Published online Jun 28, 2022. doi: 10.13105/wjma.v10.i3.130
Peer-review started: February 10, 2022
First decision: April 13, 2022
Revised: April 25, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: June 28, 2022
Processing time: 144 Days and 20.1 Hours
While extensive information exists relating cigarette smoking to the risk of lung cancer, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD) or acute myocardial infarction (AMI), and stroke, far less information is available on risks from moist snuff (“snus”) or smokeless tobacco (ST) in United States/Canada, Europe or Japan.
To summarize data from the selected countries on risks of the four diseases associated with current ST or snus use.
Publications in English in 1990-2020 were considered that, based on epidemiological studies in North America, Europe or Japan, estimated risks of lung cancer, COPD, IHD/AMI, or stroke according to use of ST or snus. The studies should involve at least 100 cases of the disease considered, and not be restricted to those with specific other diseases. Medline literature searches were conducted, selecting papers initially from examination of titles and abstracts, and then from full texts. Further papers were sought from reference lists in selected papers, reviews and meta-analyses. For each disease, relative risk estimates adjusted at least for age were extracted relating ST or snus use to risk, and combined using random-effects meta-analysis. The estimates were mainly for current vs. never or non-current use, but results for ever vs never use were also considered.
Seven publications reported results for ST use from six United States studies. The most useful results came from four studies which provided results for current vs. never use. Random-effects meta-analyses of these results showed an increased risk for each disease, clearest for lung cancer (relative risk 1.59, 95% confidence interval 1.06-2.39, based on 4 estimates) and COPD (1.57, 1.09-2.26, n = 3), but also significant (at P < 0.05) for IHD (1.26, 1.10-1.45, n = 4) and stroke (1.27, 1.03-1.57, n = 4). Also including results for ever vs. never use from two other studies increased the lung cancer estimate to 1.80 (1.23-2.64, n = 6), but had little effect on the other estimates. For snus, 16 publications described results from 12 studies, one in Norway and the rest in Sweden. There were no results for COPD, and only three for lung cancer, with these reporting a relative risk of 0.80 (0.40-1.30) for current vs never use. More extensive data were available for IHD/AMI and stroke. Using the latest results from each study, combined estimates for current vs. never use were 1.00 (0.91-1.11, n = 5) for IHD/AMI and 1.05 (0.95-1.17, n = 2) for stroke, while for current vs. non-current use they were 1.10 (0.92-1.33, n = 9) for IHD/AMI and 1.12 (0.86-1.45, n = 9) for stroke. Meta-analyses including earlier results from some studies also showed no significant association between snus use and IHD/AMI or stroke. No relevant results were found for Japan.
Risks of smoking-related diseases from snus use in Scandinavia are not demonstrated, while those from ST use in the United States are less than from smoking.
Core Tip: United States studies show that, in never users of other products, current smokeless tobacco use associates with a significant (P < 0.05) increase in risk of the four major smoking-related diseases, with relative risks, compared to never users, of almost 1.6 for lung cancer and chronic obstructive pulmonary disease (COPD) and 1.3 for ischaemic heart disease (IHD)/acute myocardial infarction (AMI) and stroke. This increase is substantially less than for smoking. In Scandinavia, current snus use, does not significantly increase risk of IHD/AMI, stroke or lung cancer, with no data for COPD. Smokers unwilling to quit might consider these smokeless products.
- Citation: Lee PN, Coombs KJ, Hamling JS. Review with meta-analysis relating North American, European and Japanese snus or smokeless tobacco use to major smoking-related diseases. World J Meta-Anal 2022; 10(3): 130-142
- URL: https://www.wjgnet.com/2308-3840/full/v10/i3/130.htm
- DOI: https://dx.doi.org/10.13105/wjma.v10.i3.130
It is well established[1,2] that cigarette smoking markedly increases the risk of a range of diseases, particularly lung cancer, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD) and acute myocardial infarction (AMI), and stroke. Meta-analyses[3] have shown that in North American and European populations, current cigarette smokers, compared with those who have never smoked cigarettes, have about a ten-fold increase in risk of lung cancer, with the extent of the increase rising with amount smoked and earlier age of starting. Relative risks (RRs) exceed three for COPD and, in younger individuals, two for cardiovascular disease[4]. Pipe and cigar smoking is also associated with a clear increase in risk of smoking-related disease[2].
Here, we study the association between current use of smokeless tobacco (ST) and four major smoking-related diseases (lung cancer, COPD, IHD/AMI, and stroke). Our analyses are based on studies published from 1990, and separate out the effects of ST as used in North America, and the effects of moist snuff (“snus”) as mainly used in Sweden and neighbouring countries. Coupled with a separate ongoing attempt to provide updated meta-analyses relating the same diseases to current cigarette, cigar and pipe smoking, our results should help to provide a good picture of the relative effects of the different nicotine products on the major smoking-related diseases.
Attention was restricted to publications in English in the years 1990 to 2020 which provide results relating use of current ST or snus) in non-smokers to the risk of lung cancer, COPD, IHD/AMI or stroke, based on epidemiological cohort or case-control studies conducted in North America, Europe or Japan, and involving at least 100 cases of the disease of interest. The studies selected should not be restricted to those with specific other diseases.
The search procedures are described in detail in Supplementary material and are summarized below. First, separate literature searches on Medline were conducted for lung cancer, COPD or cardiovascular disease, the aim being to identify from these searches not only publications that described studies satisfying the inclusion criteria, but also meta-analyses and reviews that may themselves cite other relevant publications. Then, for each of the three searches, a print-out of the Medline output for title and abstract was examined by Katharine J Coombs (Coombs KJ) to identify publications of possible relevance, the selection then being checked by Peter N Lee (Lee PN), with any disagreements resolved in discussion. The selected publications (and where relevant supplementary files and also other publications linked to them in the Medline search) were then obtained, and examined by Lee PN, and classified as either an accepted publication possibly including relevant data, a reject (giving reason), a relevant review or a relevant meta-analysis. The suggested rejects were then checked by Coombs KJ, with any disagreements resolved. Then additional accepted publications not detected by the Medline searches were sought from examination of reference lists of the accepted papers and of the relevant reviews and meta-analyses.
The accepted publications from the three searches combined were then examined to eliminate those giving results superseded by a later publication, those not providing new data, and those not providing results relating current ST or snus use specifically for the four diseases of interest.
Using standard methods[5] individual study RR estimates were combined using fixed-effect and random-effects meta-analysis, with the significance of between-study heterogeneity also estimated.
For studies on ST use in North America, preference was given to results for those who had never used cigarettes, pipes or cigars which compared current and never ST use, but results from studies which only compared ever and never ST use were also considered in some meta-analyses.
For studies on snus use, use of pipes and cigars was disregarded as this was often not reported, and such use is rare in Scandinavia. RRs comparing current snus users both with never users and with non-users (i.e. non-current users, including both former and never users) were separately considered, as a number of studies only presented results compared to non-use. In some cases these estimates were derived from data separately by current, former and never use. Only age-adjusted RR estimates were considered, with the estimates adjusted for the most other factors generally being used.
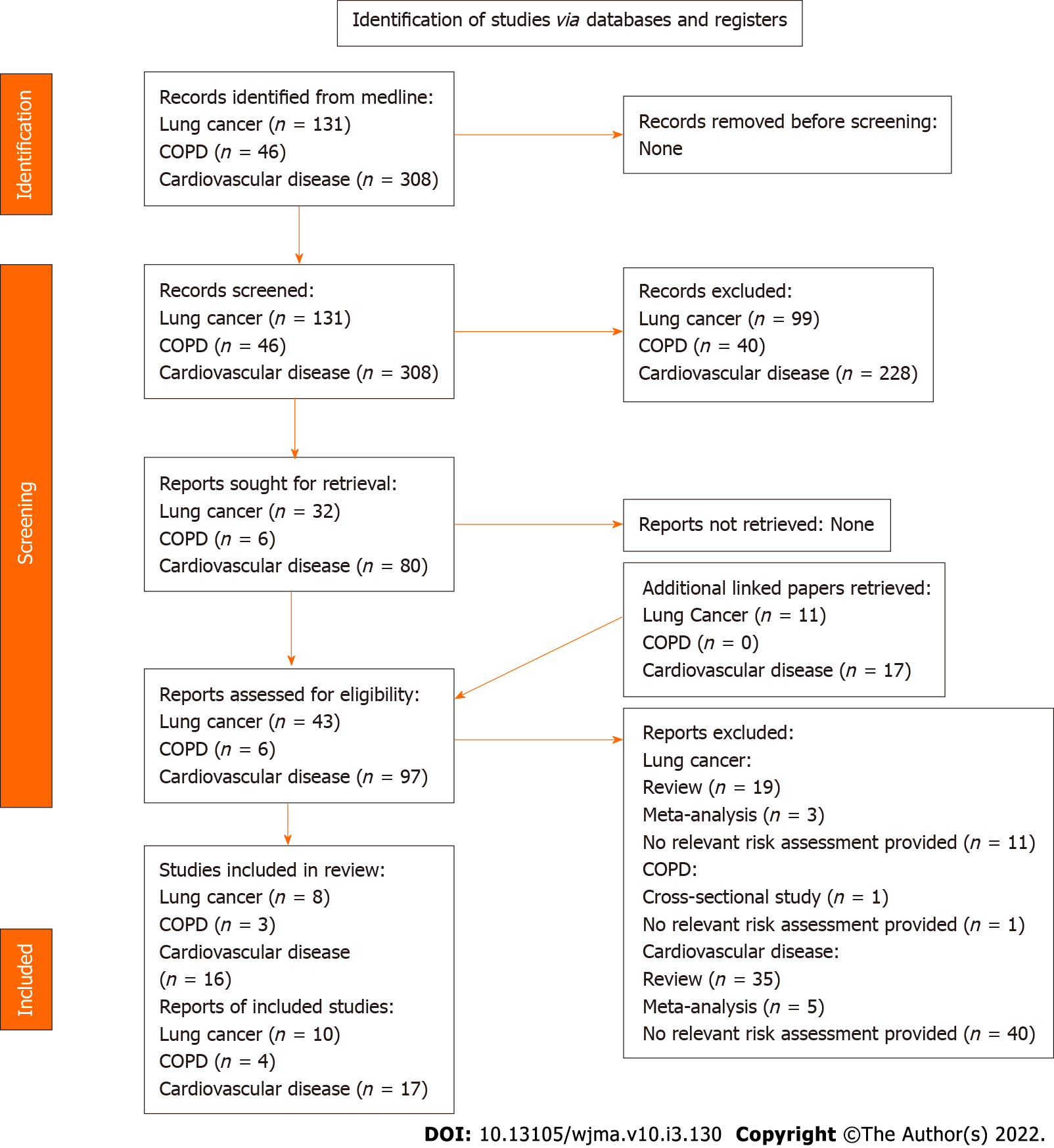
The results of the searches are given in detail in Additional File 1 and are summarized below and in Figure 1.
For lung cancer, 131 papers were identified in the Medline searches, with 32 considered possibly relevant from examination of title and abstract, and a further 12 identified from comments on these papers. Examination of the full text from the 44 papers led to 10 being accepted as providing apparently relevant study data, with 23 being reviews or meta-analyses and 11 rejected for various reasons.
For COPD, the Medline searches identified 46 papers with six initially considered possibly relevant based on title and abstract, and no further papers identified from comments. The full text examination led to one of the six papers being accepted and three rejected, with the other two being reviews.
For cardiovascular diseases, the Medline searches identified 308 papers, with 80 initially considered possibly relevant, a number extended to 97 after identification of comments on these papers. Of these 27 were accepted, with 52 being reviews or meta-analyses and 18 rejected.
