Published online Jun 18, 2022. doi: 10.5500/wjt.v12.i6.120
Peer-review started: January 10, 2022
First decision: April 13, 2022
Revised: April 24, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 18, 2022
Pre-transplant muscle wasting measured by computed tomography has been associated with adverse clinical outcomes after liver transplantation including increased rates of sepsis and hospitalisation days. Upper limb lean mass (LM) measured by dual-energy X-ray absorptiometry (DEXA) was recently identified as a novel predictor of sarcopenia-associated mortality in men waitlisted for transplantation.
To investigate the use of DEXA LM in predicting gender-stratified early post-transplant outcomes.
Liver transplant recipients who underwent pre-transplant DEXA body compo
Four hundred and sixty-nine patients met inclusion criteria of which 338 were male (72%). Median age was 55.0 years (interquartile range 47.4, 59.7) and model for end-stage liver disease (MELD) score 16. Median time from assessment to transplantation was 7 mo (3.5, 12). Upper limb LM was inversely associated with bacterial infections at 180 d post-transplant (hazard ratio = 0.42; 95% confidence interval: 0.20-0.89; P = 0.024) in males only. There was a negative correlation between upper limb LM and intensive care (τb = -0.090, P = 0.015) and total hospital length of stay (τb = -0.10, P = 0.0078) in men. In women, neither MELD nor body composition parameters were associated with post-transplant adverse outcomes or increased length of stay. Body composition parameters, MELD and age were not associated with 90-d mortality or graft failure in either gender. There were no significant predictors of early ACR.
Sarcopenia is an independent and potentially modifiable predictor of increased post-transplant bacterial infections and hospital length of stay in men with cirrhosis. DEXA upper limb LM provides a novel measure of muscle wasting that has prognostic value in this cohort. The lack of association in women requires further investigation.
Core Tip: Pre-transplant sarcopenia as measured by single-slice computed tomography has prognostic value in predicting outcomes in liver transplant recipients. In this retrospective study, we explore the association of pre-transplant dual-energy X-ray absorptiometry (DEXA) body composition analysis with early post-transplant outcomes. Low upper limb lean mass (LM) was a predictor of 180-d post-transplant bacterial infections and longer hospital and intensive care length of stay in men but not women. Upper limb LM was superior to other measures of LM including appendicular LM in predicting adverse outcomes. There was no association between pre-transplant body composition and post-transplant mortality, graft failure or early acute cellular rejection. In conclusion, pre-transplant sarcopenia is associated with adverse outcomes in men after liver transplantation. Upper limb LM provides a novel measure of muscle mass that is superior to other measures of LM on DEXA in predicting early post-transplant outcomes.
- Citation: Hey P, Hoermann R, Gow P, Hanrahan TP, Testro AG, Apostolov R, Sinclair M. Reduced upper limb lean mass on dual energy X-ray absorptiometry predicts adverse outcomes in male liver transplant recipients. World J Transplant 2022; 12(6): 120-130
- URL: https://www.wjgnet.com/2220-3230/full/v12/i6/120.htm
- DOI: https://dx.doi.org/10.5500/wjt.v12.i6.120
Sarcopenia is a syndrome defined by decreased muscle mass and reduced strength or function[1]. It is estimated to affect between 40% and 70% of patients waitlisted for liver transplantation depending on the modality used to measure muscle mass[2]. Sarcopenia is a predictor of waitlist mortality, independent of model for end-stage liver disease (MELD) score[3]. Muscle wasting measured using the cross-sectional muscle area at the third lumbar vertebrae on computed tomography (CT) is associated with longer hospital length of stay and increased risk of post-operative complications following liver transplantation[4,5].
There is emerging evidence for the role of dual-energy X-ray absorptiometry (DEXA) body composition to quantify muscle mass in cirrhosis. DEXA provides whole body compartmentalised measurements of bone mineral content, fat mass and lean tissue. It has the advantage of being a simple, reproducible, low-cost technique that can be performed easily on outpatients being worked up for transplantation. Results are readily available without the need for further analysis or dedicated software. The major limitation for the use of DEXA in cirrhosis is that it can be influenced by hydration status including the presence of ascites and oedema. To reduce the impact of ascites, appendicular lean mass (APLM), the sum of LM in arms and legs corrected for height is the preferred measure for sarcopenia in cirrhosis.
We recently identified that upper limb LM was a novel, independent predictor of sarcopenia-associated mortality in men waitlisted for transplantation[6]. Upper limb LM rather than total APLM has the advantage of being unaffected by peripheral oedema. This study aims to describe the associations between pre-transplant gender-specific body composition measurements and early post-transplant outcomes.
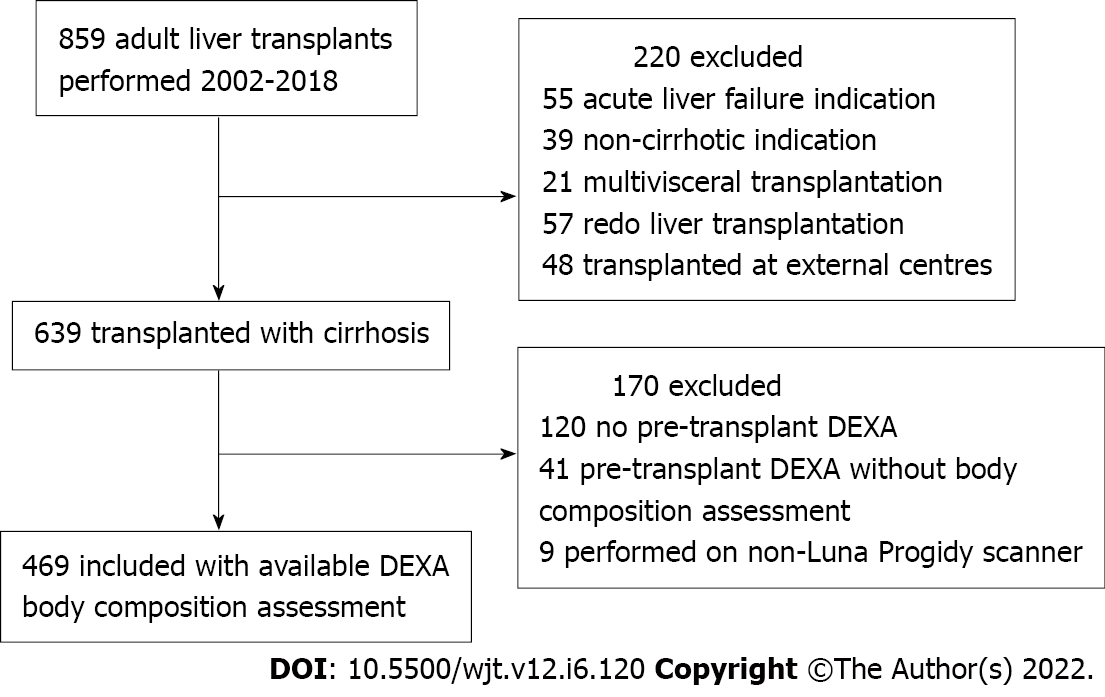
This study retrospectively analysed data of all adult patients (> 18 years) who underwent liver transplantation at a tertiary centre in Melbourne, Australia, between January 2002 and July 2018. Exclusion criteria included transplantation for non-cirrhotic indications, redo liver transplantation, multi-visceral transplants and those missing DEXA body composition data at transplant assessment. Approval was obtained by the Austin Health Human Ethics Research Committee.
Baseline demographics including age and aetiology of liver disease were recorded at transplant assessment. Clinical examination findings including presence of hepatic encephalopathy and ascites were recorded by a transplant hepatologist. Ascites was graded as requiring no treatment, diuretic therapy alone or paracentesis. Body mass index was calculated at the time of the DEXA. Biochemistry and haematology were measured at transplant assessment, all within 6 wk of the DEXA scan. Laboratory assessments included bilirubin, serum creatinine, international normalized ratio and serum albumin to enable calculation of MELD and Child Pugh Scores. Operative data at the time of liver transplantation was collected including cold and warm ischaemic time (minutes), operative time (minutes), and blood transfusion requirement (units).
DEXA body composition analysis was performed at the time of transplant assessment using a Lunar Prodigy DEXA scanner (GE Healthcare, Madison, WI. United States). This quantified compartmentalised total body composition including LM, fat mass and bone mass. Variables analysed included appendicular, upper limb, lower limb and total LM and fat mass. All measurements were corrected for height2. Sarcopenia was defined by previously reported cut-off values for APLM from the European Working Group on Sarcopenia in older people (males < 7.26 kg/m2, females < 5.5 kg/m2)[7,8].
Clinical endpoints were examined at 90 d, 180 d and 12 mo post transplantation and included mortality, graft failure, bacterial infections, and acute cellular rejection (ACR). Graft failure was defined as graft loss requiring re-transplantation or due to patient death. Bacterial infections required the identification of a causative pathogen treated with systemic antimicrobial therapy. ACR was biopsy proven, defined as a rejection activity index ≥ 4 based on Banff criteria. Other outcomes included post-transplant intensive care stay (hours), hospital length of stay (days) and discharge destination (discharge to home or subacute care). Length of stay data excluded patients who died within the early post-operative period, within 48 h of transplantation.
Orthotopic liver transplantation was performed according to unit protocol and included both donation after brain death and donation after cardiac death. Organ allocation was based on the MELD scoring system. Protocolised immunosuppression comprised intravenous corticosteroids administered from day 0 to day 5 post-transplantation followed by a weaning course of oral corticosteroids. A combination of oral calcineurin inhibitors (cyclosporin or tacrolimus) and either mycophenolate mofetil or azathioprine were initiated early post-transplantation. A gradual switch from azathioprine to mycophenolate mofetil was made following Therapeutic Goods Administration approval of the latter medication in Australia in 2012. Intravenous basiliximab was administered at day 0 and 5 in patients with impaired renal function to allow delayed commencement of calcineurin inhibitors.
Continuous variables were expressed as a median and interquartile range (25th and 75th percentile). Chi squared and fisher’s exact tests were used for categorical variables. Continuous variables were compared using students t test (normal distribution) or Mann-Whitney U test (without normal distribution). Kendall Rank correlations were used to assess correlations between pre-transplant variables and post-transplant intensive care and hospital length of stay.
Survival analysis was used to follow patients after liver transplantation until they had died, experienced a complication such as bacterial infection or graft failure, or their status had last been audited. Univariate Cox proportional hazard regression analysis was used to identify predictors of 90-d and 12-mo post-transplant mortality and graft failure. Univariate and multivariate Cox regression analyses were used to identify predictors of 90 and 180-d post-transplant bacterial infections and 90-d ACR. Two-sided P < 0.05 conferred significance for all tests. The statistical software package R 4.1.2 for Mac with the survival package 3.2-13 was used for the analyses[9,10].
Between January 2002 and December 2018, 859 adults underwent liver transplantation (Figure 1). Four-hundred and sixty-nine patients had available pre-transplant DEXA body composition data and met the inclusion criteria. Three-hundred and thirty-eight (72%) were male. The median age was 55.0 years (interquartile range 47.4, 59.7) and MELD score 16 (Table 1). The most common indications for liver transplantation were decompensated cirrhosis caused by viral hepatitis (n = 138, 29%) and alcohol (n = 51, 11%). Hepatocellular carcinoma in the context of cirrhosis was the primary indication for transplantation in 122 patients (26%). At transplant assessment, 259 (55%) patients had ascites, of which 137 (29%) had required recent paracentesis. A history of hepatic encephalopathy was reported in 220 patients (47%). The median time from assessment to transplantation was 7 mo (3.5, 12).
| Non-sarcopenic (n = 355, 75.7%) | Sarcopenic (n = 114, 24.3%) | P value | |
| Age, indication for transplantation | 55 (48, 60) | 54 (46, 58) | 0.253 |
| Viral hepatitis | 106 (30%) | 32 (28%) | 0.715 |
| Alcohol | 30 (8%) | 21 (18%) | 0.003a |
| Hepatoma | 96 (27%) | 26 (23%) | 0.370 |
| PBC/PSC/AIH | 66 (19%) | 18 (16%) | 0.497 |
| Bilirubin (μmol/L) | 57 (28, 114) | 47.5 (25, 91.5) | 0.324 |
| Albumin (g/L) | 29 (24, 33) | 30 (25, 25) | 0.172 |
| INR | 1.4 (1.2, 1.7) | 1.4 (1.2, 1.6) | 0.786 |
| Ascites | 188 (53%) | 71 (62%) | 0.082 |
| Encephalopathy | 163 (46%) | 57 (50%) | 0.401 |
| MELD score | 16 (12, 20) | 16 (12, 19) | 0.934 |
| Operative data | |||
| Total operative time (min) | 465 (397, 534) | 445 (291, 510) | 0.162 |
| Peak ALT | 884 (509, 1525) | 933 (496, 1494) | 0.991 |
| Cold ischaemic time (min) | 381 (318, 479) | 384 (303, 473) | 0.530 |
| Warm ischaemic time (min) | 45 (39, 52) | 44 (38, 50) | 0.212 |
| RBC transfusions (units) | 2 (0, 4) | 2 (0, 5) | 0.008a |
Using DEXA body composition assessment, the median APLM was 7.91 kg/m2 (7.15, 8.71) for males and 6.50 kg/m2 (5.87, 7.36) for females. Based on previously reported cut-off values[7], 95 men (28%) and 19 women (15%) were sarcopenic (Table 1). Women had higher fat mass, 7.56 kg/m2 (5.48, 9.95) compared to men, 6.41 kg/m2 (4.70, 9.31), P = 0.018.
At 90 d and 12 mo post transplantation, 15 (3.2%) and 33 (7.0%) of patients respectively had died. 12-mo post-transplant survival increased in the latter half of the period examined from 90% in 2002-2009 to 96% in 2010-2018. Pre-transplant body composition parameters, MELD and age were not associated with 90-d or 12-mo post-transplant mortality in men. Higher total LM but no other LM parameters was associated with 12-mo mortality in women [hazard ratio (HR) = 1.22; 95% confidence interval (CI): 1.04-1.44; P = 0.017]. Peri-operative blood transfusion requirements was associated with 90-d and 12-mo mortality in both men (HR = 1.21; 95%CI: 1.06-1.39; P = 0.006) and women (HR = 1.24; 95%CI: 1.10-1.40, P = 0.006). Of the 15 patients who died within 90 d of transplantation, only 3 met previously reported DEXA-based gender-specific diagnostic criteria for sarcopenia using APLM[7].
At 90 d and 12 mo post transplantation, 22 (4.6%) and 43 (9.2%) of patients respectively had graft failure. Body composition parameters, MELD and presence of ascites at workup were not associated with 90-d or 12-mo graft failure in men. Higher intra-operative blood transfusion requirement was associated with 90-d graft failure in both genders. Longer operative time was also associated with 90-d graft failure in men only (HR = 1.004; 95%CI: 0.001-1.008; P = 0.017).
At 90 d and 180 d post-transplant, 59 (17.5%) and 73 (21.6%) men respectively had suffered a bacterial infection. Reduced upper limb LM was associated with bacterial infections in men at 180 d only, HR = 0.42; 95%CI: 0.20-0.89 (Table 2). The presence of ascites at transplant assessment was associated with 90-d and 180-d post-transplant bacterial infection in men only. Body composition parameters, MELD score, ascites and operative variables did not show an association with 90-d or 180-d bacterial infections in women.
| Males (n = 338) | Females (n = 131) | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| MELD | 1.04 (1.00, 1.08) | 0.051 | 1.06 (0.99, 1.12) | 0.074 |
| APLM | 1.03 (0.87, 1.21) | 0.76 | 0.93 (0.70, 1.24) | 0.63 |
| Upper limb LM | 0.42 (0.20, 0.89) | 0.024a | 0.74 (0.19, 2.95) | 0.67 |
| Lower limb LM | 1.09 (0.92, 1.30) | 0.33 | 0.93 (0.67, 1.28) | 0.66 |
| Total LM | 1.07 (0.99, 1.15) | 0.08 | 0.89 (0.76, 1.03) | 0.12 |
| Total fat mass | 0.98 (0.91, 1.05) | 0.50 | 0.99 (0.90, 1.08) | 0.76 |
| Ascites | 2.18 (1.32, 3.59) | 0.002a | 2.14 (0.95, 4.82) | 0.06 |
At 90 d post transplantation, 105 patients (22.4%) had an episode of moderate to severe ACR. In men, 90-d ACR was negatively associated the presence of ascites (HR = 0.93; 95%CI: 0.89-0.97, P = 0.0021) and MELD sore (Table 3). Similarly, lower total lean mass (TLM) was associated with higher 90-d ACR (HR = 0.83; 95%CI: 0.75-0.92; P < 0.001) whereas APLM and upper limb mass were not. 90-d ACR was not associated with body composition parameters, MELD or the presence of ascites in women. Peri-operative blood transfusion requirement was negatively associated with 90-d ACR in men but not women (HR = 0.89; 95%CI: 0.81-0.99; P = 0.026). Other operative data was not associated with ACR in either gender.
| Males (n = 338) | Females (n = 131) | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| MELD | 0.93 (0.89, 0.97) | 0.002 | 1.04 (0.99, 1.09) | 0.14 |
| APLM | 0.87 (0.72, 1.05) | 0.16 | 1.10 (0.87. 1.4) | 0.43 |
| Upper limb LM | 1.34 (0.67, 2.68) | 0.41 | 0.43 (0.12, 1.51) | 0.19 |
| Lower limb LM | 0.80 (0.63, 1.01) | 0.063 | 1.17 (0.90, 1.52) | 0.23 |
| Total LM | 0.83 (0.74, 0.92) | < 0.001a | 1.05 (0.95, 1.17) | 0.32 |
| Total fat mass | 0.93 (0.87, 1.00) | 0.062 | 1.03 (0.95, 1.12) | 0.50 |
| BMI | 0.91 (0.86, 0.96) | < 0.001a | 1.02 (0.97, 1.08) | 0.45 |
| Ascites | 0.43 (0.26, 0.70) | < 0.001a | 1.51 (0.76, 3.00) | 0.24 |
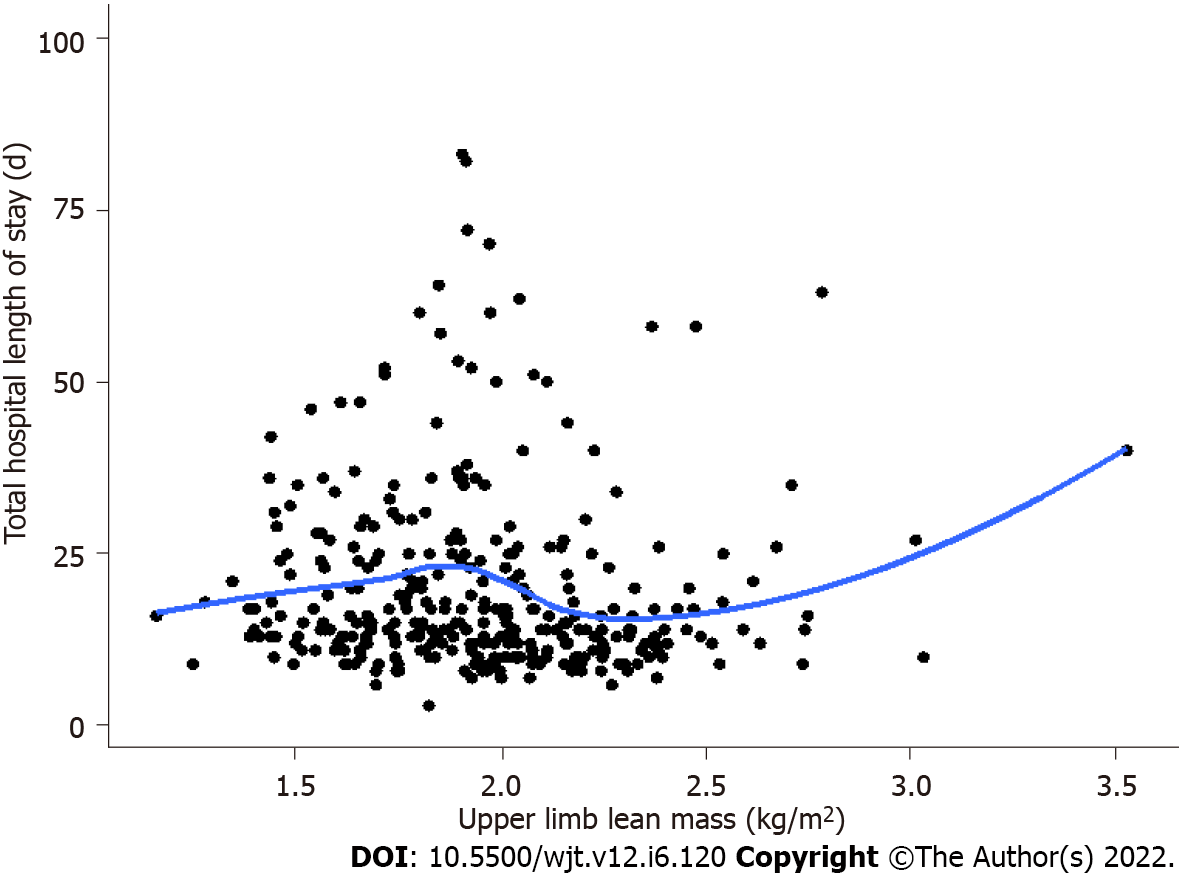
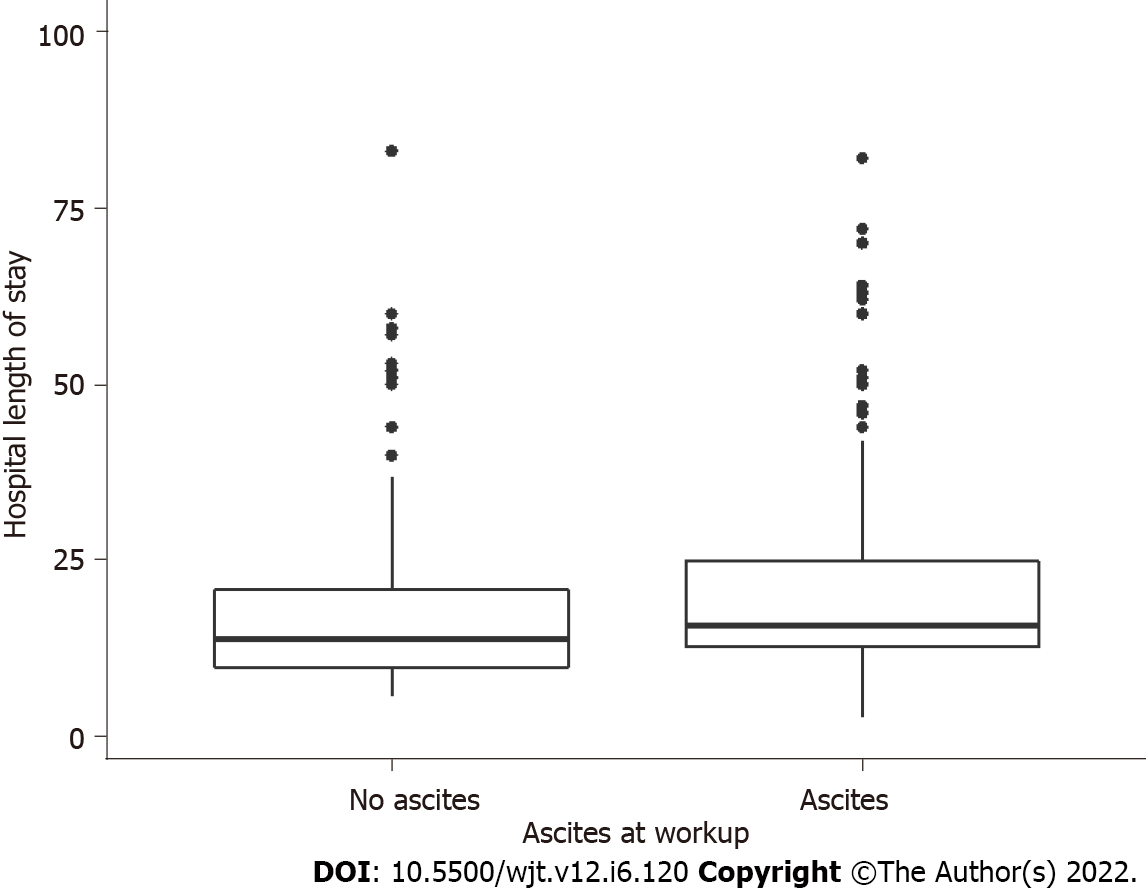
The median intensive care stay following liver transplantation was 66 and hospital length of stay was 15 d in men but not women, upper limb LM was inversely associated with longer intensive care stay (τb = -0.090, P = 0.015) and hospital length of stay (τb = -0.10, P = 0.0078) (Figure 2 and Table 4). The presence of ascites at transplant assessment was associated with longer intensive care and hospital stay in men (median 15 d vs 4 d, P = 0.024) but not women (Figure 3). In men only, a higher peak alanine transaminase also correlated with longer intensive care stay (τb = 0.13, P < 0.001), but not total hospital length of stay. There was no significant difference in intensive care or hospital length of stay in patients who were classified as sarcopenic based on gender-specific cut offs for APLM.
| Males (n = 338) | Females (n = 131) | |||||||
| Correlation1 (τb) | P value1 | Correlation2 (τb) | P value2 | Correlation1 (τb) | P value1 | Correlation2 (τb) | P value2 | |
| Age | < -0.001 | 0.98 | 0.055 | 0.14 | 0.084 | -0.18 | 0.047 | 0.44 |
| Total APLM | -0.027 | 0.48 | -0.004 | 0.91 | -0.029 | 0.65 | -0.012 | 0.84 |
| Upper limb LM | -0.10 | 0.0078a | -0.090 | 0.015a | -0.079 | 0.21 | 0.019 | 0.75 |
| Lower limb LM | < 0.001 | 0.99 | 0.017 | 0.64 | -0.018 | 0.76 | -0.018 | 0.76 |
| Total LM | 0.32 | 0.037a | 0.055 | 0.13 | -0.012 | 0.84 | -0.012 | 0.84 |
| Total fat mass | 0.036 | 0.33 | 0.048 | 0.20 | 0.039 | 0.53 | 0.039 | 0.53 |
| MELD | 0.078 | 0.045a | 0.0087 | 0.058 | -0.037 | 0.56 | 0.087 | 0.17 |
Pre-transplant MELD and the presence of ascites at work up showed differing relationships with DEXA body composition parameters.
MELD and body composition: Upper limb LM negatively correlated with increasing MELD score in men but not women (men: τb = -0.14, P < 0.001, women; τb = -0.077, P = 0.20). Increasing TLM and lower limb LM correlated with higher MELD score in both genders (Table 5).
Ascites and body composition: Compared to those without, ascites was associated with lower upper limb LM in men [median 1.83 kg/m2 (1.63, 2.03) vs 2.02 kg/m2 (1.86, 2.20), P < 0.001). Conversely, TLM was higher in those with ascites [median 20.0 kg/m2 (18.4, 22.1) vs 18.7 kg/m2 (17.2, 20.2), P < 0.001]. In women, the presence of ascites was associated with TLM only [median 16.9 kg/m2 (15.7, 19) vs 16.2 kg/m2 (14.4, 17.3), P = 0.004].
Ascites and MELD: With rising MELD, the prevalence of ascites increased (risk ratio for ascites 4.79 ± 0.58, P < 0.001).
This study investigates the impact of pre-transplant DEXA body composition on outcomes after liver transplantation. We identified reduced upper limb LM as a novel predictor of adverse outcomes including bacterial infections and longer hospital stay in men only. We did not find any significant association between body composition and post-transplant graft-failure or mortality, which suggests that prioritizing patients with sarcopenia for transplantation may be an appropriate strategy to minimize waitlist mortality without a negative impact on post-transplant survival[6].
Previous studies investigating the impact of pre-transplant sarcopenia on post-transplant survival have shown conflicting outcomes[5,11,12]. This disparity may relate to differing definitions of sarcopenia, modalities used for muscle mass assessment, severity of liver disease and inadequate power of some studies to adequately assess mortality. In this study, we describe excellent patient and graft survival of 93% and 91% respectively at 12 mo post-transplant. Era of transplantation may also be a factor as advancements in peri-operative care and immunosuppressive agents have improved post-transplant survival in the modern era. The higher 12-mo post-transplant survival observed in the latter half of the period likely reflects improvements in medical care, despite the increasing medical complexity and older age of transplant recipients. Further large-scale multi-centre studies using reproducible measures of sarcopenia that incorporate muscle function and potential deterioration on the waitlist are required to better elucidate the impact of pre-transplant sarcopenia on post-transplant survival. This will help to determine whether prioritising sarcopenic patients is appropriate and whether a threshold exists below which these patients are indeed too sick for transplantation.
Pre-transplant sarcopenia, as defined by CT imaging, has been consistently reported to be associated with increased post-transplant sepsis. In keeping with this, our study found that upper limb LM was associated with bacterial infections in men at 180-d post-transplant. No significant association was found at 90-d post-transplant, likely reflecting our relatively low infection rate of 21% at this time point as compared to other studies[13]. Our definition of bacterial infections, requiring the identification of a causative pathogen, may result in a lower incidence of early post-transplant bacterial infection leading to inadequate power to detect an association with pre-transplant muscle parameters. The influence of pre-transplant sarcopenia and frailty on early post-transplant ACR is also uncertain with conflicting reports in the literature[14,15]. This study found no association between pre-transplant sarcopenia and early ACR. This provides reassurance that optimising sarcopenia pre-transplant does not appear to result in higher rates of ACR.
While muscle area measured on transverse abdominal CT is often considered gold standard for quantifying muscle mass in cirrhosis, practice guidelines recommend against the use of CT for the sole purposes of sarcopenia assessment due to high radiation doses[16]. In addition to CT, DEXA and bioelectrical impedance are recommended by the European Working Group for Sarcopenia in Older People for assessment of muscle mass[1]. DEXA has advantages over CT due to its reproducibility, low cost and radiation and no requirement for further analysis. However, the inability of DEXA to differentiate fluid and lean tissue is particularly problematic in decompensated cirrhosis where the occurrence of ascites and peripheral oedema are high.
Current guidelines recommend the use of APLM for defining sarcopenia using DEXA with cut-off values extrapolated from non-cirrhotic cohorts for both men and women[1,7]. In a small prospective series of men with cirrhosis, APLM did not change following large volume paracentesis suggesting this is not confounded by ascites[17]. However, the influence of peripheral oedema in this population has not been well described. Similar to our prior work[6], this study demonstrates the superiority of upper limb LM in predicting post-transplant outcomes in patients with cirrhosis when compared to APLM, lower limb LM and TLM. As MELD rose, upper limb LM decreased whereas lower limb LM and TLM increased. This suggests that in decompensated cirrhosis, upper limb LM more accurately reflects true muscle mass as it is not confounded by peripheral oedema or ascites. A cut-off of upper limb LM of < 1.6 kg/m2 was the best predictor of waitlist mortality in a single-centre cohort of men with cirrhosis[6]. This cut-off requires validation in multicentre cohorts and as yet no definitions for sarcopenia using upper limb LM have been proposed for women.
A major finding in this study is the lack of association of pre-transplant muscle parameters with post-transplant outcomes in women. This remains an unanswered question in the literature. While a sex-stratified approach to diagnose sarcopenia is required, most studies fail to report on gender-specific mortality analyses. Like most studies in the field of cirrhosis, women accounted for less than a third of patients transplanted for cirrhosis in this cohort. This may lead to inadequate power to detect significant associations between sarcopenia and outcomes.
It is possible that muscle mass has greater prognostic significance in men than women. The pathogenesis of sarcopenia in cirrhosis is a complex interplay between multiple factors. Testosterone, a potent promoter of muscle growth, plays a particularly important role in the development of sarcopenia in men. Testosterone levels fall with progression of liver disease and correlate with muscle mass in men with cirrhosis[18,19]. Furthermore, there is a clear association between testosterone levels in cirrhotic men and the adverse outcomes of hepatic decompensation, need for liver transplantation and death[20]. This may explain the higher prevalence of low muscle mass in men waitlisted for transplantation compared to women[21].
Functional measures of muscle such as handgrip strength and the liver frailty index may carry better prognostic utility in women. A multi-centre study of patients waitlisted for liver transplantation in the United States found that women had higher frailty scores than men and that increased frailty was associated with higher waitlist mortality[22]. A major limitation of this study is that muscle strength was not included due to the lack of available data over the timeframe described. Larger studies describing sarcopenia-related outcomes in cirrhotic and liver transplant cohorts need to include functional measures of sarcopenia and provide gender-stratified analyses so we can better understand the role of muscle in predicting outcomes in each gender.
In conclusion, this study is the first to comprehensively describe the association of reduced muscle mass as measured by DEXA on post-liver transplant outcomes providing gender-stratified analyses. We identify upper limb LM as a novel measure of sarcopenia that is associated with adverse outcomes post-liver transplant in men, without a corresponding increase in mortality. Larger multi-centre studies that provide gender-stratified monitoring of muscle mass and function serially on the waitlist are required to assess the full impact of sarcopenia on post-transplant outcomes. This will help determine whether prioritizing patients with sarcopenia for transplantation may be an appropriate strategy to minimize waitlist mortality without compromising post-transplant survival.
Pre-transplant sarcopenia defined by reduced skeletal muscle index measured by transverse abdominal computed tomography (CT) is associated with adverse outcomes after liver transplantation. These include increased rates of sepsis, longer hospital length of stay and a possible increase in post-transplant mortality.
CT is not recommended for use solely for the purpose of diagnosing sarcopenia given the high radiation doses. Dual-energy X-ray absorptiometry (DEXA) body composition assessment provides a low radiation and reproducible alternative for measuring muscle mass with prognostic utility in the pre-transplant setting. Upper limb lean mass (LM) has recently been identified as a novel assessment of sarcopenia using DEXA.
This study investigates the use of DEXA body composition assessment in predicting gender-stratified early post-transplant outcomes.
This study retrospectively analysed liver transplant recipients who underwent pre-transplant DEXA body composition imaging between 2002 and 2017 at a single-centre. DEXA variables analysed included appendicular LM (APLM), total, upper and lower limb LM and fat mass corrected for height2. Endpoints included post-transplant mortality and graft failure, bacterial infections, acute cellular rejection and intensive care and total hospital length of stay (days).
Four hundred and sixty-nine patients met inclusion criteria of which 338 were male (72%). Upper limb LM was inversely associated with bacterial infections at 180 d post-transplant in males only. There was a negative correlation between upper limb LM and intensive care and total hospital length of stay in men. In women, neither model for end-stage liver disease (MELD) nor body composition parameters were associated with post-transplant adverse outcomes or increased length of stay. Body composition parameters, MELD and age were not associated with 90-d mortality or graft failure in either gender.
