Peer-review started: August 27, 2016
First decision: September 27, 2016
Revised: December 28, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 6, 2017
The clinical spectrum of diseases associated with monoclonal gammopathies is wide and they are most commonly the consequence of renal deposition of monoclonal immunoglobulin or its components. The differential diagnosis is difficult and renal biopsy is essential. To distinguish many of these pathologies is necessary to use techniques that are not always available, even in tertiary central hospitals. This review will discuss the clinical presentation, pathologic features, treatment, prognosis and common diagnostic difficulties of these entities.
Core tip: Monoclonal gammopathy of renal significance is a wide group of kidney diseases. We discuss the most common diagnostic difficulties and suggest an algorithm for clinical approach. Screening for monoclonal immunoglobulin and an appropriate hematologic workup are fundamental and, sometimes a difficult challenge. Kidney biopsy is required to determine the exact nature of the lesion and to evaluate the severity of renal disease. Therefore, clinical and pathologic features are also discussed.
- Citation: Correia SO, Santos S, Malheiro J, Cabrita A, Martins LS, Santos J. Monoclonal gammopathy of renal significance: Diagnostic workup. World J Nephrol 2017; 6(2): 72-78
- URL: https://www.wjgnet.com/2220-6124/full/v6/i2/72.htm
- DOI: https://dx.doi.org/10.5527/wjn.v6.i2.72
The term monoclonal gammopathy of renal significance (MGRS) is a recent concept, introduced in 2012, to distinguish the nephropathic nature of these diseases from the truly benign monoclonal gammopathy of undetermined significance[1-3].
Renal damage is caused by the deposition of secreted monoclonal immunoglobulin (MIg) or its fragments, produced by a B-cell or plasma cell clone. They are heterogeneous in nature and are not always related to the presence of a detectable M component in serum and/or urine.
The immunoglobulin (Ig) deposits associated to MGRS can be classified into two categories (Table 1). The first is characterized by organized deposits: Immunoglobulin related amyloidosis, fibrillar glomerulonephritis, immunotactoid and type I cryoglobulinemic glomerulonephritis. The second disease category is characterized by non-organized electro-dense granular deposits in clinical pathological entities as MIg deposition disease, proliferative glomerulonephritis with monoclonal IgG deposits and C3 glomerulopathy with monoclonal gammopathy. We decided to address only diseases with glomerular involvement.
| Organized immunoglobulin deposits | |
| Fibrillar deposits | Immunoglobulin related amyloidosis |
| Fibrillar glomerulonephritis | |
| Microtubular deposits | Immunotactoid glomerulopathy |
| Type I cryoglobulinemic glomerulonephritis | |
| Non-organized immunoglobulin deposits | |
| Monoclonal | Light chain deposition disease |
| Immunoglobulin | Light and heavy chain deposition disease |
| Deposition disease | Heavy deposition disease |
| Proliferative glomerulonephritis with monoclonal IgG deposits | |
| C3 glomerulopathy with monoclonal gammopathy | |
Several mechanisms that induce injury have been described, such as deposition and precipitation of MIg in the different components of the kidney (glomeruli, vessels, interstitium)[4], dysregulation of the complement pathway by the MIg[5], and the MIg itself acting like autoantibodies against complement factor or phospholipase A2 receptor[6]. Other mechanisms are still unknown.
Screening for MIg and an appropriate hematologic workup are essential. It should include serum (SPEP), urine electrophoresis, immunofixation studies and free light-chain assays (FLC) if conventional electrophoresis studies are negative. FLC is more sensitive for the detection of light chains than urine immunofixation. Results may be affected by the presence of renal failure, but a Kappa(κ)/lambda(λ) light-chain ratio > 3.0 is unlikely to be due to renal insufficiency alone.
MIg may be undetectable by these methods reflecting the “small” size of the underlying clone.
It is mandatory to characterize the clone by bone marrow aspirate and biopsy, to establish the therapeutic strategy.