Examination of reference lists in accepted papers, reviews and meta-analyses led to ten further papers being considered possibly relevant, but only one of these was a paper describing relevant results (for COPD). The total of 39 accepted papers for the diseases combined, was then reduced to 26, as three had been accepted in two separate searches, four did not give results for non-smokers, one did not separate results for IHD and stroke, and five were only comments on other accepted papers and provided no new data. Of the 26 papers, 18 gave results for snus, and eight for ST as used in the United States (US), considered separately below. No relevant results were found for Japan.
Each of the eight publications identified[6-13] reports results from a prospective study. Results from one[10] were not considered further as a later publication[11] provides corrected results from the same study.
The most relevant results, comparing risks for current vs never ST users in those who had never used cigarettes, pipes or cigars, come from four studies. For Cancer Prevention Studies I and II (CPS-I and CPS-II), separate results for each of the four diseases are available in one publication[9]. For the National Longitudinal Mortality Study (NLMS), results for IHD and stroke from one publication[13] are preferred to those from another[8], due to the longer follow-up considered, though results for lung cancer are only available from the latter publication[8]. For the National Health Interview Surveys (NHIS), the results from one publication[11] are preferred, as they provide results for all four diseases, and for a longer follow-up than do other publications[8,12].
Less useful are results from two studies. For the Agricultural Health Study (AHS), the results[7] are only for lung cancer, and only compare ever and never ST use. For the first National Health and Nutrition Examination Survey (NHANES), the results[6], for all the diseases except COPD, only compare ever and never ST use, with pipe and cigar smokers not excluded.
Table 1 gives a summary description of the six studies considered, including timing, population studied, and relevant diseases considered, as well as the ST exposure index used and whether pipe and cigar smokers are excluded from the results for never smokers.
| Study | Ref. | Study type1 | Timing | Population | Diseases considered | Excludes pipe/cigar | Exposure index |
| Main sources | |||||||
| CPS-I2 | Henley et al[9], 2005 | P | 1959 to 1971 | Families of volunteers’ friends and neighbours | LC, COPD3, IHD, Stroke | Yes | Current vs never |
| CPS-II2 | Henley et al[9], 2005 | P | 1982 to 2000 | Families of volunteers’ friends and neighbours | LC, COPD4, IHD, Stroke | Yes | Current vs never |
| NHIS5 | Inoue-Choi et al[10], 2019; Inoue-Choi et al[11], 2020 | P | 1991-2010 to 2015 | Civilian non-institutionalized | LC, COPD6, IHD, Stroke | Yes | Current vs never |
| NLMS7 | Timberlake et al[13], 2017 | P | 1985-2011 to 2011 | Civilian non-institutionalized | IHD, Stroke | Yes | Current vs never |
| NLMS7 | Fisher et al[8], 2019 | P | 1993-2005 to 2010 | Civilian non-institutionalized | LC | Yes | Current vs never |
| Other sources | |||||||
| NHANES8 | Accortt et al[6], 2002 | P | 1971-75 to 1992 | Civilian non-institutionalized | LC, IHD, Stroke | No | Ever vs never |
| AHS9 | Andreotti et al[7], 2017 | P | 1993-97 to 2010-11 | Pesticide applicators and their spouses | LC | Yes | Ever vs never |
Table 2 gives the RRs and 95% confidence intervals (CIs), both as reported for the individual studies and as estimated for the combined studies using random-effect meta-analysis, as well as the available results by sex, and the adjustment factors taken into account. Two studies report results only for males, three for sexes combined and only one for the sexes separately. All the RRs were adjusted for age and a varying list of other factors, including sex where relevant.
| Study | Sex | Lung cancer | Chronic obstructive pulmonary disease | Ischaemic heart disease | Stroke | Adjustment factors |
| Main sources | ||||||
| CPS-I | M | 1.08 (0.64-1.83) | 1.86 (1.12-3.06) | 1.12 (1.03-1.21) | 1.46 (1.31-1.64) | Age, alc, asp, bmi, edu, ex, fat, f/v, race |
| CPS-II | M | 2.00 (1.23-3.24) | 1.28 (0.71-2.32) | 1.26 (1.08-1.47) | 1.40 (1.10-1.79) | Age, alc, asp, bmi, edu, emp, ex, fat, f/v, race |
| NHIS | M + F | 1.43 (0.51-4.01) | 1.35 (0.39-4.76) | 1.66 (1.30-2.13) | 1.09 (0.56-2.11) | Age, edu, race, sex, year |
| NLMS | M + F | 2.98 (0.91-9.76) | - | 1.24 (1.05-1.46) | 0.92 (0.67-1.27) | Lung cancer: age, edu, hea, inc, race, sexIHD and CVD: age, edu, inc, race, sex |
| Random-effects meta-analysis | 1.59 (1.06-2.39) (n = 4) | 1.57 (1.09-2.26) (n = 3) | 1.26 (1.10-1.45) (n = 4) | 1.27 (1.03-1.57) (n = 4) | ||
| Other sources | ||||||
| NHANES | M | - | - | 0.6 (0.3-1.2) | 0.7 (0.2-2.0) | Lung cancer: age, alc, ex, f/v, pov, race, regIHD: age, alc, bmi, chol, ex, f/v, pov, race, sbpCVD: age, alc, ex, f/v, pov, race, sbp |
| NHANES | F | 9.1 (1.1-75.4) | - | 1.4 (0.8-2.2) | 1.0 (0.3-2.9) | |
| AHS | M + F | 2.21 (1.11-4.42) | - | - | - | Age, alc, edu, race, reg, sex |
| All sources | ||||||
| Random-effects meta-analysis | 1.80 (1.23-2.64) (n = 6) | 1.57 (1.09-2.26) (n = 3) | 1.24 (1.08-1.43) (n = 6) | 1.24 (1.02-1.52) (n = 6) | ||
The combined evidence from the main studies (CPS-I, CPS-II, NHIS, NLMS) shows a statistically significant increase in risk relating to current ST use which is somewhat greater for lung cancer (RR 1.59, 95%CI: 1.06-2.39) and COPD (1.57, 1.09-2.26) than for IHD (1.26, 1.10-1.45) and stroke (1.27, 1.03-1.57). Including also the evidence from the other two studies (AHS, NHANES) somewhat increased the combined RR estimate for lung cancer (to 1.80, 1.23-2.64) but left the RRs for the other three diseases virtually unchanged. Significant evidence of heterogeneity between the estimates was only seen in the analyses for IHD, where due to a rather higher estimate from NHIS, the associated P value was 0.019 for the estimate based only on the four main results, and 0.015 when also including the results from NHANES.
There is also information from three of the studies on variation in risk by type of ST (chewing tobacco or snuff). For CPS-II[9] RRs were reported, for lung cancer, IHD and stroke, respectively of 1.97 (95%CI: 1.10-3.54), 1.25 (1.03-1.51) and 1.38 (1.02-1.86) for exclusive chewing tobacco use, and of 2.08 (0.51-8.45), 1.59 (1.06-2.39) and 0.62 (0.23-1.67) for exclusive snuff use. For AHS[7] the RR of lung cancer for chewing tobacco of 2.20 (0.98-4.97) was similar to that of 2.21 (1.11-4.42) for overall ST use. No result was given for snuff, as there were only three cases of lung cancer in the exposed group. For NLMS[13] RRs for IHD were 1.11 (0.88-1.42) for exclusive chewing tobacco and 1.30 (1.03-1.63) for exclusive snuff use. In all three studies, the RRs did not vary significantly by type of ST.
Of the 18 publications on snus[14-31], one[16] describes results from a study in Norway, with the rest describing studies in Sweden. Most describe results from a single study, but one[14] presents separate results from two studies, while two[20,21] present results from eight studies, one for AMI and the other for stroke. All the available results are for males.
Two papers were not considered further. One[30] only reported results for ever vs never snus use, reported RRs in never smokers only for combined cardiovascular death (RR 1.15, 95%CI: 0.97-1.37) and respiratory death (0.8, 0.2-3.0), and did not separate out results for IHD/AMI, stroke or COPD. The other[14] mainly considered heart failure, the limited results for AMI being unrestricted to non-smokers and not adjusted for any potential confounding factors.
The other 16 studies all present results for snus use in non-smokers or non-regular smokers, in some where the comparison is between current and non-use rather than between current and never use, and one where it is between ever and never use. Table 3 gives details, by study and publication, of the study type, timing, population, relevant diseases considered, and the unexposed group considered. In total there are results from 12 studies, with multiple publications describing results from some studies. For no study did any of the publications present simple updates of results given in another publication. All but the Two Counties study is of prospective design, though some results from the MONICA study are based on case-control analyses.
| Study1 | Source | Study type2 | Timing | Population | Diseases considered | Unexposed snus3 |
| CWC | Bolinder et al[17], 1994 | P | 1971-74 to 1985 | Construction workers | LC, IHD, stroke | Non |
| Hergens et al[23], 2007 | 1978-93 to 2004 | AMI | Never | |||
| Luo et al[29], 2007 | 1978-92 to 2004 | LC | Never | |||
| Hergens et al[24], 2008 | 1978-92 to 2003 | CVD | Never | |||
| Hansson et al[20], 2012; Hansson et al[21], 2014 | 1978-93 to 2004 | AMI, stroke | Non | |||
| MALMÖ | Janzon and Hedblad[27], 2009 | P | 1991-96 to 2004 | Population-based, Malmö city | AMI, stroke | Non |
| Hansson et al[20], 2012; Hansson et al[21], 2014 | AMI, stroke | Non | ||||
| MONICA | Asplund et al[15], 2003 | NCC | 1986-99 to 2000 | Population-based, Norrbotten and Västerbotten counties | CVD | Non |
| Wennberg et al[31], 2007 | NCC | 1986-99 to 1999 | AMI | Never | ||
| Huhtasaari et al[25], 1992 | CC | 1989-91 | AMI | Non | ||
| Huhtasaari et al[26], 1999 | CC | 1991-93 | AMI | Non | ||
| Hansson et al[20], 2012; Hansson et al[21], 2014 | P | 1986-2004 to 2004 | AMI, stroke | Non | ||
| NMC | Hansson et al[20], 2012; Hansson et al[21], 2014 | P | 1997 to 2004 | Participant in charity walk | AMI, stroke | Non |
| NORWAY | Boffetta et al[16], 2005 | P | 1964-67 to 2001 | Population sample and relatives of emigrants | LC | Ever vs never |
| SALLS | Johansson et al[28], 2005 | P | 1988-89 to 2000 | Civilian non-institutionalized | IHD | Non |
| SALT | Hansson et al[19], 2009 | P | 1998-2002 to 2005 | Twins born in Sweden 1926-1958 | IHD, stroke | Never |
| Hansson et al[20], 2012; Hansson et al[21], 2014 | 1998-2002 to 2004 | AMI, stroke | Non | |||
| Scania-PHC | Hansson et al[20], 2012; Hansson et al[21], 2014 | P | 2002 to 2004 | Population-based, Skåne County | AMI, stroke | Non |
| Stockholm-PHC | Hansson et al[20], 2012; Hansson et al[21], 2014 | P | 2002 to 2004 | Population-based, Stockholm County | AMI, stroke | Non |
| ULF | Haglund et al[18], 2007 | P | 1988-89 to 2003 | Civilian, non-institutionalized | IHD, stroke | Non |
| Two Counties | Hergens et al[22], 2005 | CC | 1992-94 | Randomly selected, Stockholm and Västernorrland counties | AMI | Never |
| WOLF | Hansson et al[20], 2012; Hansson et al[21], 2014 | P | 1992-98 to 2004 | Employed in three counties | AMI, stroke | Non |
From Table 3 it can be seen that there are no results at all for COPD (or a closely related endpoint) and only three publications present results for lung cancer. The most useful result[29] is based on follow-up of construction workers interviewed in 1978-92, including 15 cases in current users and three in former users, with a RR of 0.80 (95%CI: 0.40-1.30) for current vs. never ST use and of 0.80 (0.45-1.45) for current vs non ST use. An earlier result from this study[17] can be ignored, as it is based on no more than three lung cancer cases in current users, and based on interviews in 1971-74, when coding of smoking status was problematic[29]. A RR of 0.96 (0.26-3.56) from the Norway study[16] is for ever vs never use and based on only three cases in ever users. No meta-analyses seemed to be worth conducting for lung cancer.
As illustrated in Table 4, much more evidence is available for IHD/AMI and stroke, both for current vs. non snus use and for current vs never use, each RR estimate being adjusted for age and varying other factors. Based on the estimate from the latest publication, where data for a study provides a choice, Table 5 shows no evidence of an increased risk in current snus users, whether the comparison group is never users (IHD/AMI: RR 1.00, 95%CI: 0.91-1.11; stroke: 1.05, 0.95-1.17), or is non users (IHD/AMI: 1.10, 0.92-1.33; stroke 1.12, 0.86-1.45). No significant association is also seen when, less satisfactorily, all available RRs are combined, regardless of whether in some studies some disease occurrences may be counted more than once.
| Study | Source1 | Current vs never | Current vs non | Adjustmentfactors2 | ||
| IHD/AMI | Stroke | IHD/AMI | Stroke | |||
| CWC | Bolinder et al[17], 1994 | - | - | 1.35 (1.13-1.62)3 | 1.29 (0.83-1.99)3 | Age, res |
| CWC | Hergens et al[23], 2007 | 1.02 (0.92-1.14) | - | 1.03 (0.93-1.15) | - | Age, BMI, res |
| CWC | Hergens et al[24], 2008 | - | 1.05 (0.95-1.17) | - | 1.06 (0.96-1.18) | Age, BMI, res |
| CWC | Hansson et al[20], 2012; Hansson et al[21], 2014 | - | - | 1.01 (0.90-1.14) | 1.03 (0.90-1.17) | Age, BMI4 |
| MALMÖ | Janzon and Hedblad[27], 2009 | - | - | 0.75 (0.30-1.80) | 0.59 (0.20-1.50) | Age, BMI, dia, hyp, mar, occ, phys |
| MALMÖ | Hansson et al[20], 2012; Hansson et al[21], 2014 | - | - | 1.00 (0.37-2.70) | 1.23 (0.50-2.99) | Age, BMI4 |
| MONICA | Asplund et al[15], 2003 | - | - | - | 0.87 (0.41-1.83) | Age, chol, cohort, edu, dia, hyp, mar, year |
| MONICA | Wennberg et al[31], 2007 | 0.82 (0.46-1.43) | - | 0.85 (0.48-1.50) | - | Age, BMI, chol, edu, phys, res, year |
| MONICA | Huhtasaari et al[25], 1992 | - | - | 0.89 (0.62-1.29) | - | Age |
| MONICA | Huhtasaari et al[26], 1999 | - | - | 0.58 (0.35-0.94) | - | Age, chol, dia, edu, her, hyp, mar, res |
| MONICA | Hansson et al[20], 2012; Hansson et al[21], 2014 | - | - | 0.77 (0.35-1.69) | 0.65 (0.23-1.80) | Age, BMI4 |
| NMC | - | - | No IHD cases in current snus users | 1.28 (0.40-4.10) | Age, BMI4 | |
| SALLS | 1.41 (0.61-3.28) | - | - | - | Age, BMI, dia, hyp, phys | |
| SALT | Hansson et al[19], 2009 | 0.85 (0.51-1.41) | 1.18 (0.67-2.08) | 0.85 (0.51-1.40) | 1.15 (0.66-2.02) | Age, chol, dia, hyp |
| SALT | Hansson et al[20], 2012; Hansson et al[21], 2014 | - | - | 1.56 (0.98-2.48) | 0.98 (0.52-1.83) | Age, BMI4 |
| Scania-PHC | - | - | 1.90 (0.90-4.00) | 3.17 (1.50-6.70) | Age, BMI4 | |
| Stockholm-PHC | - | - | 1.21 (0.48-3.08) | 0.58 (0.14-2.45) | Age, BMI4 | |
| ULF | - | - | 1.15 (0.54-2.41) | 1.01 (0.35-2.92) | Age, heal, ill, phys, res, ses | |
| Two Counties | 0.73 (0.35-1.50) | - | 0.73 (0.35-1.51) | - | Age, area | |
| WOLF | - | - | 3.30 (0.63-17.1) | 0.96 (0.28-3.30) | Age, BMI4 | |
| Disease | Comparison group | All data or latest | Random-effects meta-analysis relative risk (95%CI) | Heterogeneity | ||
| Chi sq | DF | P value | ||||
| IHD/AMI | Never | Latest | 1.00 (0.91-1.11) | 2.33 | 4 | NS |
| Non | All | 1.04 (0.92-1.18) | 24.87 | 15 | 0.052 | |
| Latest | 1.10 (0.92-1.33) | 9.18 | 8 | NS | ||
| Stroke | Never | Latest | 1.05 (0.95-1.17) | 0.16 | 1 | NS |
| Non | All | 1.06 (0.98-1.14) | 12.69 | 13 | NS | |
| Latest | 1.12 (0.86-1.45) | 10.26 | 8 | NS | ||
The results of the meta-analyses for ST use in the US show that, in those who have never used cigarettes, cigars or pipes, current use, compared to never use, is associated with a significant increase in risk of all four major smoking-related diseases studied, the increases estimated from the four main sources of data (CPS-I, CPS-II, NHIS, NLMS) being almost 30% for IHD and stroke and almost 60% for COPD and lung cancer. These increases are less than those associated with cigarette smoking, e.g.[4]) and suggest that ST, as used in the US, is a safer, but not harmless, alternative method of nicotine exposure than cigarette smoking for smokers not willing to quit. While some of the publications we consider[6,10] have concluded that an excess risk of smoking-related disease associated with ST use in the US has been shown, some are more cautious, regarding the evidence as limited[9,13].
