Copyright
©The Author(s) 2017.
World J Transplant. Feb 24, 2017; 7(1): 1-25
Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.1
Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.1
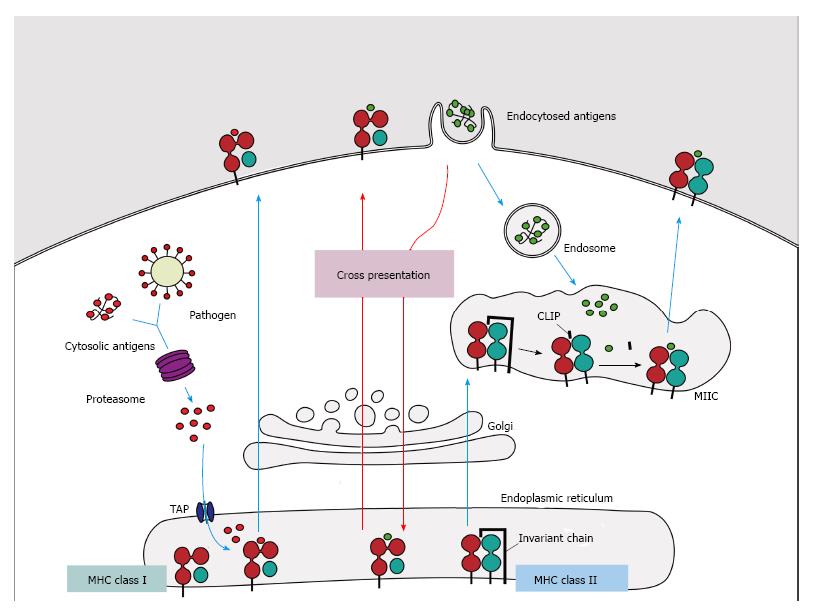
Figure 1 Major histocompatibility complex class I and II pathways.
(1) MHC class I molecules present peptides derived from proteins presented in the cytosol of endogenous or pathogen origin. The proteasome breaks down these proteins into peptides, which are then translocated to ER by the transporter associated with antigen processing (TAP) to access the MHC class I molecules. In absence of peptides, MHC class I molecule is stabilized by ER chaperones (calreticulin, PDIA3, PDI and tapasin), but when peptides with sufficient affinity bind to class I molecules, these chaperones are released and the peptide: MHC complex leaves the ER for presentation on cell surface of CD8+ T cells; (2) MHC class II molecules present peptides derived from proteins that enter the cell through endocytosis. The chains α and β are assembled in the endoplasmatic reticulum associated with the invariant-chain (li) to prevent binding of endogenous proteins. This complex (MHC:li) is translocated to MHC class II compartment (MIIC) where li is degraded to class II-associated invariant chain (CLIP). In the MIIC the MHC class II molecules acquire HLA-DM to facilitate the exchange of CLIP to specific antigen derived from degraded protein on the endosomal pathway, thus the complexes are transported to the plasma membrane to present the peptide to CD4+ T cells; (3) Cross presentation involves dendritic cells with the unique ability to present exogenous antigens via MHC class I (by a mechanism not completely understood). MHC: Major histocompatibility complex.
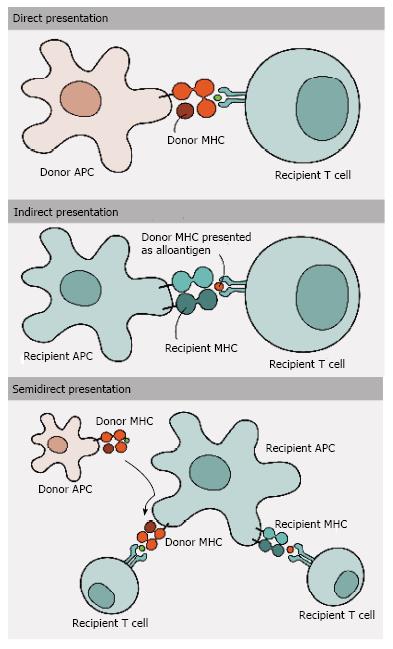
Figure 2 Antigen presentation and allorecognition.
T cells can recognize alloantigens by three different pathways of allorecognition: (1) Direct pathway involves the recognition of intact donor MHC molecules on the cell surface of donor APCs by recipient T cells; (2) In contrast, in indirect pathway donor MHC molecules are processed and presented as peptides by recipient MHC molecules to recipient T cells; and (3) Semi-direct pathway, in turn, involves the transfer of intact donor MHC molecules to recipient APCs, being presented to recipient T cells. MHC: Major histocompatibility complex; APCs: Antigen-presenting cells.
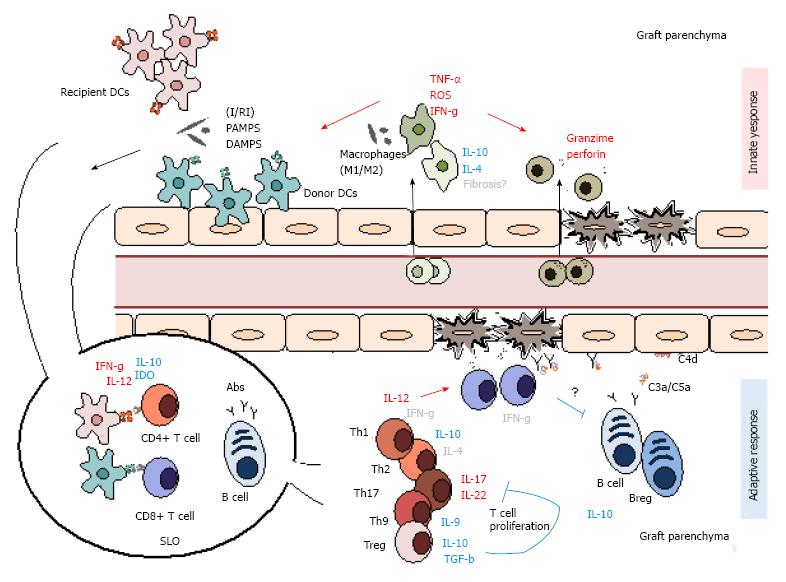
Figure 3 Summary of the main innate and adaptive mediators of graft rejection.
Alloimmune rejection is a multifaceted process that involves both innate and adaptive mediators. Initial tissue damage is mostly mediated by innate participants as macrophages and NK cells along with dendritic cells, which link the both innate and adaptive responses. With time, these gradually give way to more adaptive mediators as T and B lymphocytes and antibody production. I/RI: Isquemia/reperfusion injury; PAMPs: Pathogen-associated molecular patterns; DAMPS: Danger-associated molecular patterns; IDO: Indoleamine 2,3-dioxygenase; Abs: Antibodies; DC: Dendritic cells; SLO: Secondary lymphoid organ; ROS: Reactive oxygen species; TNF: Tumor necrosis factor. Mediator roles are represented in red (pro-inflammatory), blue (regulatory) and grey (indetermined).
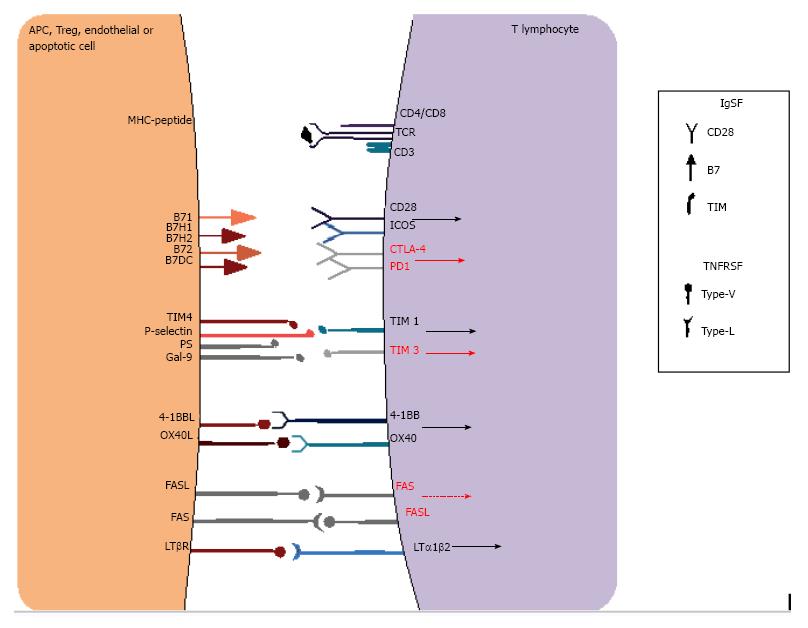
Figure 4 Examples of co-stimulatory receptors in immune synapse interactions.
T cell activation depends not only on the antigen-specific signals provided by MHC-TCR signaling but also on a complex balance of co-stimulatory signals that may enhance or inhibit activation. These receptors are mainly classified into the immunoglobulin superfamily (IgSF) or the tumor necrosis factor receptor superfamily (TNFRSF) and may provide positive (black arrows) or negative signals (red arrows), or even apoptotic signals (red dashed arrow), which in turn decide cell fate. MHC: Major histocompatibility complex; TCR: T cell receptor.
- Citation: da Silva MB, da Cunha FF, Terra FF, Camara NOS. Old game, new players: Linking classical theories to new trends in transplant immunology. World J Transplant 2017; 7(1): 1-25
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.1