Revised: May 1, 2013
Accepted: May 4, 2013
Published online: May 6, 2013
Processing time: 89 Days and 23.9 Hours
AIM: To assess the correlation between the serum hepcidin-25 level and left ventricular mass index.
METHODS: This study was a cross-sectional study conducted between March 2009 and April 2010. Demographic and biochemical data, including the serum hepcidin-25 level, were collected for chronic kidney disease (CKD) patients. Two-dimensional echocardiography was performed to determine the left ventricle mass (LVM), left ventricular mass index (LVMI), interventricular septum thickness (IVSd), left ventricle posterior wall thickness (LVPW), right ventricular dimension (RVD), left atrium (LA) and ejection fraction (EF).
RESULTS: A total of 146 patients with stage 1 to 5 CKD were enrolled. Serum hepcidin-25 levels were 16.51 ± 5.2, 17.59 ± 5.32, 17.38 ± 6.47, 19.98 ± 4.98 and 22.03 ± 4.8 ng/mL for stage 1 to 5 CKD patients, respectively. Hepcidin-25 level was independently predicted by the serum ferritin level (β = 0.6, P = 0.002) and the estimated glomerular filtration rate (β = -0.48, P = 0.04). There were negative correlations between the serum hepcidin level and the LVM and LVMI (P = 0.04 and P = 0.005, respectively). Systolic blood pressure (BP) was positively correlated with the LVMI (P = 0.005). In the multivariate analysis, a decreased serum hepcidin-25 level was independently associated with a higher LVMI (β = -0.28, 95%CI: -0.48 - -0.02, P = 0.006) after adjusting for body mass index, age and systolic BP.
CONCLUSION: A lower serum hepcidin level is associated with a higher LVMI in CKD patients. Low hepcidin levels may be independently correlated with unfavorable cardiovascular outcomes in this population.
Core tip: Cardiovascular disease is the primary cause of death among chronic kidney disease (CKD) patients, and left ventricular hypertrophy is a powerful independent predictor of mortality for end-stage renal disease patients. Low left ventricular performance is associated with iron deficiency anemia in rats with CKD. Hepcidin-25, a recently discovered biologically active 25-amino acid peptide, plays a key role in the iron homeostasis. Chronic iron deficiency may, by itself, reduce exercise capacity and cause ultrastructural alterations in cardiomyocytes. In the study, we showed that patients in higher grade of CKD stage had the higher serum hepcidin levels, and that there was negative correlation between serum hepcidin level and LVMI.
- Citation: Hsieh YP, Huang CH, Lee CY, Chen HL, Lin CY, Chang CC. Hepcidin-25 negatively predicts left ventricular mass index in chronic kidney disease patients. World J Nephrol 2013; 2(2): 38-43
- URL: https://www.wjgnet.com/2220-6124/full/v2/i2/38.htm
- DOI: https://dx.doi.org/10.5527/wjn.v2.i2.38
Hepcidin-25, a recently discovered biologically active 25-amino acid peptide, was first identified in human urine and plasma. This peptide contains four disulfide bonds[1]. It is synthesized, processed and secreted primarily by hepatocytes. In vitro, human hepcidin has anti-bacterial and antifungal activities[2]. Hepcidin regulates iron homeostasis by binding to and inducing the internalization and degradation of ferroportin, the sole cellular iron efflux channel in iron-transporting cells[3]. In experimental settings, pre-treatment with intravenous hepcidin or the induction of hepatic hepcidin expression prevents the inflammatory response due to exposure to lipopolysaccharide. Pagani et al[4] suggested that the inverse relationship between the levels of proinflammatory markers and circulating hepcidin may result from the anti-inflammatory properties of the latter[5].
Iron is essential not only for erythropoiesis but also for several bioenergetic processes in skeletal muscle. Chronic iron deficiency may, by itself, reduce exercise capacity and cause ultrastructural alterations in cardiomyocytes[6]. Using multivariable Cox models, Jankowska et al[7] found that low hepcidin was independently associated with increased 3-year mortality among systolic heart failure (HF) patients. These authors also found a gradual reduction in the serum hepcidin level during the natural course of HF, accompanied by depleted iron stores (low serum ferritin) and iron-restricted erythropoiesis (reduced hemoglobin, high RDW). However, the possible mechanisms connecting the hepcidin level and cardiac mortality have not been addressed.
