Copyright
©2012 Baishideng.
World J Nephrol. Dec 6, 2012; 1(6): 166-176
Published online Dec 6, 2012. doi: 10.5527/wjn.v1.i6.166
Published online Dec 6, 2012. doi: 10.5527/wjn.v1.i6.166
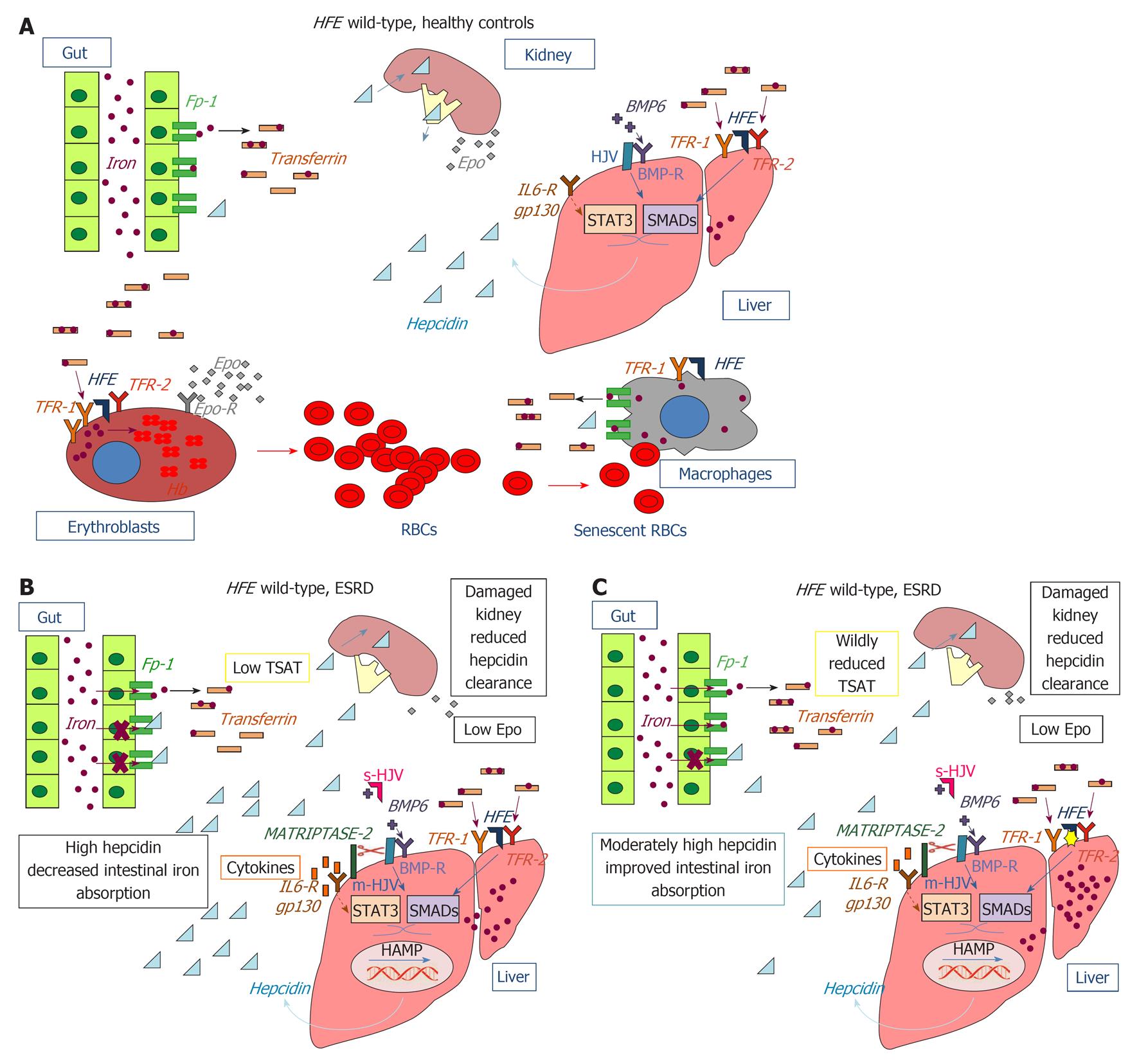
Figure 1 Role of the proteins and mediators involved in iron homeostasis and impact of hereditary hemochromatosis protein mutations in chronic kidney disease.