The M protein in light chain deposition disease can be identified by FLC, however only 25%-76% of the cases can be identified by SPEP or immunofixation studies[2,4]. In light chain amyloidosis, SPEP and immunofixation can identify the M protein in 66%-80% of the cases and FLC in 76%-88%[2].
A review of systems especially renal, cardiac, skin and nervous system should be performed when evaluating patients with monoclonal gammopathy.
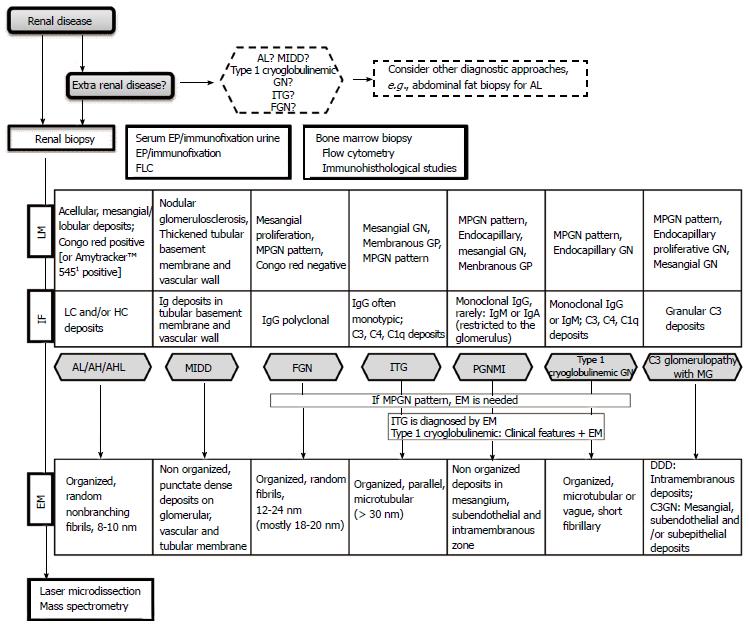
Kidney biopsy is therefore required to determine the exact nature of the lesion and severity of renal disease, and in most situations, detailed immunofluorescence (IF) and electron microscopic (EM) studies are need to allow the identification of deposits composition and pattern of organization (Figure 1).
Hence, with such difficulties diagnosing and classifying these diseases, it is easy to understand that misdiagnosis and delayed treatment can occur, with an adverse impact on renal and patient prognosis. In this review we discuss the diagnostic approach, clinical and pathologic features of MGRS lesions related to Ig deposits and the diagnostic difficulties posed in the clinical practice.
Free Ig subunits secreted by a single clone of B cells, mostly light chains (λ or κ isotype), are the cause of the most common and severe amyloidosis affecting the kidney. The fibrils in Ig light chain (AL) amyloidosis are derived from the variable region of λ light chains in approximately seventy-five percent of cases, and κ in the remaining[7,8]. The involvement of an Ig heavy chain in amyloidosis (heavy chain only - AH; and heavy chains and light chains - AHL amyloidosis) remains extremely rare.
By light microscopy, amyloid deposits are amorphous and acellular pale eosinophilic material. The glomeruli may show massive amyloid deposits, typically without increase in cellularity. Amyloid deposits may also involve arterioles, arteries, interstitium and tubules. Definitive diagnosis is made by Congo red stain detecting apple-green birefringence under polarized light[8,9]. By EM, amyloid appears as nonbranching fibrils with a diameter of 8 to 10 nm[9].
On IF microscopy, the staining for a single AL with negativity for Ig heavy chain, is diagnostic of AL. Deposition of the variable region explains why IF microscopy with anti-λ and anti-κ light chain antibodies is often weakly positive[10]. It is important to be aware that the absence of reactivity for either heavy or light chain does not rule out AL/AH/AHL disease[9].
Problematic amyloid cases, such as those with equivocal IF (which is more frequent with heavy chains than with light chains) can be accurately typed by laser microdissection and mass spectrometry. This methods can identify the type of renal amyloidosis in more than 97% of cases, and can distinguish it from non-amyloid fibrillar glomerulonephritis[8,11,12].
The majority of patients will have a detectable serum and/or urinary M protein. All patients require immunofixation of serum and urine and FLC ratio.
AL/AH/AHL are associated with a higher degree of proteinuria and a higher frequency of nephrotic syndrome compared with the other types of amyloidosis[12]. On presentation renal impairment may be present.
The goal of current treatment approaches for AL amyloidosis is to eradicate the clonal plasma cells that produce the amyloidogenic light chain. The prognosis of AL amyloidosis has improved substantially during the past decade with the increasing use of aggressive anti-plasma cell treatment[9].
Fibrillary glomerulonephritis (FGN) is a rare primary glomerular disease. The fibrillar deposits have larger thickness than amyloid and are Congo red negative[13,14]. However, size alone, is an insufficient criterion for the diagnosis[13]. Light microscopy typically shows mesangial proliferation and a membranoproliferative glomerulonephritis (MPGN) pattern. The fibrils are deposited in the mesangium, glomerular basement membranes, or both. Tubular or interstitial deposits are rare. On IF, polyclonal glomerular Ig deposits (typically IgG, and light chains) are more common than monotypic glomerular deposits[15]. Occasionally staining for IgG may occur in a membranous pattern and IgG4 is the dominant subclass. The mesangial staining suggests the specific diagnosis, confirmed by negative Congo red stain and by EM. The EM findings show the presence of randomly aligned fibrils that resemble amyloid fibrils but are larger.
In a case series report, one third of the cases occurred in patients with history of malignancy (most commonly carcinoma) or autoimmune diseases (most commonly Crohn’s disease, systemic lupus, Graves’ disease, and idiopathic thrombocytopenic purpura)[16]. These cases should not be considered MGRS. In the same case series[16], 11% stained for IgG and light chains, which can lead to believe that the FGN is also a type of MGRS. M-spike was detected by SPEP/immunofixation in only 16% of 61 patients with fibrillary glomerulonephritis from a case series of a single medical center[16].
Clinically, FGN most often presents in middle aged to older patients. Patients typically present with proteinuria, 50% within nephrotic range, with or without renal insufficiency, hematuria or hypertension[15-17]. The outcome is frequently poor, progression to end-stage renal disease occurs in approximately half of the patients within years[15-17].
The differential diagnosis between other MPGN can be difficult without EM, which can delay the treatment targeted to the B cell or plasma cell clone. However an optimal treatment are yet to be demonstrated and prospective and controlled studies are needed to determine the appropriated therapeutic regimen. There is an ongoing phase 2 clinical trial to evaluate Rituximab as a treatment option[18]. Recurrence (20%) in transplant allograft has been reported[15].
Immunotactoid (microtubular) glomerulopathy (ITG) is a glomerular disease characterized by the presence of Congo red negative organized glomerular deposits generally limited to the glomerulus, stain by IF for IgG (in most cases monoclonal) and complement. Renal biopsy shows lobular MPGN or membranous pattern[13,15,19]. The microtubular structure often measure > 30 nm in diameter by EM and are often organized in parallel arrays[17]. ITG occurs in an older population and is typically presented as a nephrotic syndrome. Hypocomplementemia is common[15,17].
Underlying hematologic malignancy is frequent, and the most common is chronic lymphocytic leukemia (in contrast to AL amyloidosis and monoclonal immunoglobulin deposition disease in which the most common is myeloma)[13,19]. Lymphoplasmacytic lymphoma and MGRS are also common[13,19].
FGN and ITG can be overlooked when EM is not performed. Even with EM, the diagnosis can be difficult in a variety of circumstances: When fibrils are subepithelial; when they have an atypical ultrastructural appearance; when deposits of cryoglobulins are microtubular and indistinguishable from these; and when fibrils or microtubules are of a smaller admeasured size[17].
Type I cryoglobulinemic glomerulonephritis
Cryoglobulinemia is defined as the presence of circulating immunoglobulins that precipitate with cold temperature and dissolve with rewarming. Type I cryoglobulinemia consist of a single monoclonal immunoglobulin (usually of IgG or IgM class), while types II and III are mixed cryoglobulinemias, with a monoclonal component in type II and only polyclonal immunoglobulins in type III[20,21]. By light microscopy typical features are membranoproliferative or endocapillary proliferative glomerulonephritis with intraluminal periodic acid-Schiff positive (hyaline-like) deposits. IF microscopy demonstrates the presence of IgM and IgG as well as complement components. On EM, deposits are predominantly subendothelial and intracapillary. They may have a vague short fibrillar substructure, and sometimes a tubular configuration.
Type I cryoglobulin is associate with plasma cell dyscrasias or B-cell lymphoproliferative disorders (multiple myeloma, Waldenstrom macroglobulinemia, chronic lymphocytic leukemia, B-cell non-Hodgkin lymphoma, MGRS, and hairy cell leukemia)[20]. Occurrence of cutaneous involvement (palpable purpura) is frequent and neurologic manifestations can vary from pure sensory axonopathy to mononeuritis multiplex[20]. Hypocomplementemia is not as frequent as in type II cryoglobulinemia[21].
The treatment of this entity is primarily directed to the underlying hematologic malignancy[20].
In clinical and pathologic terms, light-chain, light and heavy chain, and heavy chain deposition disease (LCDD, LHCDD, HCDD, respectively) are similar and may therefore be referred as monoclonal immunoglobulin deposition disease (MIDD)[4,22]. The majority of kidney diseases in MIDD are secondary to deposition of light chains (κ in most cases) instead of heavy chains or intact Ig[23]. These forms differ from amyloidosis in that the deposits lack affinity for Congo red and do not have a fibrillar organization.
Usually they show nodular sclerosing lesions and thickening of tubular basement membranes on light microscopy; a membranoproliferative pattern has also been described. Diffuse linear staining of monoclonal light/heavy chains along the glomerular and tubular basement membranes is shown on IF and punctate dense deposits along the glomerular and tubular basement membranes on EM[4,24].
The deposits in HCDD are composed of the Ig heavy chain, which typically lacks the first constant domain (CH1). IgD deposition disease was recently described based on laser microdissection and mass spectrometry in which the IF studies were negative for Ig deposits[25].
MIDD is typically diagnosed in the sixth decade, in the presence of renal insufficiency and proteinuria, often accompanied by nephrotic syndrome or hypertension. It can occur in the absence of a detectable malignant process, even after prolonged follow-up[4]. In some case series clinical evidence of dysproteinemia was frequent, with myeloma and MGRS being described[4,24]. Treatment of the underling dysproteinemia should be considered, and studies have shown that chemotherapy and stem cell transplantation are an effective therapy for renal dysfunction in MIDD[24,26,27]. Recurrence in transplant allograft has been reported[24].
Monoclonal gammopathy is an important cause of membranoproliferative glomerulonephritis pattern, which is an immune complex-mediated glomerulonephritis characterized by subendothelial and mesangial immune complexes deposition. Nars and collegues, described this entity of proliferative glomerulonephritis associated with monoclonal IgG deposition[28]. A similar entity with deposition of monoclonal IgM or IgA has been described[29,30].
IF demonstrates deposits restricted to the glomerulus that stained for a single light-chain isotype and a single heavy-chain subtype, most commonly IgG3[31]. EM reveals mesangial, subendothelial and intramembranous granular non-organized deposits.
In cases of endocapillary proliferative or membranoproliferative glomerulonephritis in which the deposits stain for IgG and a single light chain, differential diagnoses should be made with type 1 cryoglobulinemic glomerulonephritis, and ITG[28]. The diagnosis of ITG is established by EM and type 1 cryoglobulinemic glomerulonephritis should be excluded by clinical features. A specific clone was identified in 5% to 25% in some case series[28,31].
Proliferative glomerulonephritis with monoclonal IgG deposits is typically presented with proteinuria, variable degrees of hematuria, renal insufficiency and hypertension. Hypocomplementemia (mostly of the C3 component) is frequent.
Treatment recommendations are based on clinical experience with small numbers of patients. Immunosuppressive therapy have been used with variable outcomes[32].
C3 glomerulopathy is characterized by the accumulation of complement component C3 in glomeruli caused by abnormal control of complement activation, degradation or deposition.
On light microscopy it could show a variety of appearances: Mesangial proliferation, membranoproliferative pattern, endocapillary proliferation or crescent formation. C3 glomerulonephritis (C3GN) and dense deposit disease (DDD) are its subtypes and can be distinguished by EM. DDD is characterized by replacement of the basement membrane by highly electron dense deposits. C3GN is characterized by mesangial, subendothelial and/or subepithelial granular deposits that are less electron dense[33,34].
C3 glomerulopathy could be an unusual complication of plasma cell dyscrasia[35]. Monoclonal protein (which in this case, does not deposit in the glomeruli) can interfere with complement regulating proteins such as factor H, and act as a C3 nephritic factor resulting in a pathological activation of the alternative pathway of complement.
The clinical presentation is usually with hematuria, proteinuria with or without renal insufficiency. Serum C3 levels can be low.
The optimal treatment remains undefined. There have been contradictory reports in published literature, about the efficacy of treatment based on glucocorticoid, mycophenolate mofetil, and rituximab[5,33]. There are many ongoing innovative approaches using eculizumab[33]. Studies have been reported in which the use of eculizumab in dense deposit disease and C3 glomerulonephritis resulted in proteinuria reduction and/or serum albumin normalization and/or creatinine decrease[33].
The risk for recurrence of C3 glomerulopathy is high, but one must take into account that all these results are based on small data sets[33].
In order to facilitate and summarize the clinical approach of the different entities mentioned above, we decided to build up a diagnostic work up algorithm, which we here propose (Figure 1).
Monoclonal immunoglobulin can cause a variety of renal diseases resulting from the direct renal deposition and precipitation or, from an indirect mechanism, for example, via dysregulation of the complement pathway.
In this group of renal disorders the differential diagnosis can be a clinical challenge and that’s why we considered that an algorithm for the approach has to be developed and improved.
A common clinical challenge begins with the identification of the underlying clone. Standardized diagnostic evaluations need to be carried out as summarize above. Diagnosis requires a detailed hematologic evaluation and kidney biopsy. Morphologic alterations on light microscopy and immunofluorescence often need to be integrated with the changes on electron microscopy.
The lack of experience in dealing with these diseases can delay treatment. Increased cognizance and appreciation of this clinical-pathological entity and associated treatment options may improve patient outcomes.
Successful treatment is based on chemotherapy that should be adapted to the underlying clone and renal function. A multidisciplinary team consisting of nephrologists and hematologists should take responsibility for an individualized therapeutic approach as no standardized treatments based on prospective studies exist.
Manuscript source: Invited manuscript
Specialty type: Urology and nephrology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Trumper L, Watanabe T S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Leung N, Bridoux F, Hutchison CA, Nasr SH, Cockwell P, Fermand JP, Dispenzieri A, Song KW, Kyle RA. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120:4292-4295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 332] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 2. | Bridoux F, Leung N, Hutchison CA, Touchard G, Sethi S, Fermand JP, Picken MM, Herrera GA, Kastritis E, Merlini G. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87:698-711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 3. | van de Donk NW, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, Hajek R, Kleber M, Ludwig H, Morgan G. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99:984-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D’Agati VD. Renal monoclonal immunoglobulin deposition disease: the disease spectrum. J Am Soc Nephrol. 2001;12:1482-1492. [PubMed] [Cited in This Article: ] |
| 5. | Zand L, Kattah A, Fervenza FC, Smith RJ, Nasr SH, Zhang Y, Vrana JA, Leung N, Cornell LD, Sethi S. C3 glomerulonephritis associated with monoclonal gammopathy: a case series. Am J Kidney Dis. 2013;62:506-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 132] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Debiec H, Hanoy M, Francois A, Guerrot D, Ferlicot S, Johanet C, Aucouturier P, Godin M, Ronco P. Recurrent membranous nephropathy in an allograft caused by IgG3κ targeting the PLA2 receptor. J Am Soc Nephrol. 2012;23:1949-1954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Bellotti V, Merlini G, Bucciarelli E, Perfetti V, Quaglini S, Ascari E. Relevance of class, molecular weight and isoelectric point in predicting human light chain amyloidogenicity. Br J Haematol. 1990;74:65-69. [PubMed] [Cited in This Article: ] |
| 8. | Nasr SH, Said SM, Valeri AM, Sethi S, Fidler ME, Cornell LD, Gertz MA, Dispenzieri A, Buadi FK, Vrana JA. The diagnosis and characteristics of renal heavy-chain and heavy/light-chain amyloidosis and their comparison with renal light-chain amyloidosis. Kidney Int. 2013;83:463-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Dember LM. Amyloidosis-associated kidney disease. J Am Soc Nephrol. 2006;17:3458-3471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 10. | Buxbaum JN, Chuba JV, Hellman GC, Solomon A, Gallo GR. Monoclonal immunoglobulin deposition disease: light chain and light and heavy chain deposition diseases and their relation to light chain amyloidosis. Clinical features, immunopathology, and molecular analysis. Ann Intern Med. 1990;112:455-464. [PubMed] [Cited in This Article: ] |
| 11. | Sethi S, Theis JD, Leung N, Dispenzieri A, Nasr SH, Fidler ME, Cornell LD, Gamez JD, Vrana JA, Dogan A. Mass spectrometry-based proteomic diagnosis of renal immunoglobulin heavy chain amyloidosis. Clin J Am Soc Nephrol. 2010;5:2180-2187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Said SM, Sethi S, Valeri AM, Leung N, Cornell LD, Fidler ME, Herrera Hernandez L, Vrana JA, Theis JD, Quint PS. Renal amyloidosis: origin and clinicopathologic correlations of 474 recent cases. Clin J Am Soc Nephrol. 2013;8:1515-1523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Bridoux F, Hugue V, Coldefy O, Goujon JM, Bauwens M, Sechet A, Preud’Homme JL, Touchard G. Fibrillary glomerulonephritis and immunotactoid (microtubular) glomerulopathy are associated with distinct immunologic features. Kidney Int. 2002;62:1764-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Joh K. Pathology of glomerular deposition diseases and fibrillary glomerulopathies associated with paraproteinemia and haematopoietic disorder. Nephrology (Carlton). 2007;12 Suppl 3:S21-S24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D’Agati VD. Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int. 2003;63:1450-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 251] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, Leung N, Fervenza FC. Fibrillary glomerulonephritis: a report of 66 cases from a single institution. Clin J Am Soc Nephrol. 2011;6:775-784. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Alpers CE, Kowalewska J. Fibrillary glomerulonephritis and immunotactoid glomerulopathy. J Am Soc Nephrol. 2008;19:34-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Erickson SB. Mayo Clinic. A Single Center Pilot Trial of Rituximab in the Treatment of Fibrillary Glomerulonephritis. Mayo Clinic. Bethesda (MD): National Library of Medicine. Published 2014. [accessed 2016; Aug 10] Available from: http://clinicaltrials.gov/show/NCT02197767 NLM Identifier: NCT02197767. [Cited in This Article: ] |