Cardiovascular disease is the primary cause of death among chronic kidney disease (CKD) patients[8], and left ventricular hypertrophy is a powerful independent predictor of mortality for end-stage renal disease (ESRD) patients[9]. Low LV performance is associated with iron deficiency anemia in rats with CKD. Furthermore, the overproduction of HIF-1α and the activation of caspase-3 seem to be associated with iron deficiency[10]. The correlation between the serum hepcidin level and cardiovascular risk was unclear for CKD patients.
The aims of this cross-sectional study were to use a randomly selected subgroup of the CKD population: (1) to describe the variations in the serum hepcidin level by age, sex, body mass index (BMI), blood pressure (BP), and degree of renal insufficiency; and (2) to estimate the association between the serum hepcidin level and cardiovascular risk factors, including the BP, left ventricular mass (LVM) and left ventricular mass index (LVMI), in stage 1 to 5 CKD patients.
This study was a cross-sectional study conducted between March 2009 and April 2010 in the Nephrology Department of Changhua Christian Hospital and included 146 CKD patients (84 males, 62 females) in stages 1-5 who did not require dialysis. The estimated glomerular filtration rate (eGFR) was calculated by MDRD formula[11]. The stage was determined based on the eGFR and the level of proteinuria using the Kidney Disease Outcomes Quality Initiative (K/DOQI) definition. The patients had had stable renal function for at least 3 mo before recruitment. Exclusion criteria included hospitalization or infection during the past month, age below 18 years, chronic hepatitis, malignancy, active gastrointestinal bleeding or refusal to participate. All patients gave informed consent in advance, and the Institutional Review Board of the Changhua Christian Hospital (CCH) approved the protocol. This study was conducted in adherence with the Declaration of Helsinki.
Relevant clinical and biochemical data were retrieved from the patients’ records and interviews. Demographic data included age, sex, BMI, the cause of the CKD, past medical history and medication history. The BP was measured daily with a random zero sphygmomanometer after a 15-min rest in the CKD unit. Participants with a systolic BP ≥ 140 mmHg, a diastolic BP ≥ 90 mmHg, or a history of hypertension and currently using antihypertensive drugs were categorized as hypertensive. Individuals with fasting plasma glucose levels > 126 mg/dL or a history of diabetes mellitus and those currently using hypoglycemic drugs or insulin were categorized as having diabetes mellitus.
After fasting overnight for at least eight hours, a one-time blood sample was used to measure fasting blood sugar, total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), blood urea nitrogen (BUN), creatinine, albumin, calcium, phosphate and hemoglobin. Samples were centrifuged immediately, and the supernatants were stored at -70 °C until further analysis. Two major types of hepcidin assays were available: mass spectrometry and ELISA. The plasma level of bioactive hepcidin-25 was measured in duplicate using a novel validated competitive ELISA (Peninsula Laboratories, LLC, United States).
Echocardiography was performed by a qualified cardiologist using a Hewlett Packard Sonos 2000® (Hewlett-Packard Inc., Andover, MA) Phased-Array Imaging System with built-in software. The cardiologist was blind to the clinical data. The LVM, interventricular septum thickness (IVSd), left ventricle posterior wall thickness (LVPW), right ventricular dimension (RVD), left atrium (LA) and ejection fraction (EF) were determined according to the method established by the American Society of Echocardiography. We calculated the LVMI by dividing the LVM by height to the power of 2.7(g•m -2.7) because this formula has proven to be more accurate than the formula based on body surface area for predicting cardiovascular events. In this study, all echocardiographic measurements were performed by the same cardiologist at a single center to minimize the inter-observer variation.
Values were expressed as the means ± SD. Linear regression analysis was conducted to assess the relationships between the serum hepcidin level and other relevant parameters. Correlations were assessed using Spearman’s or Pearson’s test. To identify independent predictors of the serum hepcidin level, multiple regression analysis was performed. The parameters of different groups were compared using the Jonckheere-Terpstra or the Kruskal-Wallis test for non-parametric continuous data and an independent t-test for parametric data. Statistical analysis was performed using SPSS for Windows software, version 15.0. All tests were two-tailed, and a P-value < 0.05 was considered statistically significant.