A: Hereditary hemochromatosis protein (HFE) wild-type, healthy controls; B: HFE wild-type, end-stage renal disease (ESRD); C: HFE mutated, ESRD. Bone morphogenetic protein 6 (BMP-6) binds to the co-receptor membrane hemojuvelin (m-HJV) and to the bone morphogenetic protein receptor (BMP-R) on the membrane of hepatocytes. This initiates phosphorylation of small mother against decapentaplegic (SMAD) proteins and the assembly of heteromeric complexes. After nuclear translocation, the heteromeric SMAD complexes stimulate transcription of the HAMP gene for hepcidin. Another pathway responsible for hepcidin expression involves interleukine (IL)-6/gp130 signaling via signal transducer and activator of transcription 3 (STAT3) nuclear translocation. Negative regulation of the BMP-HJV-hepcidin pathway is achieved through the proteolytic processing of membrane-HJV by matriptase-2 (TMPRSS6). Additional negative regulation of hepcidin transcription is provided by soluble-HJV, which acts as an antagonist of the BMP pathway by competing with m-HJV for BMP-6 binding. The hepcidin regulatory signaling pathway is also regulated by HFE, which interacts either with transferrin receptor 1 (TFR1) or transferrin receptor 1 (TFR2) according to serum transferrin saturation: in the presence of a high transferrin saturation HFE dissociates from TFR1 and binds TFR2 forming an iron sensing complex influencing hepcidin expression via SMAD/ERK signaling. Hepcidin secreted by the liver binds to the extracellular region of ferroportin 1 (Fp-1) on the basolateral membrane of duodenal enterocytes causing Fp-1 internalization, ubiquitination and degradation and consequently impaired intestinal iron absorption. EPO: Erythropoietin; Epo-R: Erythropoietin receptor; s-HJV: Serum hemojuvelin; TSAT: Transferrin saturation.
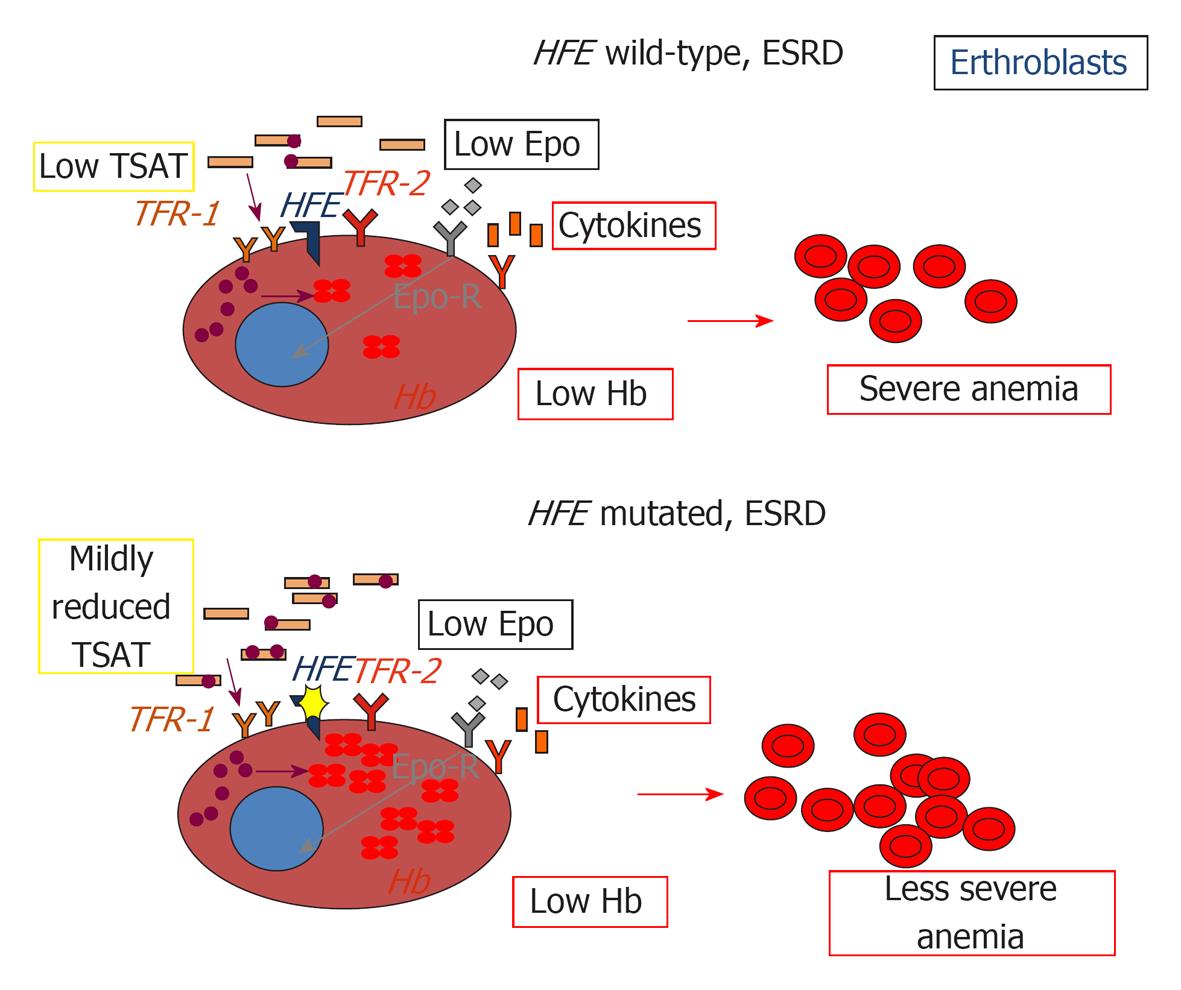
Figure 2 Impact of HFE mutations on erythropoiesis in chronic kidney disease.
EPO: Erythropoietin, Epo-R: Erythropoietin receptor; TFR1: Transferrin receptor 1; TFR2: Transferrin receptor 2; HFE: Hereditary hemochromatosis protein; TSAT: Transferrin saturation; Hb: Hemoglobin.
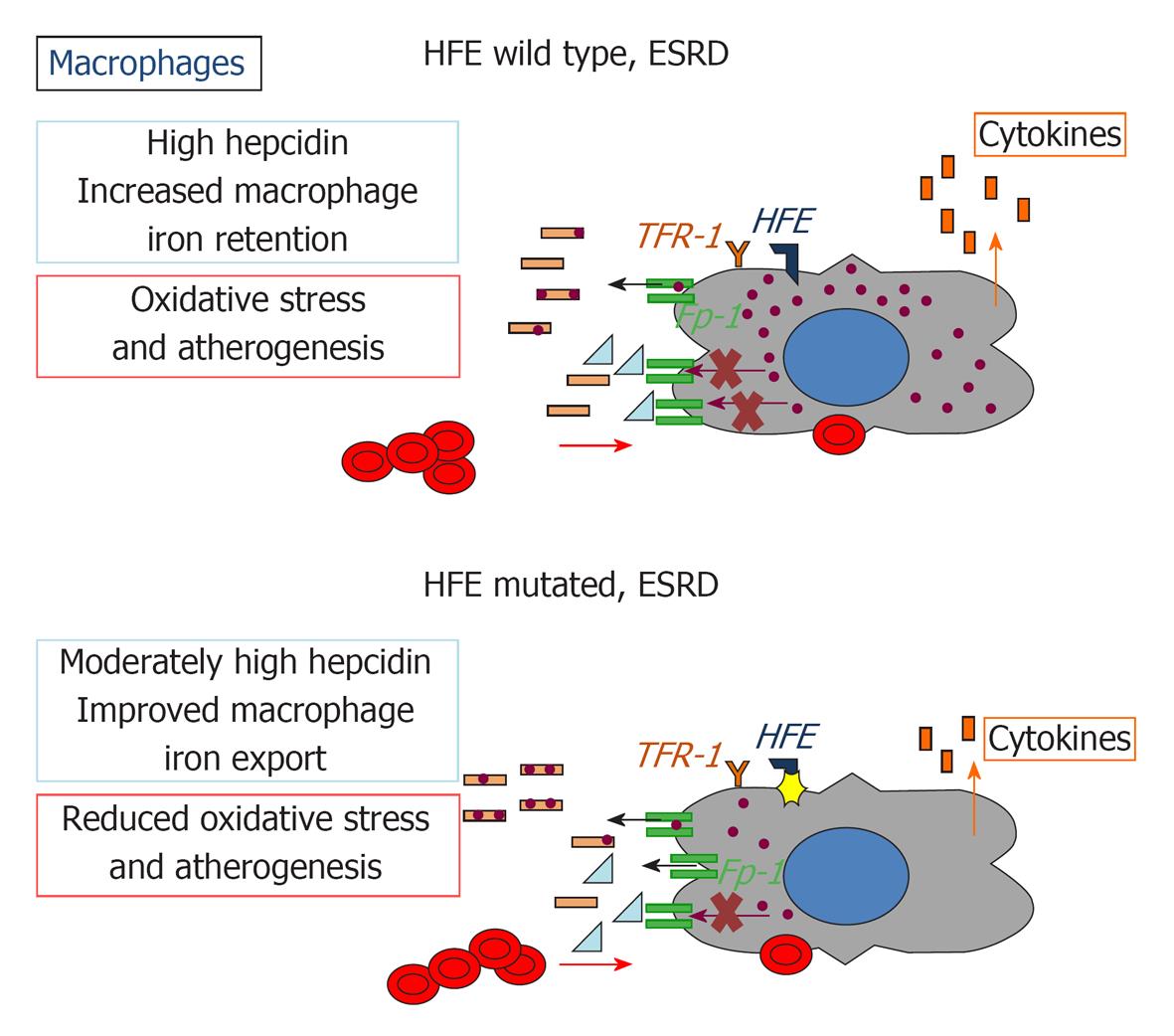
Figure 3 Impact of HFE mutations on macrophage iron recycling, oxidative stress, and atherogenesis in chronic kidney disease.
Fp-1: Ferroportin 1; TFR1: Transferrin receptor 1; HFE: Hereditary hemochromatosis protein.
- Citation: Canavesi E, Alfieri C, Pelusi S, Valenti L. Hepcidin and HFE protein: Iron metabolism as a target for the anemia of chronic kidney disease. World J Nephrol 2012; 1(6): 166-176
- URL: https://www.wjgnet.com/2220-6124/full/v1/i6/166.htm
- DOI: https://dx.doi.org/10.5527/wjn.v1.i6.166