Upper limb LM measured on DEXA is a novel measure of sarcopenia with better prognostic value compared to APLM in predicting adverse outcomes after liver transplantation. Reduced upper limb LM was a predictor of post-transplant bacterial infection and longer length of stay in men only, but was not associated with increased mortality or graft failure. The lack of association in women requires further investigation.
Larger multi-centre studies that provide gender-stratified analysis of muscle mass and function serially on the waitlist are required to assess the full impact of pre-transplant sarcopenia on post-transplant outcomes. This will help determine whether prioritizing patients with sarcopenia for transplantation may be an appropriate strategy to minimize waitlist mortality without compromising post-transplant survival.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Transplantation
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li HL, China; Schemmer P, Austria A-Editor: Yao QG, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6053] [Cited by in F6Publishing: 5686] [Article Influence: 1137.2] [Reference Citation Analysis (0)] |
| 2. | Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One. 2017;12:e0186990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 3. | Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870-1879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 343] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 4. | Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Okajima H, Uemoto S. Impact of Skeletal Muscle Mass Index, Intramuscular Adipose Tissue Content, and Visceral to Subcutaneous Adipose Tissue Area Ratio on Early Mortality of Living Donor Liver Transplantation. Transplantation. 2017;101:565-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB, Kneteman N. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 217] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 6. | Sinclair M, Hoermann R, Peterson A, Testro A, Angus PW, Hey P, Chapman B, Gow PJ. Use of Dual X-ray Absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int. 2019;39:1089-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2670] [Cited by in F6Publishing: 2611] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 8. | Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6987] [Cited by in F6Publishing: 7602] [Article Influence: 543.0] [Reference Citation Analysis (0)] |
| 9. | R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna. 2021. [cited 13 December 2021]. Available from: https://www.R-project.org/. [Cited in This Article: ] |
| 10. | Therneau TM, Grambsch PM. Modelling Survival Data: Extending the Cox Model. New York: Springer, 2000. [Cited in This Article: ] |
| 11. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 267] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 12. | van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant. 2016;16:2277-2292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 13. | Kalafateli M, Mantzoukis K, Choi Yau Y, Mohammad AO, Arora S, Rodrigues S, de Vos M, Papadimitriou K, Thorburn D, O'Beirne J, Patch D, Pinzani M, Morgan MY, Agarwal B, Yu D, Burroughs AK, Tsochatzis EA. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 14. | Wakabayashi T, Shinoda M, Obara H, Kitago M, Yagi H, Abe Y, Matsubara K, Yamada Y, Oshima G, Hirukawa K, Mizota T, Hibi T, Itano O, Hoshino K, Kuroda T, Kitagawa Y. Decreased Incidence of Acute Cellular Rejection in Low-Muscle-Mass Recipients After Living-donor Liver Transplantation. Transplant Proc. 2018;50:3626-3634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Fozouni L, Mohamad Y, Lebsack A, Freise C, Stock P, Lai JC. Frailty Is Associated With Increased Rates of Acute Cellular Rejection Within 3 Months After Liver Transplantation. Liver Transpl. 2020;26:390-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611-1644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 228] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 17. | Belarmino G, Gonzalez MC, Sala P, Torrinhas RS, Andraus W, D'Albuquerque LAC, Pereira RMR, Caparbo VF, Ferrioli E, Pfrimer K, Damiani L, Heymsfield SB, Waitzberg DL. Diagnosing Sarcopenia in Male Patients With Cirrhosis by Dual-Energy X-Ray Absorptiometry Estimates of Appendicular Skeletal Muscle Mass. JPEN J Parenter Enteral Nutr. 2018;42:24-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Grossmann M, Hoermann R, Gani L, Chan I, Cheung A, Gow PJ, Li A, Zajac JD, Angus P. Low testosterone levels as an independent predictor of mortality in men with chronic liver disease. Clin Endocrinol (Oxf). 2012;77:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Sinclair M, Grossmann M, Angus PW, Hoermann R, Hey P, Scodellaro T, Gow PJ. Low testosterone as a better predictor of mortality than sarcopenia in men with advanced liver disease. J Gastroenterol Hepatol. 2016;31:661-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Deng N, Mallepally N, Peng FB, Kanji A, Marcelli M, Hernaez R. Serum testosterone levels and testosterone supplementation in cirrhosis: A systematic review. Liver Int. 2021;41:2358-2370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, Bain VG, Esfandiari N, Baracos V, Montano-Loza AJ, Myers RP. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18:1209-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 400] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 22. | Lai JC, Ganger DR, Volk ML, Dodge JL, Dunn MA, Duarte-Rojo A, Kappus MR, Rahimi RS, Ladner DP, Boyarsky B, McAdams-DeMarco M, Segev DL, McCulloch CE, Verna EC. Association of Frailty and Sex With Wait List Mortality in Liver Transplant Candidates in the Multicenter Functional Assessment in Liver Transplantation (FrAILT) Study. JAMA Surg. 2021;156:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |