Copyright
©The Author(s) 2016.
World J Transplant. Sep 24, 2016; 6(3): 505-516
Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.505
Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.505
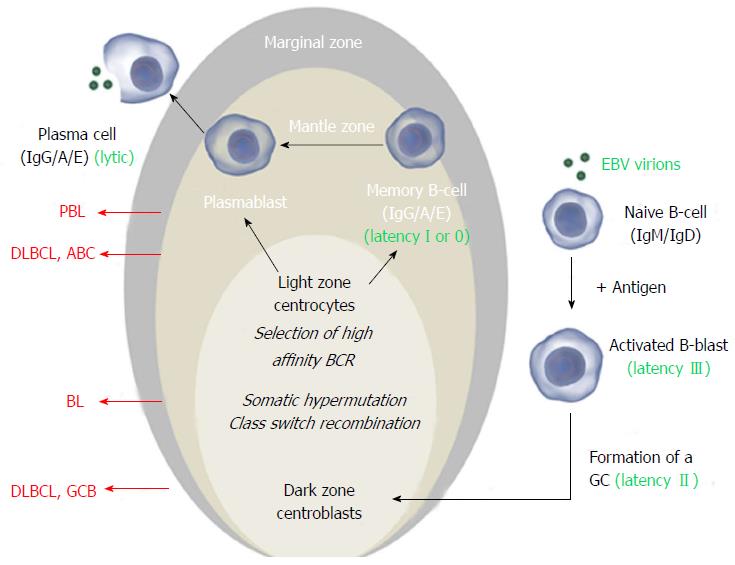
Figure 1 The Epstein-Barr virus exploits normal B-cell activation pathways.
Activation of a naive B-cell (that expresses IgM and IgD on its surface) by its cognate antigen results in B-cell activation and differentiation into a memory B-cell or a plasma cell, most commonly via T-cell dependent activation. The antigen-activated B-cell enters a primary follicle in lymph node or spleen and forms a germinal center (GC), transforming the primary follicle into a secondary follicle. This structure is composed of three distinct regions. The marginal zone[1], which consists mainly of activated B-cells and GC-matured IgM+ B-cells, the mantle zone or corona[2], which comprises naïve and memory B-cells surrounds the GC[3]. The GC consists of a dark zone and a light zone. In the dark zone, the activated B-cells (centroblasts) proliferate and downregulate expression of IgM and IgD to allow somatic hypermutation (SHM) and class switch recombination (CSR), increasing the antibody’s affinity, specificity and functional versatility. In the light zone of the GC, the B-cells (centrocytes) with the best antibody are selected and ultimately mature into memory B-cells or plasma cells. Instead of IgM and IgD, these express high affinity IgG, IgA or IgE antibodies. Classically, Epstein-Barr virus (EBV) infects naïve B-cells that are stimulated to form a GC. In the activated blast, viral latency III (LMP1+/EBNA2+) is expressed and induces proliferation. In the GC, latency II (LMP1+/EBNA2-) is expressed and infected centroblasts presumably undergo SHM and CSR, involved in antibody maturation. After leaving the GC, they differentiate into plasma cells or (mainly) memory cells (latency I, EBNA1+ or latency 0, no expression of viral proteins). In vitro and in vivo, plasma cell differentiation results in activation of the EBV lytic cycle. In all stages, the viral DNA (circle in the nucleus) is maintained as an episome. Different stages of this process can give rise to malignancy resulting in different lymphoma subtypes that have features of their normal counterpart. Here the stages at which EBV+ and EBV- B-cell lymphoma may arise are shown for the most common subtypes. Images from http://www.somersault1824.com were used in this figure. PBL: Plasmablastic lymphoma; DLBCL: Diffuse large B-cell lymphoma; ABC: Activated B-cell; GCB: Germinal center B-cell.
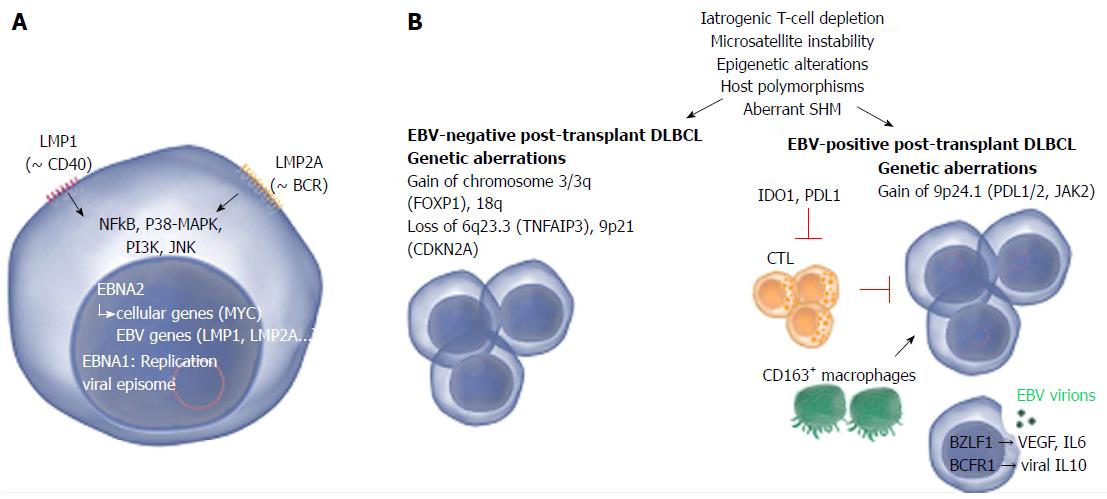
Figure 2 Common and distinct pathogenetic mechanisms in Epstein-Barr virus-positive and -negative post-transplant diffuse large B-cell lymphoma.
A: Two Epstein-Barr virus (EBV) proteins that are thought to play a role in EBV-driven lymphomagenesis are LMP1 and LMP2A. LMP1 is analogous to CD40 and promotes cell transformation by inducing NF-κB, that in turn upregulates BCL-2, A20 and C-FLIP, all involved in blocking apoptosis. LMP2A mimics a chronically active B-cell receptor (BCR) and prevents BCR-mediated activation of EBV lytic replication. LMP2A also provides the necessary survival signals which can compensate for the loss of a functional BCR. Other pathways that are induced comprise janus kinase, p38-MAPK and PI3K signaling. Nuclear EBNA1 and EBNA2 are involved in replication of the viral episome and induction of viral as well as cellular genes respectively; B: The pathogenesis of EBV-positive and -negative lymphoma is marked by a number of common as well as distinct pathogenetic mechanisms. Mechanisms that contribute to both EBV-positive and -negative lymphoma involve iatrogenic T-cell suppression, microsatellite instability (resulting in accumulation of mutations), epigenetic alterations (mainly hypermethylation), host polymorphisms (in particular in genes encoding proteins involved in immunity), aberrant somatic hypermutation (SHM, resulting in accumulation of point mutations) and aberrant up- or down-regulation of host miRNAs which may substantially impact gene expression. EBV-negative PT-DLBCL is characterized by genetic aberrations found in EBV-negative DLBCL arising in the general population, e.g., alterations involving FOXP1. EBV-positive PT-DLBCL on the other hand harbors fewer genetic lesions. Gain of 9p24.1 (harboring PDL1/2, JAK2) has been detected and may contribute to tumor immune evasion. A minority of the EBV-positive cells actively produce viral particles. This lytic replication may promote lymphoma growth by expression of IL-6 and VEGF. Also viral IL-10 (vIL10) is expressed which contributes to suppression of anti-tumor responses by antagonizing IFN-γ. The expression of EBV proteins attracts cytotoxic T-cells (CTLs) to the site of the tumor however the question remains whether effective anti-tumor responses can be produced as also tolerant immune responses are induced. IDO1 (expressed in tumor cells and dendritic cells) and PDL1 (expressed in tumor cells and macrophages) suppress T-cells and may substantially impair the activity of CTLs. Also CD163+ macrophages (thought to be immunotolerant M2 macrophages) may play a role in immune evasion. Images from http://www.somersault1824.com were used in this figure. PT-DLBCL: Post-transplant diffuse large B-cell lymphoma; IL: Interleukin; IFN: Interferon; VEGF: Vascular endothelial growth factor; BCR: B-cell Receptor.
- Citation: Morscio J, Tousseyn T. Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders. World J Transplant 2016; 6(3): 505-516
- URL: https://www.wjgnet.com/2220-3230/full/v6/i3/505.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i3.505