Table 1 presents the baseline demographic and clinical characteristics for all study participants (n = 146). According to the National Kidney Foundation’s classification of CKD, the patients were in the following stages: stage 1 for 14 patients, stage 2 for 16, stage 3 for 38, stage 4 for 34 and stage 5 for 44. The overall mean of eGFR was 38.13 mL/min per 1.73 m2, and 30 patients were diabetic. Subjects with stage 4 or 5 disease had higher serum BUN, creatinine, phosphate, iPTH and hepcidin levels and lower a eGFR and hemoglobin level than subjects with earlier CKD stages (P < 0.05 for all).
| Baseline clinical | Laboratory data |
| Age (yr) | 61.7 ± 15.24 |
| Female sex | 62 (41.89%) |
| Systolic blood pressure (mmHg) | 131.18 ± 13.2 |
| Diastolic blood pressure (mmHg) | 75.27 ± 10.51 |
| Body mass index (kg/m2)2 | 24.99 ± 4.7 |
| Hemoglobin (g/dL) | 11.14 ± 2.16 |
| BUN (mg/dL) | 43.16 ± 23.56 |
| Cr (mg/dL) | 3.63 ± 3.19 |
| eGFR-MDRD (mL/min per 1.73 m2 of Body surface area3) | 38.13 ± 35.24 |
| CRP (mg/L) | 0.223 ±0.362 |
| Ferritin (μg/mL) | 254.59 ± 293.41 |
| Iron (μg/dL) | 74.6 ± 37.95 |
| TSAT-percent4 | 0.28 ± 0.15 |
| Albumin (g/dL) | 3.96 ± 0.59 |
| Fasting blood sugar (mg/dL) | 116.9 ± 41.48 |
| Uric acid (mg/dL) | 7.71 ± 1.91 |
| Calcium (mg/dL) | 8.9 ± 0.56 |
| Phosphate (mg/dL) | 4.2 ± 1.57 |
| Intact PTH (pg/mL) | 291.97 ± 473.35 |
| Total cholesterol (mg/dL) | 176.31 ± 39.2 |
| LDL cholesterol (mg/dL) | 108.37 ± 35.8 |
| HDL cholesterol (mg/dL) | 49.35 ± 15.8 |
| Triglycerides (mg/dL) | 129.07 ± 73.96 |
| IL-6 (ng/mL) | 722.37 ± 205.53 |
| Hepcidin (ng/mL) | 19.42 ± 5.68 |
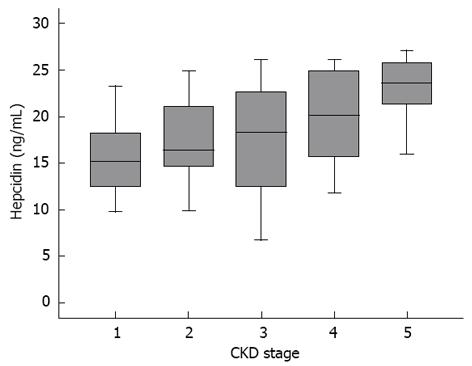
The serum hepcidin-25 levels were 16.51 ± 5.2 ng/mL, 17.59 ± 5.32 ng/mL, 17.38 ± 6.47 ng/mL, 19.98 ± 4.98 ng/mL and 22.03 ± 4.8 ng/mL for subjects with stage 1, 2, 3, 4 and 5 CKD, respectively. There were marked differences in the serum hepcidin level between patients with different CKD stages, as determined by the Kruskal Wallis test (P = 0.039). The serum hepcidin levels for patients in each CKD stage were significantly lower than those for patients in higher CKD stages, as determined by the Jonckheere-Terpstra test (P = 0.002); i.e., subjects in higher stages had significantly higher serum hepcidin-25 levels with a stepwise relationship (Figure 1). By contrast, there was no significant correlation, via the Kruskal Wallis and Jonckheere-Terpstra test, between each CKD stage and LVM and LVMI in our study. (Data not shown)
Pearson correlation analysis illustrated that the serum hepcidin level was strongly positively correlated with the serum BUN, creatinine, ferrous iron, phosphate, HDL and LDL cholesterol levels and negatively correlated with the eGFR and serum hemoglobin, albumin, and fasting blood sugar levels. There was no significant correlation between the serum hepcidin-25 level and the IL-6 or hsCRP level in our stable CKD patients (r = 0.183, P = 0.425; r = 0.19, P = 0.514, respectively). The multiple linear regression model plotted serum hepcidin vs BUN, creatinine, the iron profile, the eGFR, phosphate, HDL, LDL, hemoglobin, albumin and the fasting blood sugar level. Hepcidin was independently predicted by the serum ferritin level (β = 0.6, P = 0.002), the eGFR (β = -0.48, P = 0.04) and a CKD stage of 4-5 (β = 5.317, P = 0.035, vs CKD 1-3).
Table 2 shows the negative correlations between the serum hepcidin level and the LVM and LVMI (P = 0.04 and P = 0.005, respectively). No significant correlations with the IVSd, LVPW, RVD, LA and ejection fraction (EF) were found. The systolic BP was positively correlated with the LVMI (P = 0.005) and inversely correlated with the serum hepcidin level. No significant association between the hepcidin level and the diastolic BP was observed. After adjustment for BMI, age and systolic BP in the multivariate analysis, a decreased serum hepcidin level was only independently associated with a higher LVMI [β = -0.28, 95%CI: -0.48 - -0.02, P = 0.006].
The major findings of our study are the following: (1) there were negative correlations between the serum hepcidin level and both the LVM and LVMI; and (2) the hepcidin level was independently predicted by the serum ferritin level, the eGFR. By contrast, we did not find that there was significant correlation between each CKD stage and LVM and LVMI.
It is well-known that hepcidin expression is induced by inflammation or infection and is suppressed by anemia, hypoxia, iron and the use of erythropoiesis-stimulating agents[12,13]. However, there is an increasing number of emerging studies that show that iron deficiency itself inhibits the expression of hepcidin and its release into the circulation[14-16]. Weber et al[17] reported that hepcidin levels in the lower tertile were strongly associated with iron deficiency. As shown in the study, we also found that the hepcidin-25 level was positively correlated with the level of serum ferritin, a biomarker of iron loading and inflammation, in CKD patients. The subjects in our study population did not exhibit any signs of inflammation in the clinical and laboratory tests (hsCRP: 0.223 ± 0.362 mg/L). We hypothesized that a high circulating hepcidin-25 level can be interpreted predominantly as a response to excess iron, and this hypothesis is consistent with the results of a recent study[7].
Iron is essential not only for erythropoiesis but also for several bioenergetic processes in skeletal muscle. Chronic iron deficiency may, by itself, reduce exercise capacity and cause ultrastructural alterations in cardiomyocytes[6]. Iron constitutes an important element of the enzymatic system of cardiomyocytes and can be stored inside these cells. Turner and colleagues have shown that rats with induced iron deficiency have increased cardiac output and sympathetic activation that subsequently result in left ventricular hypertrophy[18]. Dong et al[19] reported that left ventricular hypertrophy and dilatation occurring in iron-deficient (ID) rats is accompanied by mitochondrial swelling, irregular sarcomere organization, increased mitochondrial cytochrome c release and increased reactive nitrogen species production in cardiomyocytes. They found that after 20 wk of ID anemia, the rats developed maladapted cardiac hypertrophy with severe left ventricular dysfunction and exhibited signs of decomposition with lung congestion[19]. Dilated ventricles were characterized by myocardial interstitial fibrosis and increased myocardial expression of A-type natriuretic peptide (ANP) and BNP, as well as a reduced myocardial expression of collagen type 3 genes[20]. In the present study, we demonstrated that CKD patients with lower serum hepcidin-25 levels had greater LVMIs and LVMs. The LVMI is the most reliable index of left ventricular hypertrophy (LVH), which can be diagnosed by either echocardiography or electrocardiography, and this condition is associated with cardiovascular morbidity and mortality[21,22]. Low LV performance is associated with iron deficiency anemia in rats with CKD[10]. To our knowledge, our study is the first work to analyze the serum hepcidin-25 level and echocardiography results in a group of adults with CKD.
Our study demonstrated that the serum hepcidin-25 level progressively increased with increase CKD severity, a finding consistent with the reports by Ashby et al[23] and Zaritsky et al[24] on the correlation between the hepcidin level and the eGFR. However, Peters et al[25] measured the hepcidin level using radioimmunoassay and mass spectrometry-based methods, which were shown to be in close agreement (R = 0.96, n = 99) in a comparison study. In contrast, when using mass spectrometry to measure the hepcidin level, Peters et al[26] concluded that the serum hepcidin-25 levels in CKD patients were independent of the GFR, and they attributed the discrepancy to the inability of the immunoassay used by Ashby to differentiate between isoforms and to differences in the study populations. We believe that further efforts to standardize the hepcidin assays are needed to resolve these differences.
The cytokine IL-6 is a key inducer of hepcidin synthesis during inflammation, both in mouse and human models[3,27]. No correlation was found between the hepcidin and TNF-α levels. Hepcidin levels in the lower tertile were found to be strongly associated with iron deficiency (OR = 16.5, 95%CI: 2.2-121.2; P < 0.01)[17]. Our findings are in accordance with these data: we found no significant correlation between the serum hepcidin-25 level and the IL-6 or hsCRP level in our stable CKD patients.
There are several limitations that needed to be recognized in the present study. Firstly, exogenous administration of erythropoiesis-stimulating agents (commonly for those with advanced CKD) promotes RBC synthesis, thus inhibiting hepcidin gene expression. In the present study, the impact of exogenous erythropoietin was not taken into account. Secondly, this study was limited by its cross-sectional nature, thus preventing the evaluation of causation in the association between the serum hepcidin level and cardiovascular risk. This shortcoming will be overcome as the larger participants in the future CKD study continue longitudinal follow-up with repeated measurements of the serum hepcidin-25 level.
In conclusion, this preliminary cross-sectional analysis demonstrated that the increase in the serum hepcidin level with CKD progression in adults is associated with a decreasing LVMI. Lower hepcidin-25 level may be independently associated with unfavorable cardiovascular outcomes for this population.
Cardiovascular disease is the primary cause of death among chronic kidney disease (CKD) patients, and left ventricular hypertrophy is a powerful independent predictor of mortality for end-stage renal disease patients. Low left ventricular performance is associated with iron deficiency anemia in rats with CKD. Hepcidin-25, a recently discovered biologically active 25-amino acid peptide, plays a key role in the iron homeostasis. Chronic iron deficiency may, by itself, reduce exercise capacity and cause ultrastructural alterations in cardiomyocytes. However, the correlation between the serum hepcidin level and cardiovascular risk was unclear for CKD patients.
Iron deficiency is frequent among patients with stable systolic heart failure, and associated with serious unfavourable clinical consequences. Jankowska et al found an early stage of heart failure was characterized by increased circulating hepcidin level and the progression of heart failure is connected with the decline in circulating hepcidin and the development of iron deficiency. In addition, low hepcidin was independently associated with increased 3-year mortality among heart failure patients. The studies addressing the issue of iron status and cardiac performance were sparse in CKD patients.
The serum hepcidin level increases gradually with the increment of CKD severity, suggesting the decreased excretion of hepcidin due to the decline in renal function. This is in accordance with previous researches. Left ventricular mass index, a powerful predictor of cardiovascular mortality, was shown to be negatively correlated with serum hepcidin level among our CKD patients comprising all 5 stages of CKD. Hepcidin is a key regulator of iron metabolism but accumulates with the decline in renal function. The complex mechanisms linking hepcidin and left ventricular mass index (LVMI) in CKD warrants further investigation.
The study results that there was a negative correlation between LVMI and serum hepcidin level in CKD patients suggests hepcidin is a potential therapeutic target for the treatment and prevention of left ventricular hypertrophy in the future.
LVMI: left ventricular hypertrophy (LVH) is a common finding in patients with chronic kidney disease. echocardiographic criteria for the diagnosis of left ventricular hypertrophy were largely based on M-mode echocardiography and included a left ventricular mass index ≥ 134 and ≥ 110 g/m2 body surface area in men and women, respectively. The presence of LVH (on electrocardiogram or echocardiography) is usually associated with increases in the incidence of heart failure, ventricular arrhythmias, decreased LV ejection fraction, sudden cardiac death, and aortic root dilation.
This interesting study demonstrates that serum hepcidin level is associated with left ventricular mass index, a powerful predictor of cardiovascular mortality in the general population. The linkage between hepcidin and left ventriclular performance provides a potential therapeutic target to improve survival in CKD patients.
P- Reviewers Bellomo G, Fujigaki Y S- Editor Wen LL L- Editor A E- Editor Lu YJ
| 1. | Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147-150. [PubMed] |
| 2. | Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806-7810. [PubMed] |
| 3. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. [PubMed] |
| 4. | Pagani A, Nai A, Corna G, Bosurgi L, Rovere-Querini P, Camaschella C, Silvestri L. Low hepcidin accounts for the proinflammatory status associated with iron deficiency. Blood. 2011;118:736-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | De Domenico I, Zhang TY, Koening CL, Branch RW, London N, Lo E, Daynes RA, Kushner JP, Li D, Ward DM. Hepcidin mediates transcriptional changes that modulate acute cytokine-induced inflammatory responses in mice. J Clin Invest. 2010;120:2395-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Brownlie T, Utermohlen V, Hinton PS, Haas JD. Tissue iron deficiency without anemia impairs adaptation in endurance capacity after aerobic training in previously untrained women. Am J Clin Nutr. 2004;79:437-443. [PubMed] |
| 7. | Jankowska EA, Malyszko J, Ardehali H, Koc-Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD. Iron status in patients with chronic heart failure. Eur Heart J. 2013;34:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 8. | Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112-S119. [PubMed] |
| 9. | Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11:1277-1285. [PubMed] |
| 10. | Toblli JE, Cao G, Rivas C, Kulaksiz H. Heart and iron deficiency anaemia in rats with renal insufficiency: the role of hepcidin. Nephrology (Carlton). 2008;13:636-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1-266. [PubMed] |
| 12. | Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037-1044. [PubMed] |
| 13. | Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730-3735. [PubMed] |
| 14. | Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55:726-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 16. | Nemeth E, Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 17. | Weber CS, Beck-da-Silva L, Goldraich LA, Biolo A, Clausell N. Anemia in heart failure: association of hepcidin levels to iron deficiency in stable outpatients. Acta Haematol. 2013;129:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Turner LR, Premo DA, Gibbs BJ, Hearthway ML, Motsko M, Sappington A, Walker L, Mullendore ME, Chew HG. Adaptations to iron deficiency: cardiac functional responsiveness to norepinephrine, arterial remodeling, and the effect of beta-blockade on cardiac hypertrophy. BMC Physiol. 2002;2:1. [PubMed] |
| 19. | Dong F, Zhang X, Culver B, Chew HG, Kelley RO, Ren J. Dietary iron deficiency induces ventricular dilation, mitochondrial ultrastructural aberrations and cytochrome c release: involvement of nitric oxide synthase and protein tyrosine nitration. Clin Sci (Lond). 2005;109:277-286. [PubMed] |
| 20. | Naito Y, Tsujino T, Matsumoto M, Sakoda T, Ohyanagi M, Masuyama T. Adaptive response of the heart to long-term anemia induced by iron deficiency. Am J Physiol Heart Circ Physiol. 2009;296:H585-H593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89-105. [PubMed] |
| 22. | de Simone G, Gottdiener JS, Chinali M, Maurer MS. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FW, Chapman RS. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 24. | Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, Nemeth E, Ganz T, Rivera S, Nissenson AR, Salusky IB. Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 229] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 25. | Peters HP, Laarakkers CM, Wetzels JF, Swinkels DW. Hepcidin levels in patients with renal disease. Kidney Int. 2009;76:680; author reply 680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Peters HP, Laarakkers CM, Swinkels DW, Wetzels JF. Serum hepcidin-25 levels in patients with chronic kidney disease are independent of glomerular filtration rate. Nephrol Dial Transplant. 2010;25:848-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |