsrainer A, Mirza DF, Otto G, Mazzaferro V, Neuhaus P, Schlitt HJ. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomized, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100:116-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 50. | Plessier A, Codes L, Consigny Y, Sommacale D, Dondero F, Cortes A, Degos F, Brillet PY, Vilgrain V, Paradis V, Belghiti J, Durand F. Underestimation of the influence of satellite nodules as a risk factor for post-transplantation recurrence in patients with small hepatocellular carcinoma. Liver Transpl. 2004;10:S86-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Pelizzaro F, Gambato M, Gringeri E, Vitale A, Cillo U, Farinati F, Burra P, Russo FP. Management of Hepatocellular Carcinoma Recurrence after Liver Transplantation. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |