Published online Nov 20, 2021. doi: 10.5662/wjm.v11.i6.294
Peer-review started: January 2, 2021
First decision: July 8, 2021
Revised: July 16, 2021
Accepted: August 31, 2021
Article in press: August 31, 2021
Published online: November 20, 2021
Serologic cross-reactivity between hantaviruses often complicates the interpre
To analyze the diagnostic value of indirect immunofluorescence assay (IFA) and western blot (WB) in the diagnosis of hantavirus infections.
One hundred eighty-eight serum samples from Puumala (PUUV) and Dobrava (DOBV) orthohantavirus infected patients were analyzed. Serology was perfor
Using IFA, 49.5% of acute-phase samples showed a monotypic response to PUUV, while 50.5% cross-reacted with other hantaviruses. The overall cross-reactivity was higher for immunoglobulin G (IgG) (50.0%) than for immunoglobulin M (IgM) (25.5%). PUUV IgM/IgG antibodies showed low/moderate reactivity with orthohantaviruses Hantaan (12.3%/31.5%), Seoul (7.5%/17.8%), DOBV (5.4%/ 28.1%), and Saaremaa (4.8%/15.7%). Both DOBV IgM and IgG antibodies were broadly reactive with Hantaan (76.2%/95.2%), Saaremaa (80.9%/83.3%), and Seoul (78.6%/85.7%) and moderate with PUUV (28.5%/38.1%). Using a WB, serotyping was successful in most cross-reactive samples (89.5%).
The presented results indicate that WB is more specific than IFA in the diagnosis of hantavirus infections, confirming serotype in most IFA cross-reactive samples.
Core Tip: Serologic cross-reactivity among hantaviruses often complicates the interpretation of the results. The overall cross-reactivity is generally higher for immunoglobulin G antibodies than for immunoglobulin M antibodies. Western blot seems to be a more specific serology method than indirect immunofluorescence assay in the diagnosis of hantavirus infections, confirming serotype in the majority of cross-reactive samples detected by indirect immunofluorescence assay.
- Citation: Vilibic-Cavlek T, Barbic L, Stevanovic V, Savic V, Mrzljak A, Bogdanic M, Tabain I. Comparison of indirect immunofluorescence and western blot method in the diagnosis of hantavirus infections. World J Methodol 2021; 11(6): 294-301
- URL: https://www.wjgnet.com/2222-0682/full/v11/i6/294.htm
- DOI: https://dx.doi.org/10.5662/wjm.v11.i6.294
Hantaviruses represent a group of serologically related rodent-borne RNA viruses that belong to the genus Orthohantavirus of the family Hantaviridae. Two different diseases, hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS), are caused by hantaviruses in humans[1]. Orthohantaviruses Hantaan (HTNV), Dobrava (DOBV), Puumala (PUUV), Seoul (SEOV), and Saaremaa (SAAV) cause HFRS with varying degrees of severity. While HTNV and DOBV cause a severe form of HFRS in Asia and Europe, SEOV causes less severe disease worldwide[2,3]. SAAV is also found to be responsible for a relatively mild human disease in Europe[4]. PUUV is a causative agent of nephropathia epidemica, the mildest form of the disease, endemic in Western Europe and Scandinavia[2].
Diagnostic methods for hantavirus infections include serology, reverse transcrip
Vero E6 cell culture has been used to isolate hantaviruses causing HFRS and HPS. Hantaviruses usually are not cytopathic in cultured cells; therefore, the detection of infection is confirmed using an immunofluorescence antibody test for viral antigen. Virus isolation is not performed as part of routine hantavirus diagnostics, since it is laborious and time-consuming and requires biosafety level 3 and 4 laboratories[6].
Serology is the main method for the diagnosis due to the hazardous nature of hantaviruses and a short-term viremia in infected humans[7,8]. Enzyme-linked immunosorbent assay and indirect immunofluorescence assay (IFA) are broadly used serologic tests used for detection of hantavirus immunoglobulin M (IgM) and immunoglobulin G (IgG) antibodies[9]. Immunoblot tests [western blot (WB) and line immunoassay] are also used in some laboratories[10].
Hantavirus nucleocapsid (N) protein is the major antigen in early humoral response in patients with hantavirus infection[11,12]. N protein is highly cross-reactive between different hantaviruses due to its conserved nature[11,13]. Overall, serologic cross-reactivity within the genus Orthohantavirus is the highest among viruses associated with (phylo)genetically closely related rodent species. DOBV is genetically and anti-genetically related to other orthohantaviruses transmitted by Murinae rodents (Old World mice and rats) such as HTNV, SEOV, and SAAV. PUUV is more distantly related to this group since its reservoirs belong to the Arvicollinae rodents (voles and lemmings)[14-16]. The interpretation of serology results is often complicated by the cross-reactivity, especially in areas where different hantaviruses co-circulate. Virus neutralization test is still the gold standard serologic test. Since this test has to be performed in biosafety level 3 laboratory, it is confined mainly to the reference laboratories[17].
Molecular diagnostic methods, including classic and real-time RT-PCR, are also widely used for the diagnosis of hantaviruses. Hantavirus RNA is detectable in blood early after the onset of symptoms; therefore, RT-PCR is a sensitive method for detecting hantavirus infections before the appearance of IgM antibodies. Primers specific for the hantavirus S and M segments have been used in different studies. The advantage of the molecular methods is that the RT-PCR product may be sequenced to identify the virus and perform phylogenetic analysis[5,18].
In Croatia, PUUV and DOBV have been demonstrated in humans[19-23], while SAAV and Tula orthohantavirus were also documented in rodents[24,25]. This study aimed to analyze the diagnostic value of IFA and WB methods in the diagnosis of hantavirus infections.
A total of 188 serum samples from patients with serologically confirmed acute hantavirus infection (2015-2019) tested at the National Reference Laboratory for Arboviruses and Hantaviruses, Croatian Institute of Public Health were included in the study. Serologic tests were performed using a commercial IFA (Hantavirus mosaic; Euroimmun, Lübeck, Germany) to detect IgM/IgG antibodies of the most common hantaviruses: PUUV, DOBV, HTNV, SEOV, and SAAV. A fluorescence occurring as fine droplets in the cytoplasm of infected cells in a dilution 1:100 was considered a positive result.
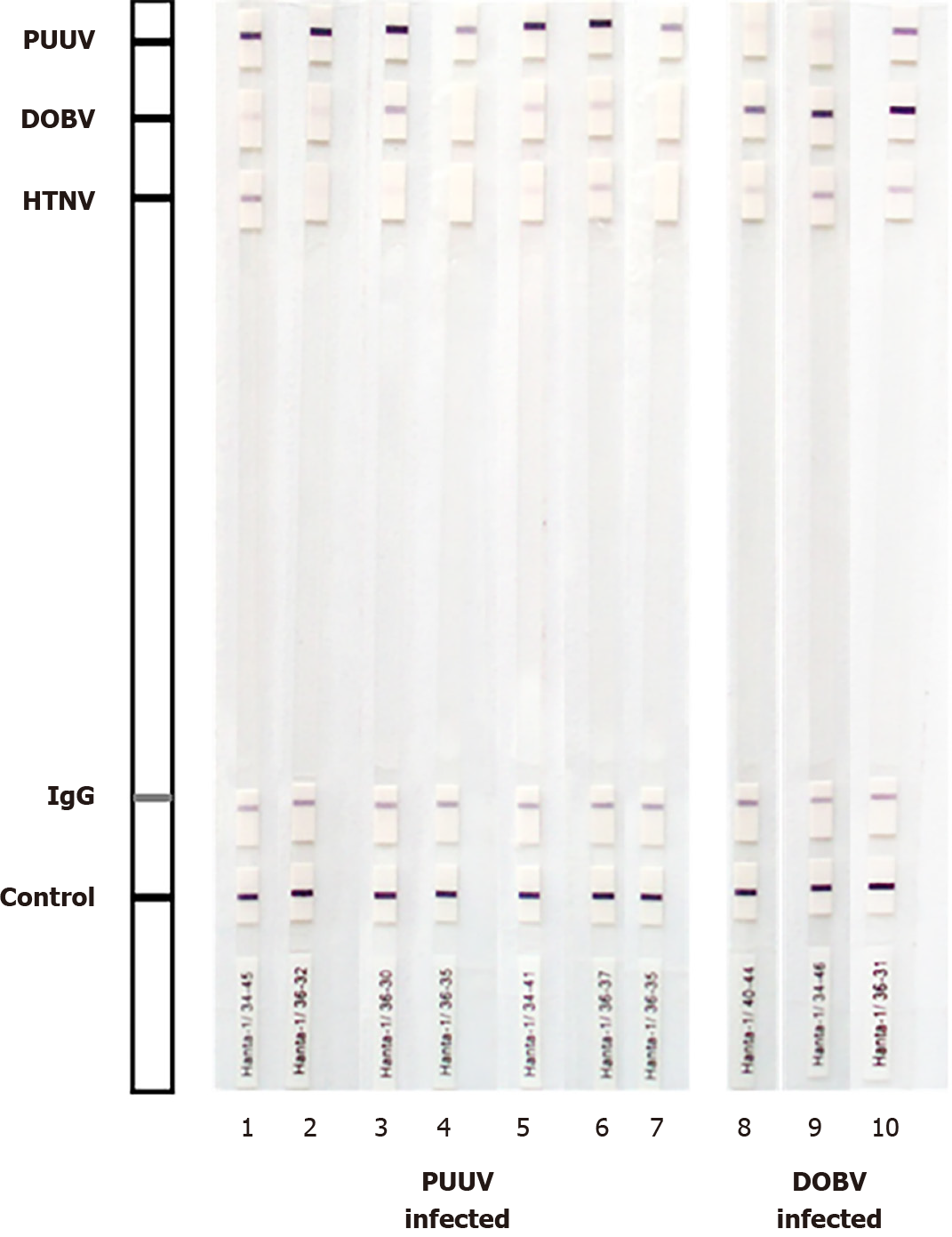
Cross-reactive samples were further tested for hantavirus IgG antibodies using a WB (Euroline Hantavirus profile, Euroimmun). WB test strips were coated with nucleocapsid PUUV; DOBV and HTNV antigens. Band signal intensity at least as of IgG control was considered a positive result. According to the band intensity, results were interpreted as follows: strong positive-very strong band (+++); positive-medium to strong band (+/++); borderline-very weak band (+/-).
The study was approved by the Ethics Committee of the Croatian Institute of Public Health (Decision number: 030-02/17-10/1). Informed consent was obtained from all subjects included in the study.
PUUV was confirmed in 146 (77.6%) and DOBV in 42 (32.4%). Using IFA, 93 (49.5%) of 188 acute-phase serum samples reacted only with the homologous PUUV antigen, while in 95 (50.5%) samples, cross-reactive IgM and/or IgG antibodies were found. The overall cross-reactivity was higher for IgG antibodies (94/188; 50.0%) than for IgM antibodies (48/188; 25.5%). Among 95 cross-reactive samples, 55 (57.9%) were confirmed as PUUV and 30 (31.6%) samples as DOBV using a WB.
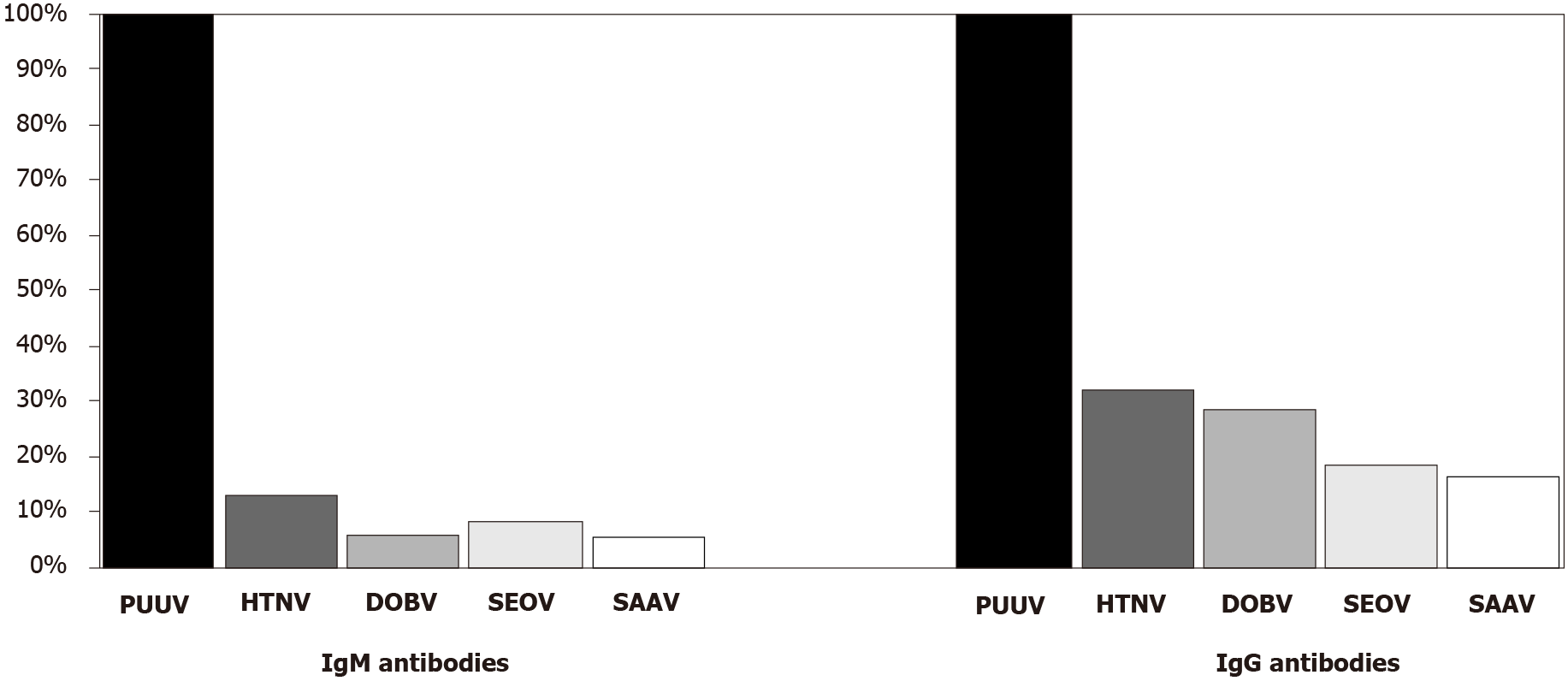
Cross-reactive patterns to different hantavirus antigens in PUUV- and DOBV-infected patients detected using IFA are presented in Figures 1 and 2. Among PUUV positive samples, a low/very low IgM reactivity was observed with HTNV (18/146; 12.3%), SEOV (11/146; 7.5%), DOBV (8/146; 5.4%), and SAAV (7/146; 4.8%). PUUV IgG antibodies showed a moderate reactivity with HTNV (46/146; 31.5%) and DOBV (41/146; 28.1%), while reactivity with SEOV and SAAV was low (26/146; 17.8% and 23/146; 15.7%, respectively).
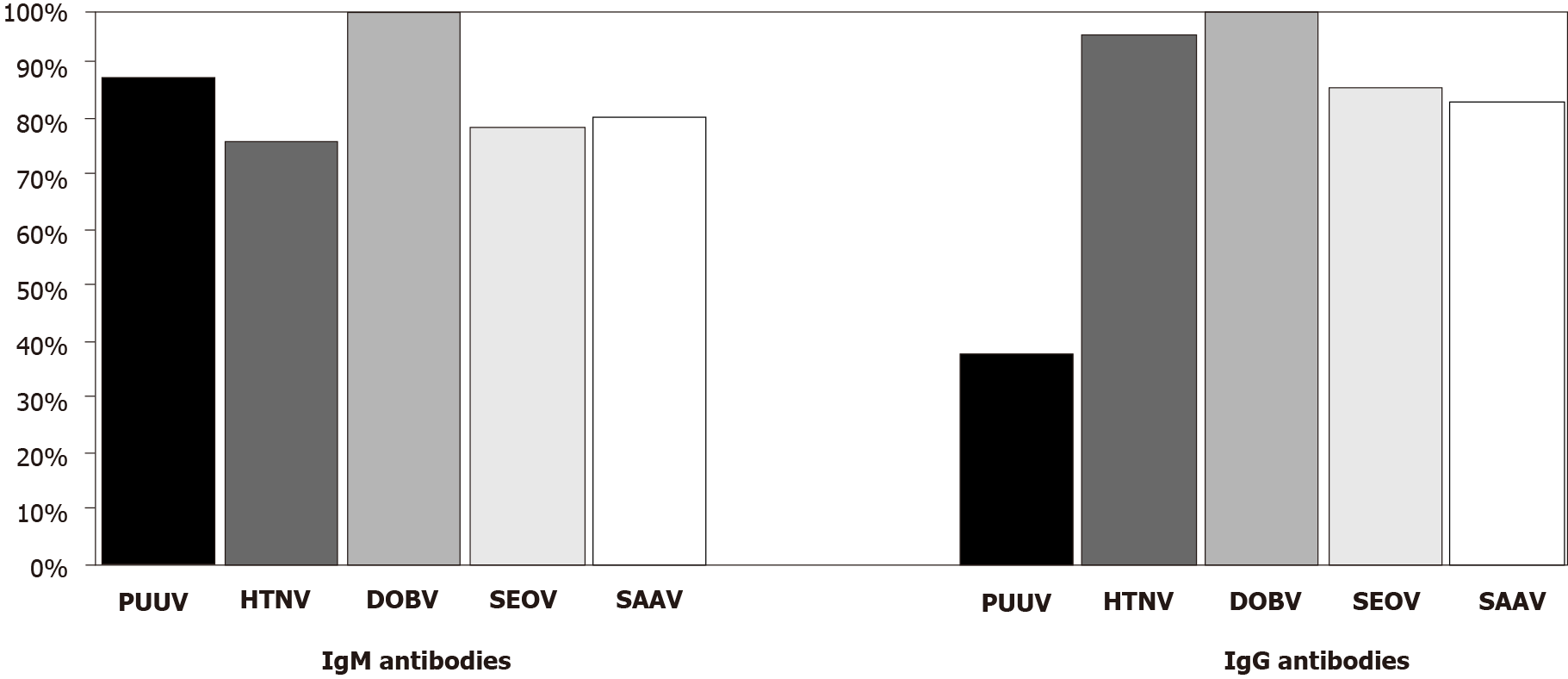
In DOBV positive samples, both IgM and IgG antibodies showed a high degree of cross-reactivity. Among IgM positive samples, the highest cross-reactivity was observed with SAAV (34/42; 80.9%), 33/42 (78.6%) with SEOV, and 32/42 (76.2%) with HTNV. In 12 samples (28.5%), cross-reactive antibodies with PUUV were found. DOBV IgG antibodies showed the highest reactivity with HTNV (40/42; 95.2%). Almost equally high reactivity was found with SEOV and SAAV (36/42; 85.7% and 35/42, 83.3%, respectively), and moderate reactivity was found with PUUV (16/42; 38.1%). The majority of DOBV-positive samples (IgM 24/42, 57.1%; IgG 35/42; 83.3%) showed reactivity with all three hantavirus antigens (HTNV + SEOV + SAAV).
Forty-six of 172 (24.5%) IgG-positive samples cross-reacted with other hantaviruses by WB. However, based on signal intensity, a very strong band to the homologous viral antigen was observed in most cross-reactive samples compared to a weak/me
| Band intensity | PUUV | HTNV | DOBV |
| PUUV-infected patients (n = 146) | |||
| Strong positive (+++)1 | - | 0 (0%) | 8 (5.5%) |
| Positive (+, ++)2 | - | 0 (0%) | 0 (0%) |
| Borderline (+/-)3 | - | 8 (5.5%) | 10 (6.8%) |
| DOBV-infected patients (n = 42) | |||
| Strong positive (+++)1 | 0 (0%) | 2 (4.7%) | - |
| Positive (+, ++)2 | 1 (2.4%) | 8 (19.0%) | - |
| Borderline (+/-)3 | 4 (9.5%) | 11 (26.2%) | - |
Results of this study indicated broadly cross-reactive patterns of hantaviruses detected by IFA, which were found to be much higher for DOBV compared to PUUV. One published multicenter study on the simultaneous detection of hantaviruses showed a high cross-reactivity of serum samples from DOBV-infected patients with SAAV, HTNV, and SEOV (60%-100%), while cross-reactivity with PUUV was moderate (up to 43%) using IFA[26]. This study observed a remarkably high cross-reactivity for both DOBV IgM/IgG antibodies with SAAV, HTNV, and SEOV antigens (IgM 76.2%-80.9%, IgG 83.3%-95.2%). In addition, 57.1% IgM and 83.3% IgG positive samples cross-reacted with all three hantavirus antigens. These results are in accordance with the phylogenetic relatedness of hantaviruses. However, a substantial cross-reactivity was also found with PUUV (IgM 28.5%, IgG 38.1%), although PUUV is phylogenetically distantly from DOBV.
IgM/IgG antibodies of PUUV-infected Croatian patients reacted moderately with HTNV (12.3%/31.5%). In a study by Lederer et al[26], even higher cross-reactivity between PUUV and HTNV IgM/IgG was found (49%/79%), while the reactivity to other tested hantaviruses was low, similar to our results.
In this study, a lower degree of cross-reactivity was also found by WB (24.5%). However, in all but 8 samples, differentiation of hantavirus serotype was possible based on powerful signal intensity to homologous antigen compared to weak/me
Since the clinical course and prognosis differ in PUUV and DOBV infection, the determination of hantavirus serotype is important for diagnosing acute HFRS cases. In addition, due to specific rodent hosts, identification of currently circulating hantavirus serotype is also useful for planning rodent control programs. Using IFA, serotype identification in seroepidemiological studies is often difficult because of extensive cross-reactivity among IgG antibodies. In DOBV infected individuals, considerable cross-reactivity was also observed between IgM antibodies. Using WB, differentiation of hantavirus serotype was possible in most cases by comparing the signal intensity in most IFA cross-reactive samples.
Although cross-reactivity among hantaviruses was detected in both IFA and WB, the results of this study showed that WB seems to be more specific than IFA, confirming hantavirus serotype in 89.5% of cross-reactive samples detected by IFA.
The cross-reactivity among hantaviruses often complicates the interpretation of serology results, especially in areas where different hantaviruses co-circulate.
Data on the comparison of different serologic methods in the diagnosis of hantaviruses are scarce.
This study aimed to analyze the diagnostic value of indirect immunofluorescence (IFA) and western blot (WB) methods in diagnosing hantavirus infections.
A commercial IFA was used to detect immunoglobulin M (IgM)/immunoglobulin G (IgG) antibodies to the most common orthohantaviruses: Puumala (PUUV), Dobrava (DOBV), Hantaan (HTNV), Seoul (SEOV), and Saaremaa (SAAV). Cross-reactive samples were additionally tested by a commercial WB using PUUV, DOBV, and HTNV antigens.
Using IFA, 49.5% of acute-phase serum samples reacted only with the homologous PUUV antigen, while in 50.5% samples, cross-reactive IgM and/or IgG antibodies were found. PUUV IgM/IgG antibodies cross-reacted with HTNV (12.3%/31.5%), SEOV (7.5%/17.8%), DOBV (5.4%/28.1%), and SAAV (4.8%/15.7%). Both DOBV IgM and IgG antibodies were broadly reactive with HTNV (76.2%/95.2%), SAAV (80.9%/83.3%), and SEOV (78.6%/85.7%) and moderate with PUUV (28.5%/38.1%). Using a WB, serotyping was successful in 89.5% cross-reactive samples.
WB seems to be more specific than IFA, confirming hantavirus serotype in the majority of cross-reactive samples detected by IFA.
Further studies on a large sample caused by different hantavirus serotypes are needed.
Provenance and peer review: Invited article; Externally peer reviewed
Specialty type: Medical laboratory technology
Country/Territory of origin: Croatia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan S S-Editor: Fan JR L-Editor: Filipodia P-Editor: Guo X
| 1. | Manigold T, Vial P. Human hantavirus infections: epidemiology, clinical features, pathogenesis and immunology. Swiss Med Wkly. 2014;144:w13937. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Fulhorst CF, Bowen MD. Hantaviruses. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. DC, Washington: ASM Press, 2011: 1504-13. [Cited in This Article: ] |
| 3. | Heyman P, Baert K, Plyusnina A, Cochez C, Lundkvist A, Esbroeck MV, Goossens E, Vandenvelde C, Plyusnin A, Stuyck J. Serological and genetic evidence for the presence of Seoul hantavirus in Rattus norvegicus in Flanders, Belgium. Scand J Infect Dis. 2009;41:51-56. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Plyusnin A, Vaheri A, Lundkvist A. Saaremaa hantavirus should not be confused with its dangerous relative, Dobrava virus. J Clin Microbiol. 2006;44:1608-9; author reply 1609. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Mattar S, Guzmán C, Figueiredo LT. Diagnosis of hantavirus infection in humans. Expert Rev Anti Infect Ther. 2015;13:939-946. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Galeno H, Mora J, Villagra E, Fernandez J, Hernandez J, Mertz GJ, Ramirez E. First human isolate of Hantavirus (Andes virus) in the Americas. Emerg Infect Dis. 2002;8:657-661. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Hujakka H, Koistinen V, Kuronen I, Eerikäinen P, Parviainen M, Lundkvist A, Vaheri A, Vapalahti O, Närvänen A. Diagnostic rapid tests for acute hantavirus infections: specific tests for Hantaan, Dobrava and Puumala viruses vs a hantavirus combination test. J Virol Methods. 2003;108:117-122. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Meisel H, Wolbert A, Razanskiene A, Marg A, Kazaks A, Sasnauskas K, Pauli G, Ulrich R, Krüger DH. Development of novel immunoglobulin G (IgG), IgA, and IgM enzyme immunoassays based on recombinant Puumala and Dobrava hantavirus nucleocapsid proteins. Clin Vaccine Immunol. 2006;13:1349-1357. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Biel SS, Donoso Mantke O, Lemmer K, Vaheri A, Lundkvist A, Emmerich P, Hukic M, Niedrig M. Quality control measures for the serological diagnosis of hantavirus infections. J Clin Virol. 2003;28:248-256. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Oldal M, Németh V, Madai M, Kemenesi G, Dallos B, Péterfi Z, Sebők J, Wittmann I, Bányai K, Jakab F. Identification of hantavirus infection by Western blot assay and TaqMan PCR in patients hospitalized with acute kidney injury. Diagn Microbiol Infect Dis. 2014;79:166-170. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Kallio-Kokko H, Leveelahti R, Brummer-Korvenkontio M, Lundkvist A, Vaheri A, Vapalahti O. Human immune response to Puumala virus glycoproteins and nucleocapsid protein expressed in mammalian cells. J Med Virol. 2001;65:605-613. [PubMed] [Cited in This Article: ] |
| 12. | Tischler ND, Rosemblatt M, Valenzuela PD. Characterization of cross-reactive and serotype-specific epitopes on the nucleocapsid proteins of hantaviruses. Virus Res. 2008;135:1-9. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Lindkvist M, Näslund J, Ahlm C, Bucht G. Cross-reactive and serospecific epitopes of nucleocapsid proteins of three hantaviruses: prospects for new diagnostic tools. Virus Res. 2008;137:97-105. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Plyusnin A. Genetics of hantaviruses: implications to taxonomy. Arch Virol. 2002;147:665-682. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Yoshimatsu K, Arikawa J. Antigenic properties of N protein of hantavirus. Viruses. 2014;6:3097-3109. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Yoshimatsu K, Arikawa J. Serological diagnosis with recombinant N antigen for hantavirus infection. Virus Res. 2014;187:77-83. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Li W, Cao S, Zhang Q, Li J, Zhang S, Wu W, Qu J, Li C, Liang M, Li D. Comparison of serological assays to titrate Hantaan and Seoul hantavirus-specific antibodies. Virol J. 2017;14:133. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Evander M, Eriksson I, Pettersson L, Juto P, Ahlm C, Olsson GE, Bucht G, Allard A. Puumala hantavirus viremia diagnosed by real-time reverse transcriptase PCR using samples from patients with hemorrhagic fever and renal syndrome. J Clin Microbiol. 2007;45:2491-2497. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Kuzman I, Markotić A, Turcinov D, Beus I. [An epidemic of hemorrhagic fever with renal syndrome in Croatia in 1995]. Lijec Vjesn. 1997;119:311-315. [PubMed] [Cited in This Article: ] |
| 20. | Markotić A, Nichol ST, Kuzman I, Sanchez AJ, Ksiazek TG, Gagro A, Rabatić S, Zgorelec R, Avsic-Zupanc T, Beus I, Dekaris D. Characteristics of Puumala and Dobrava infections in Croatia. J Med Virol. 2002;66:542-551. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Kuzman I, Puljiz I, Turcinov D, Markotić A, Turković B, Aleraj B, Andrić Z, Petković D, Tutek V, Herendić B, Iskra M, Pandak N, Misetić Z, Perić L, Jelaska D, Majetić-Sekovanić M, Ledina D, Misić-Majerus L, Radonić R. [The biggest epidemic of hemorrhagic fever with renal syndrome in Croatia]. Acta Med Croatica. 2003;57:337-346. [PubMed] [Cited in This Article: ] |
| 22. | Vilibic-Cavlek T, Furic A, Barbic L, Tabain I, Stevanovic V, Mlinaric-Galinovic G. Clinical and virological characteristics of hantavirus infections in a 2014 Croatian outbreak. J Infect Dev Ctries. 2017;11:73-80. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Lovrić Z, Kolarić B, Kosanović Ličina ML, Tomljenović M, Đaković Rode O, Danis K, Kaić B, Tešić V. An outbreak of haemorrhagic fever with renal syndrome linked with mountain recreational activities in Zagreb, Croatia, 2017. Epidemiol Infect. 2018;146:1236-1239. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Plyusnina A, Krajinović LC, Margaletić J, Niemimaa J, Nemirov K, Lundkvist Å, Markotić A, Miletić-Medved M, Avšič-Županc T, Henttonen H, Plyusnin A. Genetic evidence for the presence of two distinct hantaviruses associated with Apodemus mice in Croatia and analysis of local strains. J Med Virol. 2011;83:108-114. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Scharninghausen JJ, Pfeffer M, Meyer H, Davis DS, Honeycutt RL, Faulde M. Genetic evidence for tula virus in Microtus arvalis and Microtus agrestis populations in Croatia. Vector Borne Zoonotic Dis. 2002;2:19-27. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Lederer S, Lattwein E, Hanke M, Sonnenberg K, Stoecker W, Lundkvist Å, Vaheri A, Vapalahti O, Chan PK, Feldmann H, Dick D, Schmidt-Chanasit J, Padula P, Vial PA, Panculescu-Gatej R, Ceianu C, Heyman P, Avšič-Županc T, Niedrig M. Indirect immunofluorescence assay for the simultaneous detection of antibodies against clinically important old and new world hantaviruses. PLoS Negl Trop Dis. 2013;7:e2157. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Escadafal C, Avšič-Županc T, Vapalahti O, Niklasson B, Teichmann A, Niedrig M, Donoso-Mantke O. Second external quality assurance study for the serological diagnosis of hantaviruses in Europe. PLoS Negl Trop Dis. 2012;6:e1607. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Engler O, Klingstrom J, Aliyev E, Niederhauser C, Fontana S, Strasser M, Portmann J, Signer J, Bankoul S, Frey F, Hatz C, Stutz A, Tschaggelar A, Mutsch M. Seroprevalence of hantavirus infections in Switzerland in 2009: difficulties in determining prevalence in a country with low endemicity. Euro Surveill. 2013;18:20660. [PubMed] [DOI] [Cited in This Article: ] |