Limitations of the evidence for US ST include the fact that a number of the studies considered are quite old, with three of the seven studies summarized in Table 1 involving follow-up periods ending over 20 years ago, ignoring the possibility that the nature of the products studied may have changed over time. Another limitation is the fairly sparse evidence comparing risk by type of ST product. Although this does not suggest any marked differences in risk between those who use chewing tobacco or use snuff, the data are insufficient to reliably detect smaller differences. Also, it is possible that some misclassification of smoking status has taken place, with some of the effects attributed to ST use actually being a consequence of unreported current or past smoking of cigarettes, pipes or cigars.
Even if the magnitude of the effect on risk of current ST use in the US may be somewhat inaccurately measured in our meta-analyses, there seems little doubt that it is substantially less than that for cigarette smoking. For lung cancer, for example, RRs for current cigarette smoking for the US have been estimated as 11.68 in one meta-analysis[3], with RRs increasing with increasing amount smoked and earlier age of starting to smoke, and higher for squamous cell carcinoma than for adenocarcinoma. While we have not attempted to quantify risk of ST use in the US by amount or duration of use, or by subdivision of the diseases considered, this does not affect the conclusion that the risks of the four diseases for ST are less than for cigarette smoking.
The results of our meta-analyses for current snus use, based on studies in Scandinavia, show no clear evidence of any increased risk, whether the comparison group is never or non-users. While there is little evidence for lung cancer, and there are no useful results for COPD, the evidence for cardiovascular disease is based on as many as 12 studies, the results from some being reported in multiple publications (see Table 4). As shown in Table 5, RR estimates for IHD/AMI and for stroke vary only from 1.00 to 1.12, and none are statistically significant. Though a lack of effect cannot be demonstrated, and it is possible that there is a true small increase in risk by perhaps about 5%, it seems likely that any increase is less than for US ST, and much less than that for cigarette smoking. Certainly the great majority of the publications from which we derived data[14-16,18-22,25-31] considered that no increased risk in current snus users had been demonstrated for any of the smoking-related diseases we considered, many concluding that components of tobacco smoke other than nicotine appear to be involved in the relationship of smoking with heart disease and stroke. However, possible effects were noted for cardiovascular disease[17] based on early and unreliable data[29], fatal AMI and fatal stroke[23,24] and for heart failure[14]. The at most very weak association of snus with the smoking-related diseases considered was also the conclusion of a review of the evidence on snus[32], though this review also noted a possible effect of snus on reduced survival from AMI and on heart failure, arguing that further investigation was needed to investigate possible confounding by socio-economic status or other factors.
In the last few years there have been a number of reviews and meta-analyses on the effects of ST, e.g.[33-42], many unrestricted to effects in the US and Scandinavia, and some restricted to specific diseases. Where effects are claimed, they often relate to products used in Africa or Asia, e.g.[42], or to other diseases, such as oral or pancreatic cancer. For oral cancer, however, evidence of an increased risk from snus has not emerged from meta-analyses[32], while for US ST any increase is mainly evident in studies before 1980[43]. Also, for pancreatic cancer, claims of any increased risk associated with snus use[33,34] are weakly based, with the evidence for any association with ST use essentially disappearing[32] following publication of pooled analyses[44,45]. For lung cancer, the reviews, e.g.[33,34,38,46] generally consider that no increased risk from snus has been demonstrated, though one[39] points to increased risk from US ST. COPD is little considered in the reviews, though one[39] does refer to the increased risk seen in the CPS-I study shown in Table 2. The risks of IHD/AMI and stroke are more extensively considered in the reviews, and some, e.g.[35] refer to a possible increase in risk of fatal AMI and stroke. However, this increase is mainly dependent on the results for US ST, where we have found a significant increase in our analyses. For snus, where the evidence considered derives from studies of fatal cases only, of non-fatal cases only, or of first occurrences of a case (fatal or non-fatal), where separate results are not always reported by fatality, there is no clear evidence of an increased risk specifically in fatal cases[32]. As noted in this review, confounding may occur due to snus users reporting disease later, or having less medical care when they do. Even if, for some reason, there is a slight adverse effect of snus on fatal AMI and stroke, it is clearly less than for cigarette smoking. This conclusion is consistent with a recent follow-up of almost 75000 patients admitted with a first percutaneous intervention, which found that snus use was not associated with increased mortality, new revascularisation or hospitalisation for heart failure[47].
Taken as a whole, the conclusions reached in the reviews are consistent with our findings that, for the four major diseases considered, effects of the smokeless products commonly used in the US are less than those for cigarette smoking, and they are not clearly evident for Swedish snus. Our analyses provide no information on risks from ST as used in Africa and Asia.
Studies in the US show that, in those who never used other tobacco products, current ST use is associated with an increased risk of the four major smoking-related diseases. However, this increase, though statistically significant (at P < 0.05), is much less than for cigarette smoking. Scandinavian studies show no significant increase in risk of IHD/AMI, stroke or lung cancer in current snus users, with no data available for COPD. Though the data have limitations, providing information only on risks from the major smoking-related diseases, and none on risks from the smokeless products used in Africa or Asia, our findings clearly show that risks of the diseases considered from US ST and snus use are much less than for smoking.
There are extensive data on the risks from cigarette smoking, but far less on the risks from moist snuff (“snus”) or smokeless tobacco (ST) as used in Western populations and Japan.
To obtain recent evidence as part of a project comparing risks from use of various tobacco products.
To summarize data relating snus and ST use in North America, Europe and Japan to risk of the four main smoking related diseases – lung cancer, chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD) (including acute myocardial infarction (AMI) and stroke.
Medline searches sought English publications in 1990-2020 providing data on risks of each of the diseases relating to current (or ever) use of snus or ST in the selected regions. The studies had to include at least 100 cases of the disease considered, and not be based on individuals with specific diseases. Relative risk estimates adjusted at least for age were extracted for each study and combined using random-effects meta-analyses.
Six United States studies provided ST results. For current vs. never use (4 studies), significant increases were seen for each disease, with the RRs higher for lung cancer (1.59) and COPD (1.57) than for IHD/AMI (1.26) and stroke (1.25). Including also results for ever vs. never use, increased the lung cancer RR to 1.80, but little affected the other RRs. Twelve Scandinavian studies provided snus results, with no data on COPD. For the other diseases, RRs for current vs. never use were never significant, the highest RR being 1.05 for stroke. There were no relevant studies in Japan.
Risks from ST use in North America are much less than for smoking, while no risks were demonstrated for snus.
The results suggest that smokers unwilling to give up nicotine may substantially reduce their risk of the four diseases by switching to ST (as used in North America) or snus.
We thank Yvonne Cooper for typing the various drafts of the paper and obtaining the relevant references.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Statistics and probability
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aggarwal P, India; Seid AA, Ethiopia A-Editor: Zhu JQ; China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | International Agency for Research on Cancer. Tobacco smoking. Vol 38. IARC Monogr Eval Carcinog Risk Chem Hum Lyon, France: IARC, 1986: 421. Available from: http://monographs.iarc.fr/ENG/Monographs/vol1-42/mono38.pdf. |
| 2. | US Surgeon General. The health consequences of smoking - 50 years of progress: a report of the Surgeon General. Vol Atlanta, Georgia: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014: 944. Available from: https://www.ncbi.nlm.nih.gov/books/NBK179276. |
| 3. | Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer. 2012;12:385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 4. | Lee PN, Fry JS, Hamling JF, Sponsiello-Wang Z, Baker G, Weitkunat R. Estimating the effect of differing assumptions on the population health impact of introducing a Reduced Risk Tobacco Product in the USA. Regul Toxicol Pharmacol. 2017;88:192-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Fleiss JL, Gross AJ. Meta-analysis in epidemiology, with special reference to studies of the association between exposure to environmental tobacco smoke and lung cancer: a critique. J Clin Epidemiol. 1991;44:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 6. | Accortt NA, Waterbor JW, Beall C, Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. Am J Epidemiol. 2002;156:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Andreotti G, Freedman ND, Silverman DT, Lerro CC, Koutros S, Hartge P, Alavanja MC, Sandler DP, Freeman LB. Tobacco Use and Cancer Risk in the Agricultural Health Study. Cancer Epidemiol Biomarkers Prev. 2017;26:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Fisher MT, Tan-Torres SM, Gaworski CL, Black RA, Sarkar MA. Smokeless tobacco mortality risks: an analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct J. 2019;16:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States). Cancer Causes Control. 2005;16:347-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Inoue-Choi M, Shiels MS, McNeel TS, Graubard BI, Hatsukami D, Freedman ND. Contemporary Associations of Exclusive Cigarette, Cigar, Pipe, and Smokeless Tobacco Use With Overall and Cause-Specific Mortality in the United States. JNCI Cancer Spectr. 2019;3:pkz036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | . Corrigendum to "Contemporary Associations of Exclusive Cigarette, Cigar, Pipe, and Smokeless Tobacco Use With Overall and Cause-Specific Mortality in the United States". JNCI Cancer Spectr. 2020;4:pkz105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Rodu B, Plurphanswat N. Mortality among male smokers and smokeless tobacco users in the USA. Harm Reduct J. 2019;16:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Timberlake DS, Nikitin D, Johnson NJ, Altekruse SF. A longitudinal study of smokeless tobacco use and mortality in the United States. Int J Cancer. 2017;141:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Arefalk G, Hergens MP, Ingelsson E, Arnlöv J, Michaëlsson K, Lind L, Ye W, Nyrén O, Lambe M, Sundström J. Smokeless tobacco (snus) and risk of heart failure: results from two Swedish cohorts. Eur J Prev Cardiol. 2012;19:1120-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Asplund K, Nasic S, Janlert U, Stegmayr B. Smokeless tobacco as a possible risk factor for stroke in men: a nested case-control study. Stroke. 2003;34:1754-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Boffetta P, Aagnes B, Weiderpass E, Andersen A. Smokeless tobacco use and risk of cancer of the pancreas and other organs. Int J Cancer. 2005;114:992-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am J Public Health. 1994;84:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 202] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Haglund B, Eliasson M, Stenbeck M, Rosén M. Is moist snuff use associated with excess risk of IHD or stroke? Scand J Public Health. 2007;35:618-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Hansson J, Pedersen NL, Galanti MR, Andersson T, Ahlbom A, Hallqvist J, Magnusson C. Use of snus and risk for cardiovascular disease: results from the Swedish Twin Registry. J Intern Med. 2009;265:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Hansson J, Galanti MR, Hergens MP, Fredlund P, Ahlbom A, Alfredsson L, Bellocco R, Eriksson M, Hallqvist J, Hedblad B, Jansson JH, Nilsson P, Pedersen N, Trolle Lagerros Y, Ostergren PO, Magnusson C. Use of snus and acute myocardial infarction: pooled analysis of eight prospective observational studies. Eur J Epidemiol. 2012;27:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Hansson J, Galanti MR, Hergens MP, Fredlund P, Ahlbom A, Alfredsson L, Bellocco R, Engström G, Eriksson M, Hallqvist J, Hedblad B, Jansson JH, Pedersen NL, Trolle Lagerros Y, Ostergren PO, Magnusson C. Snus (Swedish smokeless tobacco) use and risk of stroke: pooled analyses of incidence and survival. J Intern Med. 2014;276:87-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Hergens MP, Ahlbom A, Andersson T, Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16:12-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Hergens MP, Alfredsson L, Bolinder G, Lambe M, Pershagen G, Ye W. Long-term use of Swedish moist snuff and the risk of myocardial infarction amongst men. J Intern Med. 2007;262:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Hergens MP, Lambe M, Pershagen G, Terent A, Ye W. Smokeless tobacco and the risk of stroke. Epidemiology. 2008;19:794-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 25. | Huhtasaari F, Asplund K, Lundberg V, Stegmayr B, Wester PO. Tobacco and myocardial infarction: is snuff less dangerous than cigarettes? BMJ. 1992;305:1252-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Huhtasaari F, Lundberg V, Eliasson M, Janlert U, Asplund K. Smokeless tobacco as a possible risk factor for myocardial infarction: a population-based study in middle-aged men. J Am Coll Cardiol. 1999;34:1784-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Janzon E, Hedblad B. Swedish snuff and incidence of cardiovascular disease. A population-based cohort study. BMC Cardiovasc Disord. 2009;9:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Johansson SE, Sundquist K, Qvist J, Sundquist J. Smokeless tobacco and coronary heart disease: a 12-year follow-up study. Eur J Cardiovasc Prev Rehabil. 2005;12:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Luo J, Ye W, Zendehdel K, Adami J, Adami HO, Boffetta P, Nyrén O. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: a retrospective cohort study. Lancet. 2007;369:2015-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 30. | Roosaar A, Johansson AL, Sandborgh-Englund G, Axéll T, Nyrén O. Cancer and mortality among users and nonusers of snus. Int J Cancer. 2008;123:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 31. | Wennberg P, Eliasson M, Hallmans G, Johansson L, Boman K, Jansson JH. The risk of myocardial infarction and sudden cardiac death amongst snuff users with or without a previous history of smoking. J Intern Med. 2007;262:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Lee PN. Epidemiological evidence relating snus to health--an updated review based on recent publications. Harm Reduct J. 2013;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Health effects of smokeless tobacco products. Vol Brussels: European Commission, Health & Consumer Protection Directorate-General, 2008: 157. Available from: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_013.pdf. |
| 34. | Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncol. 2008;9:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 420] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 35. | Boffetta P, Straif K. Use of smokeless tobacco and risk of myocardial infarction and stroke: systematic review with meta-analysis. BMJ. 2009;339:b3060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 36. | Piano MR, Benowitz NL, Fitzgerald GA, Corbridge S, Heath J, Hahn E, Pechacek TF, Howard G; American Heart Association Council on Cardiovascular Nursing. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment: a policy statement from the American Heart Association. Circulation. 2010;122:1520-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | International Agency for Research on Cancer. Smokeless tobacco. A review of human carcinogens: Part E: Personal habits and indoor combustions. Vol 100. IARC Monographs on the evaluation of carcinogenic risks to humans Lyon, France: IARC, 2012: 267-321. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100E/mono100E.pdf. |
| 38. | National Cancer Institute and Centers for Disease Control and Prevention. Smokeless tobacco and public health: A global perspective. NIH Publication No.14-7983. Vol Bethesda, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention and National Institutes of Health, National Cancer Institute, 2014: 558. Available from: http://nccd.cdc.gov/gtssdata/Ancillary/Publications.aspx. |
| 39. | Schivo M, Avdalovic MV, Murin S. Non-cigarette tobacco and the lung. Clin Rev Allergy Immunol. 2014;46:34-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Gupta R, Gupta S, Sharma S, Sinha DN, Mehrotra R. A systematic review on association between smokeless tobacco & cardiovascular diseases. Indian J Med Res. 2018;148:77-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Murkett R, Rugh M and Ding B. Nicotine products relative risk assessment: a systematic review and meta-analysis [version 1; peer review: 1 approved]. F1000Res 2020; 9: 1225. Available from: https://doi.org/10.12688/f1000research.26762.1. |
| 42. | Hajat C, Stein E, Ramstrom L, Shantikumar S, Polosa R. The health impact of smokeless tobacco products: a systematic review. Harm Reduct J. 2021;18:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 43. | Weitkunat R, Sanders E, Lee PN. Meta-analysis of the relation between European and American smokeless tobacco and oral cancer. BMC Public Health. 2007;7:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 44. | Araghi M, Rosaria Galanti M, Lundberg M, Lager A, Engström G, Alfredsson L, Knutsson A, Norberg M, Sund M, Wennberg P, Trolle Lagerros Y, Bellocco R, Pedersen NL, Östergren PO, Magnusson C. Use of moist oral snuff (snus) and pancreatic cancer: Pooled analysis of nine prospective observational studies. Int J Cancer. 2017;141:687-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Bertuccio P, La Vecchia C, Silverman DT, Petersen GM, Bracci PM, Negri E, Li D, Risch HA, Olson SH, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham ET, Bamlet WR, Holly EA, Lucenteforte E, Hassan M, Yu H, Kurtz RC, Cotterchio M, Su J, Maisonneuve P, Duell EJ, Bosetti C, Boffetta P. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol. 2011;22:1420-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | International Agency for Research on Cancer. A review of human carcinogens: Part E: Personal habits and indoor combustions. Vol 100. IARC Monogr Eval Carcinog Risks Hum Lyon, France: IARC, 2012: 602. |
| 47. | Frobert O, Reitan C, Hatsukami DK, Pernow J, Omerovic E, Andell P. Smokeless tobacco, snus, at admission for percutaneous coronary intervention and future risk for cardiac events. Open Heart. 2019;6:e001109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |