Published online Oct 28, 2022. doi: 10.13105/wjma.v10.i5.238
Peer-review started: August 4, 2022
First decision: August 19, 2022
Revised: August 23, 2022
Accepted: September 21, 2022
Article in press: September 21, 2022
Published online: October 28, 2022
Processing time: 84 Days and 20.4 Hours
Helicobacter pylori (H. pylori) infection occurs in almost half of the world's population, most of whom are merely carriers of this microorganism. H. pylori is shown to be detected more frequently in patients with diabetes mellitus (DM) than in the general population, which is accompanied by a significantly increased risk of developing H. pylori-associated diseases. In addition, eradication therapy shows a low efficiency for H. pylori infection in patients with DM. There is a relationship between the level of chronic hyperglycemia and a higher detection rate of H. pylori as well as a lower efficiency of eradication therapy in patients with DM. The exact mechanisms of these phenomena are unknown. The authors make a hypothesis that explains the relationship between chronic hyperglycemia and the increased detection rate of H. pylori, as well as the mechanisms contributing to the improved survival of this bacterium in patients with DM during eradication therapy.
Core Tip: The authors hypothesize that in patients with diabetes mellitus (DM), Helicobacter pylori (H. pylori) are most likely to rely on both amino acids and glucose for its vital activity. The hypothesis makes it possible to explain the high detection rate of H. pylori in patients with DM, as well as the lower efficiency of eradication therapy in them.
- Citation: Reshetnyak VI, Maev IV. Maintaining the metabolic homeostasis of Helicobacter pylori through chronic hyperglycemia in diabetes mellitus: A hypothesis. World J Meta-Anal 2022; 10(5): 238-243
- URL: https://www.wjgnet.com/2308-3840/full/v10/i5/238.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i5.238
Forty years have passed since the description of Helicobacter pylori (H. pylori) as a pathogen in the development of atrophic gastritis and peptic ulcer disease[1-3]. It has been shown that H. pylori infection occurs in almost half of the population in the world, most of whom are merely carriers of this microorganism[4,5]. In addition, many researchers have indicated that H. pylori are detected more frequently in patients with diabetes mellitus (DM) than in the general population[6-11]. This is accompanied by a substantial increase in the risk of developing H. pylori-associated diseases[6,11,12]. At the same time, there are studies which report reverse results about the incidence of type 2 DM (T2DM) in H. pylori-positive patients[13-15]. However, the relationship between H. pylori infection and the risk of develo
There is a clear correlation between the higher detection rate of H. pylori in diabetic patients and lower efficacy of eradication therapy, depending on the level of hyperglycemia[10,13,29]. Uncontrolled diabetes with the development of chronic hyperglycemia causes a number of metabolic changes[30]. Chronic hyperglycemia in turn leads to increased susceptibility to infective agents in diabetic patients[9,10,30,31]. The exact mechanisms underlying the link of chronic hyperglycemia and the higher detection rate of H. pylori, as well as the mechanisms that improve the survival of this bacterium in diabetic patients during eradication therapy remain unknown. An understanding of how chronic hyperglycemia is related to the maintenance of the metabolic homeostasis of H. pylori for its vital activity and reproduction in diabetic patients is of great scientific and practical importance.
It is hypothesized that chronic hyperglycemia is associated with: (1) The increased detection rate of H. pylori; (2) possible metabolic changes in the bacterial cells ; and (3) the results of eradication therapy.
It is well known that H. pylori colonizes the gastric mucosa. To establish long-term colonization, the bacterium must sense and adapt to the nutritional conditions that exist in its habitat. Surprisingly, little attention has been paid to the preferred sources of nutrients and energy for the life, growth, and reproduction of H. pylori, as well as changes in the source of food ingredients and energy for H. pylori in diabetic patients. The available data suggest that for its life, growth, and reproduction, H. pylori utilizes amino acids and carboxylic acids, which are produced in sufficient quantities in the stomach as a result of hydrolysis of food proteins[32-34]. H. pylori catabolize a large amount of amino acids with the most substantial being alanine, arginine, asparagine, aspartate, glutamate, glutamine, proline, and serine[32,35-37]. H. pylori can also catabolize fumaric acid[38], malic acid[35], and lactic acid[39]. Thus, amino acids and carboxylic acids are sources of carbon, nitrogen, and energy.
In a healthy individual, H. pylori are almost independent of sugars, such as glucose[32-34]. However, glucose is known to be one of the most important carbohydrates, which is used for life by many microorganisms, including inhabitants in the digestive system. Moreover, Wang et al[40] believe that glucose plays a key role in the outcome of bacterial infection in humans. A question is raised as to whether H. pylori can utilize glucose as a plastic and energy material. Studies conducted in the 1990s and later indicate that H. pylori has enzyme systems capable of utilizing carbohydrates, D-glucose in particular[41-43]. These data suggest that in its evolutionary phylogenetic development and adaptation to life and reproduction in the stomach, H. pylori not only acquire the ability to restructure its metabolism for the use of amino acids as a plastic and energy material, but most probably retain the ability to utilize carbohydrates for their life activity. There are experimental data showing that adding glucose to the nutrient medium when growing H. pylori, enhances its growth[29,44].
Chronic hyperglycemia in diabetic patients involves compensatory mechanisms aimed at normalizing the blood level of glucose[5]. To remove excess glucose in patients with DM and chronic hyperglycemia, it is most likely that the extradigestive (excretory) function of the gastric mucosa is switched on. This leads to the fact that in patients with DM and chronic hyperglycemia, H. pylori gain advantages for its growth, reproduction, and survival as it can use not only amino acids for its life, but also glucose available in excess in patients with DM. This hypothesis may explain the more frequent detection of H. pylori in patients with DM than in the general population.
Based on this hypothesis, it is possible to explain also the lower efficiency of eradication therapy in patients with DM.
H. pylori eradication regimens contain antibacterial drugs (clarithromycin, metronidazole, bismuths, etc.) and agents that reduce hydrochloric acid production. The use of antacids aimed at creating optimal conditions for acid-dependent antibacterial agents[45-48]. The data presented in recent studies suggest that it is extremely important to determine gastric pH for H. pylori eradication[45,46]. In addition, the antacids have a double effect on H. pylori with an opposite effect. Increased gastric pH is a favorable factor for the vital activity of H. pylori. But at the same time, the antacids deprive H. pylori of nutrients. Exposure to hydrochloric acid in the stomach causes denaturation of food proteins and initiates their hydrolysis by the gastric juice enzymes pepsin and gastrixin. This gives rise to oligopeptides with different lengths and to a certain amount of amino acids, which are utilized by H. pylori for its life activity. Taking antacids practically does not lead to denaturation of food proteins. Consequently, the rate of protein hydrolysis is considerably reduced. As a result, the stomach practically does not produce amino acids that are essential for maintaining the vital activity of H. pylori. The lack of nutrients and the intake of antibacterial drugs result in the death of the microorganism or in its transition to a dormant form[49]. The latter is rare during powerful antibiotic therapy.
There is an opportunity for H. pylori to utilize glucose as an energy and plastic material in diabetic patients receiving eradication therapy against the underlying chronic hyperglycemia and amino acid deficiency. It is likely that this mechanism enables this microorganism to successfully survive the extreme conditions of eradication. But this can happen only in the presence of chronic hyperglycemia. That is to say, the survival of H. pylori under extreme conditions of eradication should depend on the level of hyperglycemia. And the longer period of hyperglycemia is, the more likely H. pylori survive the extreme conditions of eradication.
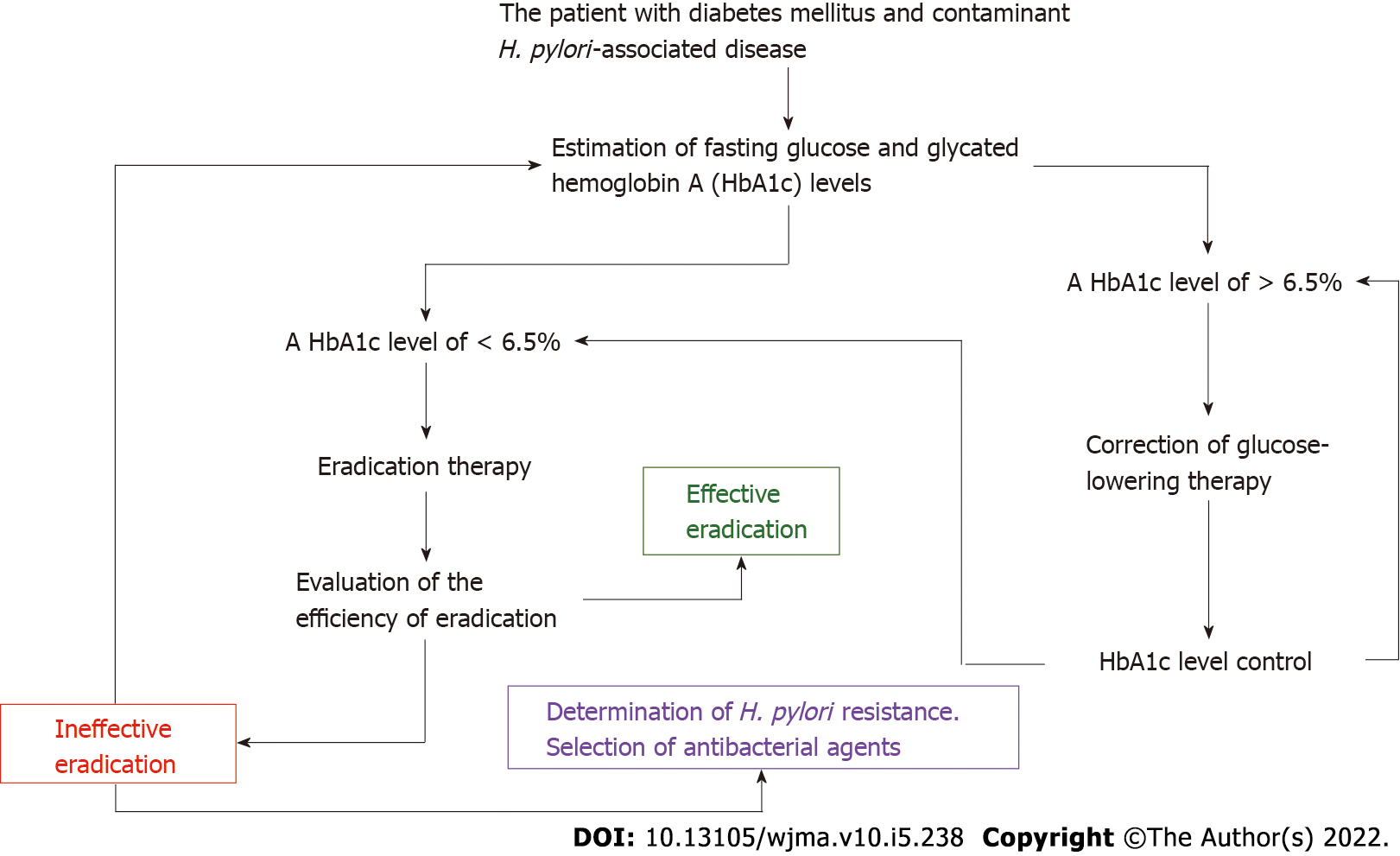
Chronic hyperglycemia can be assessed by the blood level of glycated hemoglobin A (HbA1c) (Figure 1). The HbA1c level is the result of nonenzymatic glycosylation of hemoglobin, with the formation of a bond between glucose and the free N-terminal proline amino group in the hemoglobin β-chain[50]. The indicator plays an important role in monitoring the time course of changes in blood glucose levels in diabetic patients and for evaluation of the efficacy of hypoglycemic drugs[51]. In 2011, the World Health Organization officially recommended an HbA1c level of ≥ 6.5% as a diagnostic cut-off value for DM[52]. This indicator reflects the integrated blood glucose level for the last 3-4 mo[53-55]. The association between H. pylori infection and HbA1c in diabetic patients has been confirmed in many studies[51,56,57]. Glycated hemoglobin A levels were significantly higher in patients with DM and H. pylori infection than in those with DM and without H. pylori infection (WMD = 0.50, 95%CI: 0.28-0.72, P < 0.001)[51]. Subgroup analysis by the subtype of DM has revealed a correlation between H. pylori infection and an elevated glycated hemoglobin A level in type 1 DM (I2 = 74%, P < 0.001, WMD = 0.46, 95%CI: 0.12-0.80) and in T2DM (I2 = 90%, P < 0.001, WMD = 0.59, 95%CI: 0.28-0.90, P < 0.001)[51].
Bektemirova et al[58] used the HbA1c level to evaluate the efficacy of hypoglycemic drugs taken by 83 patients with T2DM and H. pylori-associated diseases during eradication therapy. Glycated hemoglobin A was shown to reach a target level of < 6.5% in 62 of the 83 examinees, while it remained elevated (> 7.0%) in 21 patients. This means that despite the use of hypoglycemic drugs, the level of hyperglycemia persisted in these patients for at least 2-3 mo. And it was in these patients who did not reach the target HbA1c level had a significantly (P < 0.017) lower efficiency of eradication therapy than those who achieved the target level of HbA1c < 6.5%. The data obtained by Bektemirova et al[58] indirectly suggest that H. pylori most likely take advantage of chronic hyperglycemia to survive under the extreme conditions of eradication.
According to Tseng, the use of insulin to normalize blood glucose levels in patients with T2DM substantially increases the rate of H. pylori eradication compared to those with DM without insulin administration[25]. The higher efficiency of H. pylori eradication in T2DM patients taking insulin suggests that these patients are more likely to normalize their blood glucose levels during insulin therapy. And this is most likely to cause an increase in the efficiency of H. pylori eradication.
The data available in the literature advance the following hypothesis that in diabetic patients, H. pylori are most likely to utilize both amino acids and glucose for its vital activity. The hypothesis makes it possible to explain the high detection rate of H. pylori in diabetic patients, as well as their lower eradication therapy efficiency. Undoubtedly, this hypothesis requires further conformations by biochemical, microbiological, molecular genetics, and other studies. Further multicenter studies are needed to confirm this hypothesis. But if this hypothesis is correct, then before H. pylori are eradicated in DM patients, there is a need for mandatory monitoring and targeted correction of blood glucose and HbA1c levels according to the algorithm given in Figure 1. The algorithm can be used for the management of patients with DM and concomitant H. pylori-associated diseases, which is of great practical importance for their successful eradication therapy.
The authors express their gratitude to Alexandr Igorevich Burmistrov for technical assistance in preparing this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Lauro D, Italy; Rwegerera GM, Botswana S-Editor: Liu JH L-Editor: Ma JY-MedE P-Editor: Liu JH
| 1. | Malnick SD, Melzer E, Attali M, Duek G, Yahav J. Helicobacter pylori: friend or foe? World J Gastroenterol. 2014;20:8979-8985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 2. | Li J, Perez-Perez GI. Helicobacter pylori the Latent Human Pathogen or an Ancestral Commensal Organism. Front Microbiol. 2018;9:609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Reshetnyak VI, Burmistrov AI, Maev IV. Helicobacter pylori: Commensal, symbiont or pathogen? World J Gastroenterol. 2021;27:545-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (3)] |
| 4. | Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, Malfertheiner P, Graham DY, Wong VWS, Wu JCY, Chan FKL, Sung JJY, Kaplan GG, Ng SC. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 2051] [Article Influence: 256.4] [Reference Citation Analysis (0)] |
| 5. | Keilberg D, Steele N, Fan S, Yang Ch, Zavros Y, Ottemann KM. Gastric metabolomics analysis supports H. pylori’s catabolism of organic and amino acids in both the corpus and antrum. bioRxiv. 2020;183533. [DOI] [Full Text] |
| 6. | Mkrtumyan AM, Kazyulin AN, Bairova KI. Incidence and severity of Helicobacter infection in patients with type 2 diabetes mellitus. Diabetes mellitus. 2010;13:77-79 (In Russ.). [DOI] [Full Text] |
| 7. | Devrajani BR, Shah SZ, Soomro AA, Devrajani T. Type 2 diabetes mellitus: A risk factor for Helicobacter pylori infection: A hospital based case-control study. Int J Diabetes Dev Ctries. 2010;30:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Talebi-Taher M, Mashayekhi M, Hashemi MH, Bahrani V. Helicobacter pylori in diabetic and non-diabetic patients with dyspepsia. Acta Med Iran. 2012;50:315-318. [PubMed] |
| 9. | Vafaeimanesh J, Parham M, Bagherzadeh M. Helicobacter pylori infection prevalence: Is it different in diabetics and nondiabetics? Indian J Endocrinol Metab. 2015;19:364-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Abd-El-Kareem Younus H, Alkabeer AM M, Nuser MM, Mohammed AS, Saleh MW. Study of the relation between glycemic control in Egyptian patients with type-2 diabetes mellitus and Helicobacter pylori infection. Int J Multidiscip Res Dev. 2018;5: 249-256. |
| 11. | Mansori K, Moradi Y, Naderpour S, Rashti R, Moghaddam AB, Saed L, Mohammadi H. Helicobacter pylori infection as a risk factor for diabetes: a meta-analysis of case-control studies. BMC Gastroenterol. 2020;20:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Kouitcheu Mabeku LB, Noundjeu Ngamga ML, Leundji H. Helicobacter pylori infection, a risk factor for Type 2 diabetes mellitus: a hospital-based cross-sectional study among dyspeptic patients in Douala-Cameroon. Sci Rep. 2020;10:12141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Hsieh MC, Wang SS, Hsieh YT, Kuo FC, Soon MS, Wu DC. Helicobacter pylori infection associated with high HbA1c and type 2 diabetes. Eur J Clin Invest. 2013;43:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Han X, Li Y, Wang J, Liu B, Hu H, Li X, Yang K, Yuan J, Yao P, Wei S, Wang Y, Liang Y, Miao X, Zhang X, Guo H, Yang H, Wu T, He M. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age Chinese population. Diabetes Metab Res Rev. 2016;32:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Ko GT, Chan FK, Chan WB, Sung JJ, Tsoi CL, To KF, Lai CW, Cockram CS. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res. 2001;27:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Anastasios R, Goritsas C, Papamihail C, Trigidou R, Garzonis P, Ferti A. Helicobacter pylori infection in diabetic patients: prevalence and endoscopic findings. Eur J Intern Med. 2002;13:376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Howard BV, Best L, Comuzzie A, Ebbesson SO, Epstein SE, Fabsitz RR, Howard WJ, Silverman A, Wang H, Zhu J, Umans J. C-Reactive protein, insulin resistance, and metabolic syndrome in a population with a high burden of subclinical infection: insights from the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Diabetes Care. 2008;31:2312-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Lutsey PL, Pankow JS, Bertoni AG, Szklo M, Folsom AR. Serological evidence of infections and Type 2 diabetes: the MultiEthnic Study of Atherosclerosis. Diabet Med. 2009;26:149-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Tamura T, Morita E, Kawai S, Sasakabe T, Sugimoto Y, Fukuda N, Suma S, Nakagawa H, Okada R, Hishida A, Naito M, Hamajima N, Wakai K. No association between Helicobacter pylori infection and diabetes mellitus among a general Japanese population: a cross-sectional study. Springerplus. 2015;4:602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Sargýn M, Uygur-Bayramicli O, Sargýn H, Orbay E, Yavuzer D, Yayla A. Type 2 diabetes mellitus affects eradication rate of Helicobacter pylori. World J Gastroenterol. 2003;9:1126-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Demir M, Gokturk HS, Ozturk NA, Serin E, Yilmaz U. Efficacy of two different Helicobacter pylori eradication regimens in patients with type 2 diabetes and the effect of Helicobacter pylori eradication on dyspeptic symptoms in patients with diabetes: a randomized controlled study. Am J Med Sci. 2009;338:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Selinger C, Robinson A. Helicobacter pylori eradication in diabetic patients: still far off the treatment targets. South Med J. 2010;103:975-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Tseng CH. Diabetes, insulin use and Helicobacter pylori eradication: a retrospective cohort study. BMC Gastroenterol. 2012;12:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Maev IV, Mkrtumyan AM, Bektemirova LG, Andreev DN, Dicheva DT. The effectiveness of first-line eradication therapy for Helicobacter pylori infection in patients with type 2 diabetes mellitus. Ter Arkh (in Rus.). 2022;94:209-215. [DOI] [Full Text] |
| 27. | Ataseven H, Demir M, Gen R. Effect of sequential treatment as a first-line therapy for Helicobacter pylori eradication in patients with diabetes mellitus. South Med J. 2010;103:988-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99:200-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Sheu SM, Cheng H, Kao CY, Yang YJ, Wu JJ, Sheu BS. Higher glucose level can enhance the H. pylori adhesion and virulence related with type IV secretion system in AGS cells. J Biomed Sci. 2014;21:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Chávez-Reyes J, Escárcega-González CE, Chavira-Suárez E, León-Buitimea A, Vázquez-León P, Morones-Ramírez JR, Villalón CM, Quintanar-Stephano A and Marichal-Cancino BA. Susceptibility for Some Infectious Diseases in Patients With Diabetes: The Key Role of Glycemia. Front Public Health. 2021;9:559595. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Narayan KMV. Diabetes mellitus in Native Americans: the problem and its implications. Popul Res Policy Rev. 1997;116:169-192. [DOI] [Full Text] |
| 32. | Mendz GL, Hazell SL. Aminoacid utilization by Helicobacter pylori. Int J Biochem Cell Biol. 1995;27:1085-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Kelly DJ. The physiology and metabolism of the human gastric pathogen Helicobacter pylori. Adv Microb Physiol. 1998;40:137-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Marais A, Mendz GL, Hazell SL, Mégraud F. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol Mol Biol Rev. 1999;63:642-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | Lee WC, Goh KL, Loke MF, Vadivelu J. Elucidation of the Metabolic Network of Helicobacter pylori J99 and Malaysian Clinical Strains by Phenotype Microarray. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Stark RM, Suleiman MS, Hassan IJ, Greenman J, Millar MR. Amino acid utilisation and deamination of glutamine and asparagine by Helicobacter pylori. J Med Microbiol. 1997;46:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Nagata K, Nagata Y, Sato T, Fujino MA, Nakajima K, Tamura T. L-Serine, D- and L-proline and alanine as respiratory substrates of Helicobacter pylori: correlation between in vitro and in vivo amino acid levels. Microbiology (Reading). 2003;149:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Mendz GL, Hazell SL. Fumarate catabolism in Helicobacter pylori. Biochem Mol Biol Int. 1993;31:325-332. [PubMed] |
| 39. | Iwatani S, Nagashima H, Reddy R, Shiota S, Graham DY, Yamaoka Y. Identification of the genes that contribute to lactate utilization in Helicobacter pylori. PLoS One. 2014;9:e103506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing Effects of Fasting Metabolism on Tissue Tolerance in Bacterial and Viral Inflammation. Cell. 2016;166:1512-1525.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 41. | Mendz GL, Hazell SL. Glucose phosphorylation in Helicobacter pylori. Arch Biochem Biophys. 1993;300:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Mendz GL, Hazell SL, Burns BP. Glucose utilization and lactate production by Helicobacter pylori. J Gen Microbiol. 1993;139:3023-3028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Som S, De A, Banik GD, Maity A, Ghosh C, Pal M, Daschakraborty SB, Chaudhuri S, Jana S, Pradhan M. Mechanisms linking metabolism of Helicobacter pylori to (18)O and (13)C-isotopes of human breath CO2. Sci Rep. 2015;5:10936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Reynolds DJ, Penn CW. Characteristics of Helicobacter pylori growth in a defined medium and determination of its amino acid requirements. Microbiology (Reading). 1994;140 (Pt 10):2649-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Ho CY, Liu TW, Lin YS, Chen YP, Chen MJ, Wang HY, Liou TC. Factors Affecting the Intraluminal Therapy for Helicobacter pylori Infection. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Wang YC, Chen YP, Ho CY, Liu TW, Chu CH, Wang HY, Liou TC. The Impact of Gastric Juice pH on the Intraluminal Therapy for Helicobacter pylori Infection. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, Ishizaki T, Hishida A. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 48. | Marcus EA, Inatomi N, Nagami GT, Sachs G, Scott DR. The effects of varying acidity on Helicobacter pylori growth and the bactericidal efficacy of ampicillin. Aliment Pharmacol Ther. 2012;36:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Reshetnyak VI, Reshetnyak TM. Significance of dormant forms of Helicobacter pylori in ulcerogenesis. World J Gastroenterol. 2017;23:4867-4878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (5)] |
| 50. | Weykamp C, John WG, Mosca A. A review of the challenge in measuring hemoglobin A1c. J Diabetes Sci Technol. 2009;3:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Chen J, Xing Y, Zhao L, Ma H. The Association between Helicobacter pylori Infection and Glycated Hemoglobin A in Diabetes: A Meta-Analysis. J Diabetes Res. 2019;2019:3705264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Mbanya JC, Henry RR, Smith U. Presidents' statement on WHO recommendation on HbA1c for diabetes diagnosis. Diabetes Res Clin Pract. 2011;93:310-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999 2004 NHANES population. Diabetes Care. 2007;30:2233-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Herman WH, Engelgau MM, Zhang Y, Brown MB. Use of GHb (HbA(1c)) to screen for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:1207-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000;23:187-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 259] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 56. | Begue RE, Mirza A, Compton T, Gomez R, Vargas A. Helicobacter pylori infection and insulin requirement among children with type 1 diabetes mellitus. Pediatrics. 1999;103:e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 57. | Akın S, Erdem ME, Kazan S, Aliustaoğlu M. The relationship between Helicobacter pylori infection and glycemic regulation in type 2 diabetic patients. Nobel Med. 2014;10:32-35. |
| 58. | Bektemirova L, Mkrtumyan A, Rymareva E, Dicheva D, Chernavskij S. Efficacy of first-line eradication therapy in patients with Helicobacter pylori associated pathology of the upper gastrointestinal tract and type 2 diabetes depending on a level of glycated hemoglobin. Medical Bulletin of the Ministry of Internal Affairs. (in Rus.) 2022; 119: 27–31. [DOI] [Full Text] |