| 19. | Nasr SH, Fidler ME, Cornell LD, Leung N, Cosio FG, Sheikh SS, Amir AA, Vrana JA, Theis JD, Dogan A. Immunotactoid glomerulopathy: clinicopathologic and proteomic study. Nephrol Dial Transplant. 2012;27:4137-4146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Terrier B, Karras A, Kahn JE, Le Guenno G, Marie I, Benarous L, Lacraz A, Diot E, Hermine O, de Saint-Martin L. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore). 2013;92:61-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Nasr SH, Markowitz GS, Reddy BS, Maesaka J, Swidler MA, D’Agati VD. Dysproteinemia, proteinuria, and glomerulonephritis. Kidney Int. 2006;69:772-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Kapoulas S, Raptis V, Papaioannou M. New aspects on the pathogenesis of renal disorders related to monoclonal gammopathies. Nephrol Ther. 2015;11:135-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Sanders PW, Herrera GA. Monoclonal immunoglobulin light chain-related renal diseases. Semin Nephrol. 1993;13:324-341. [PubMed] [Cited in This Article: ] |
| 24. | Nasr SH, Valeri AM, Cornell LD, Fidler ME, Sethi S, D’Agati VD, Leung N. Renal monoclonal immunoglobulin deposition disease: a report of 64 patients from a single institution. Clin J Am Soc Nephrol. 2012;7:231-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 25. | Royal V, Quint P, Leblanc M, LeBlanc R, Duncanson GF, Perrizo RL, Fervenza FC, Kurtin P, Sethi S. IgD heavy-chain deposition disease: detection by laser microdissection and mass spectrometry. J Am Soc Nephrol. 2015;26:784-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Tovar N, Cibeira MT, Rosiñol L, Solé M, de Larrea CF, Escoda L, Rovira M, Bladé J. Bortezomib/dexamethasone followed by autologous stem cell transplantation as front line treatment for light-chain deposition disease. Eur J Haematol. 2012;89:340-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Cohen C, Royer B, Javaugue V, Szalat R, El Karoui K, Caulier A, Knebelmann B, Jaccard A, Chevret S, Touchard G. Bortezomib produces high hematological response rates with prolonged renal survival in monoclonal immunoglobulin deposition disease. Kidney Int. 2015;88:1135-1143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 28. | Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D’Agati VD. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20:2055-2064. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 270] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 29. | Soares SM, Lager DJ, Leung N, Haugen EN, Fervenza FC. A proliferative glomerulonephritis secondary to a monoclonal IgA. Am J Kidney Dis. 2006;47:342-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Yahata M, Nakaya I, Takahashi S, Sakuma T, Sato H, Soma J. Proliferative glomerulonephritis with monoclonal IgM deposits without Waldenström’s macroglobulinemia: case report and review of the literature. Clin Nephrol. 2012;77:254-260. [PubMed] [Cited in This Article: ] |
| 31. | Bhutani G, Nasr SH, Said SM, Sethi S, Fervenza FC, Morice WG, Kurtin PJ, Buadi FK, Dingli D, Dispenzieri A. Hematologic characteristics of proliferative glomerulonephritides with nonorganized monoclonal immunoglobulin deposits. Mayo Clin Proc. 2015;90:587-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 32. | Hogan JJ, Weiss BM. Bridging the Divide: An Onco-Nephrologic Approach to the Monoclonal Gammopathies of Renal Significance. Clin J Am Soc Nephrol. 2016;11:1681-1691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 387] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 34. | Barbour TD, Ruseva MM, Pickering MC. Update on C3 glomerulopathy. Nephrol Dial Transplant. 2016;31:717-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 35. | Sethi S, Sukov WR, Zhang Y, Fervenza FC, Lager DJ, Miller DV, Cornell LD, Krishnan SG, Smith RJ. Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis. 2010;56:977-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Sjölander D, Röcken C, Westermark P, Westermark GT, Nilsson KP, Hammarström P. Establishing the fluorescent amyloid ligand h-FTAA for studying human tissues with systemic and localized amyloid. Amyloid. 2016;23:98-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |