Copyright
©The Author(s) 2021.
World J Transplant. Mar 18, 2021; 11(3): 54-69
Published online Mar 18, 2021. doi: 10.5500/wjt.v11.i3.54
Published online Mar 18, 2021. doi: 10.5500/wjt.v11.i3.54
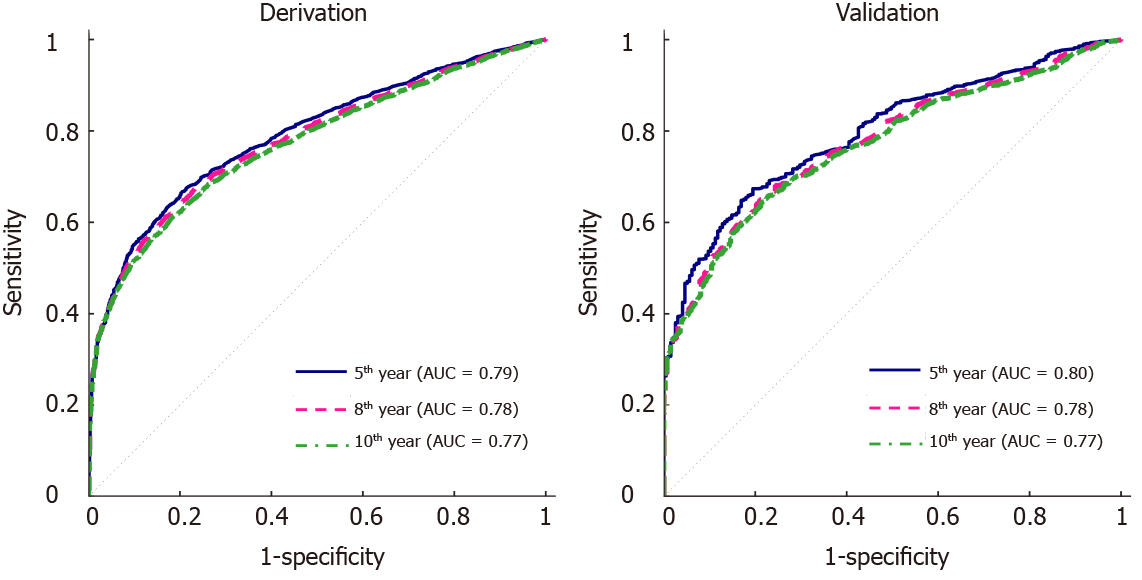
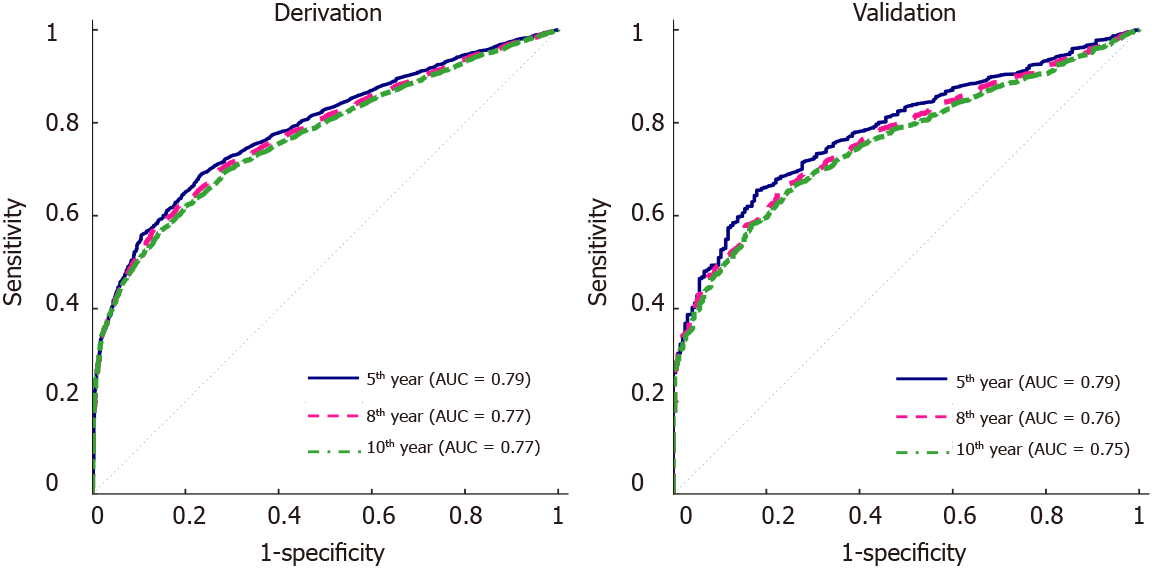
Figure 1 Receiver operating characteristics curves of the multivariate model for the 5-yr, 8-yr and 10-yr post-transplant cutaneous squamous cell carcinoma prediction.
A: The derivation set; B: The validation set. AUC: Area under the curve.
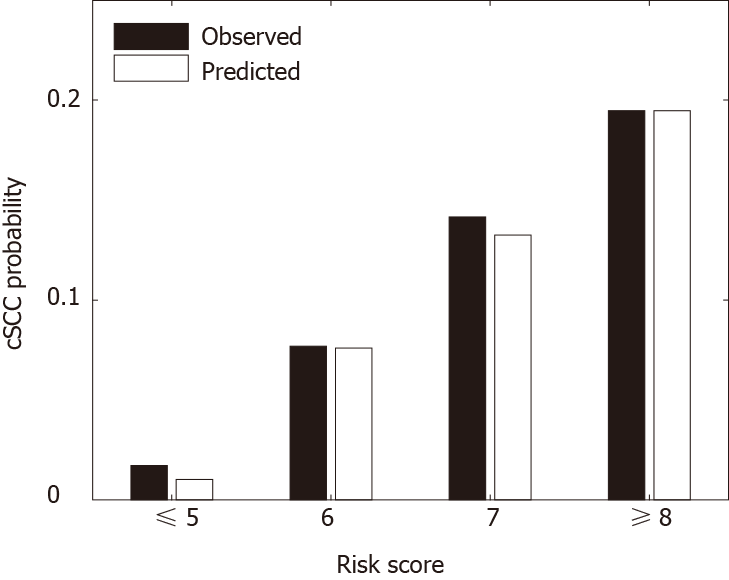
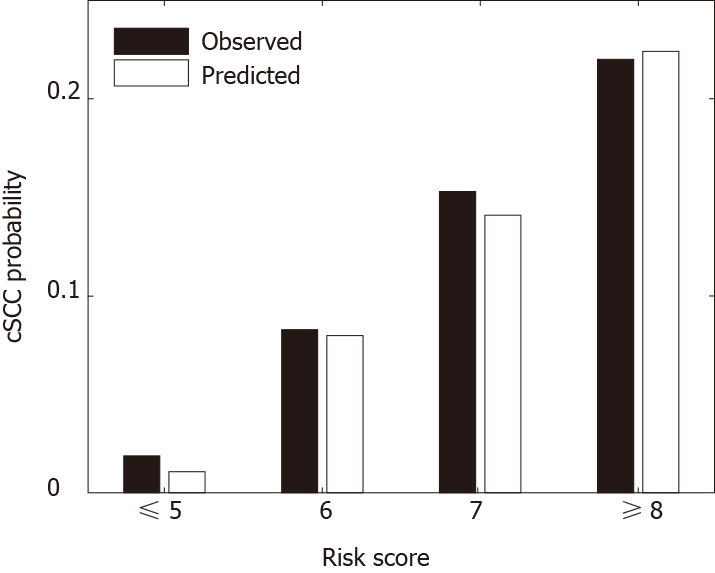
Figure 2 Predicted vs observed probabilities of developing cSCC 5 yr after transplant in different risk groups: very low-risk group (score ≤ 5), low-risk group (score = 6), medium-risk group (score = 7), high-risk group (score ≥ 8).
cSCC: Cutaneous squamous cell carcinoma.
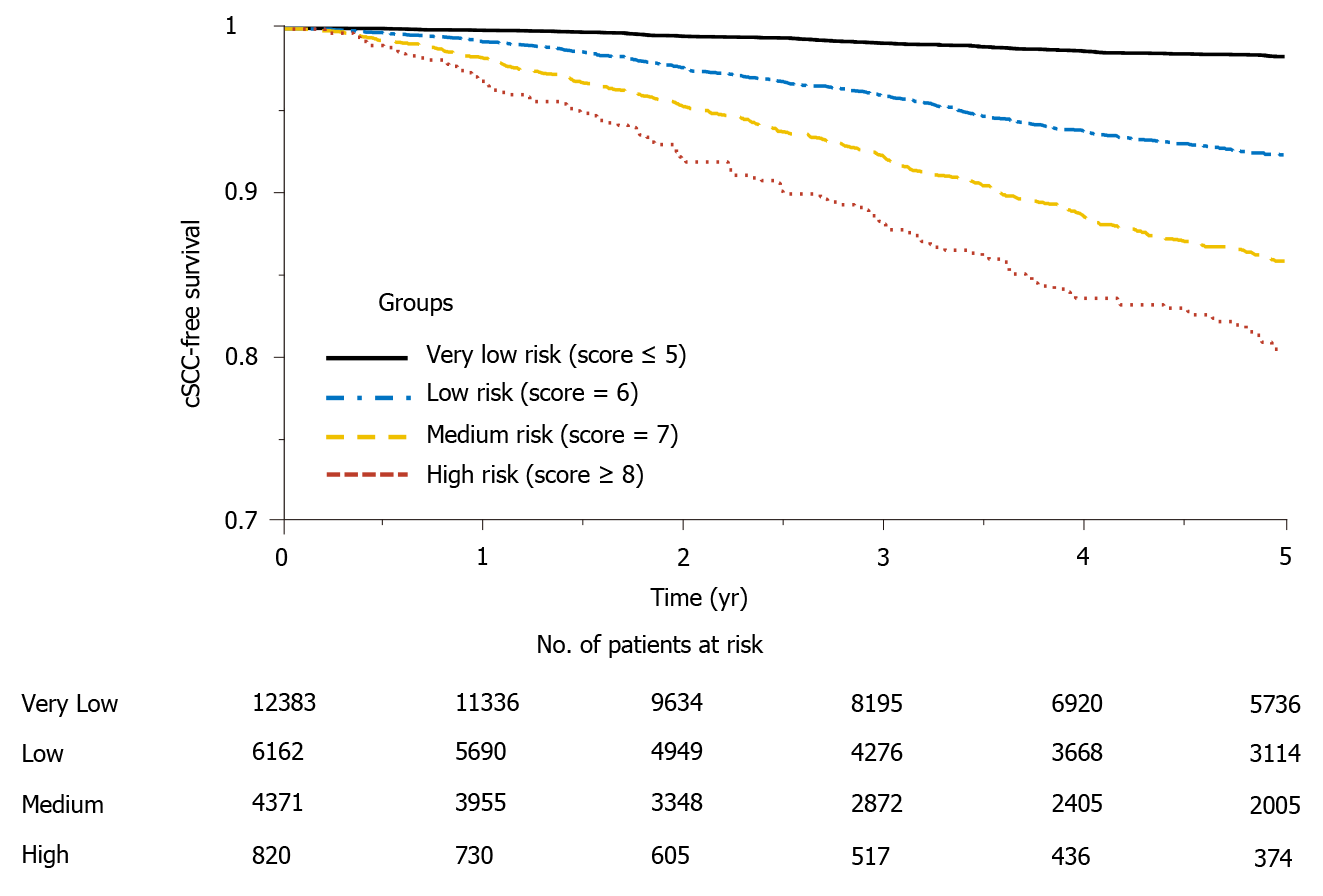
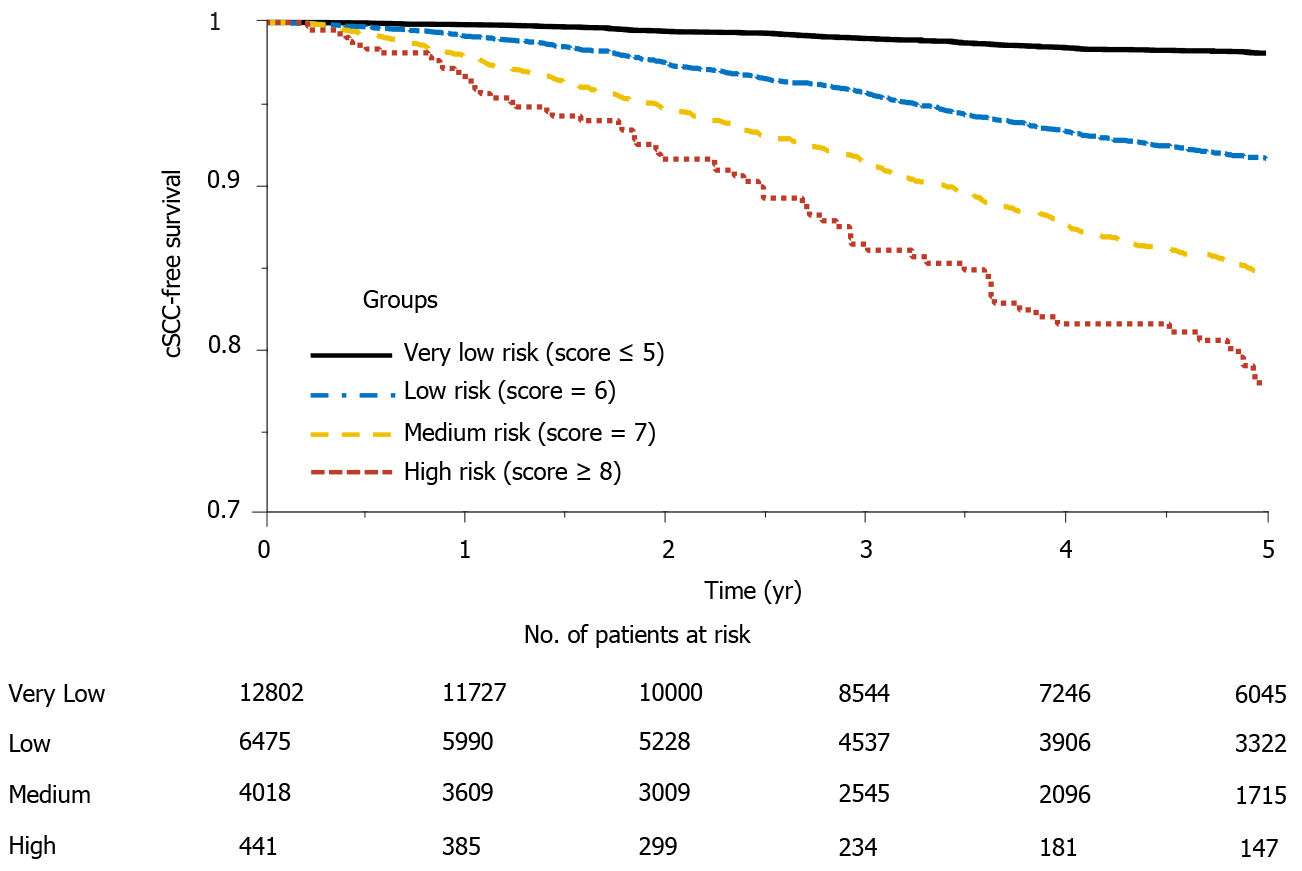
Figure 3 Cumulative cSCC-free survival curves for different risk groups.
cSCC: Cutaneous squamous cell carcinoma.
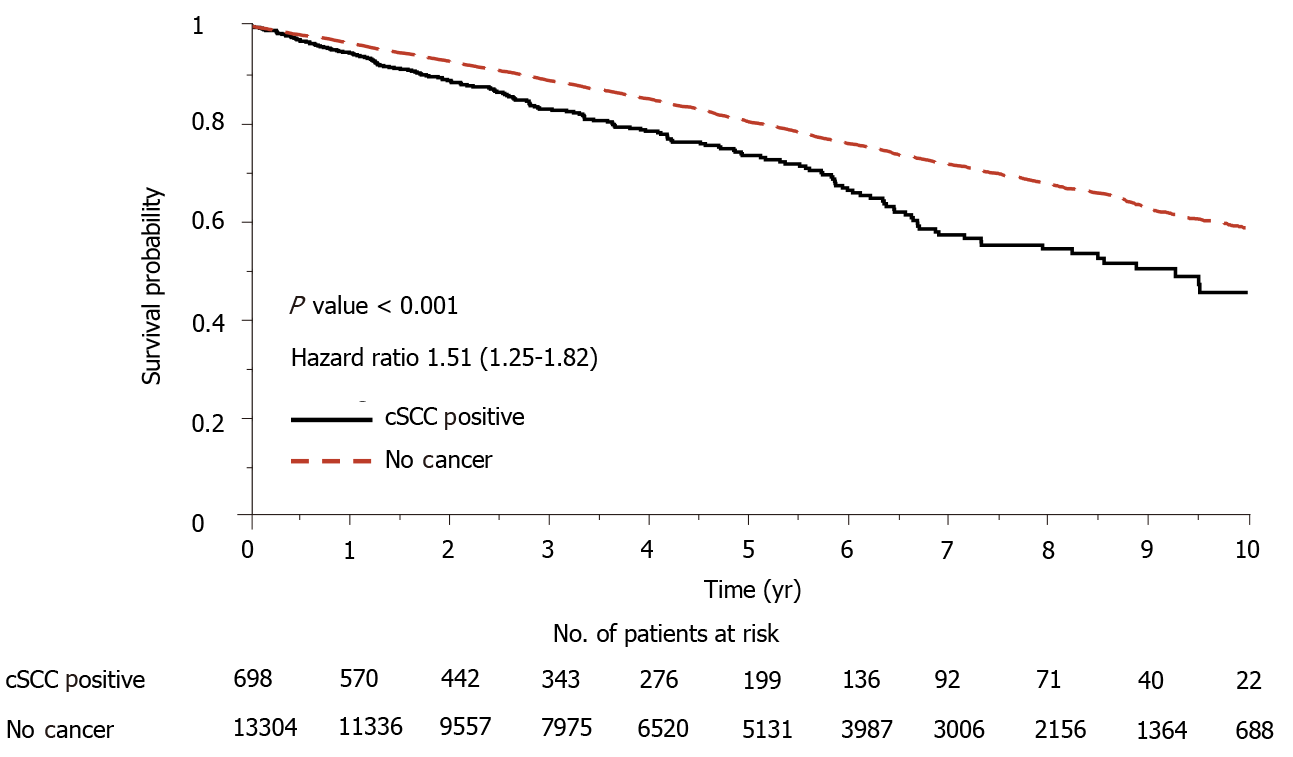
Figure 4 Cumulative survival curves for heart transplant recipients with cSCC and with no cancer.
cSCC: Cutaneous squamous cell carcinoma.
Figure 5 Receiver operating characteristics curves of the multivariate model without OKT3 and daclizumab for the 5-yr, 8-yr and 10-yr post-transplant cutaneous squamous cell carcinoma prediction.
A: The derivation set; B: The validation set. AUC: Area under the curve.
Figure 6 Predicted vs observed probabilities of developing cSCC 5 yr after transplant in different risk groups where patients were divided using the scoring system without OKT3 and daclizumab: very low-risk group (score ≤ 5), low-risk group (score = 6), medium-risk group (score = 7), high-risk group (score ≥ 8).
cSCC: Cutaneous squamous cell carcinoma.
Figure 7 Cumulative cSCC-free survival curves for different risk groups where patients were divided using the scoring system without OKT3 and daclizumab.
cSCC: Cutaneous squamous cell carcinoma.
- Citation: Nair N, Hu Z, Du D, Gongora E. Risk prediction model for cutaneous squamous cell carcinoma in adult cardiac allograft recipients. World J Transplant 2021; 11(3): 54-69
- URL: https://www.wjgnet.com/2220-3230/full/v11/i3/54.htm
- DOI: https://dx.doi.org/10.5500/wjt.v11.i3.54