Copyright
©2012 Baishideng Publishing Group Co.
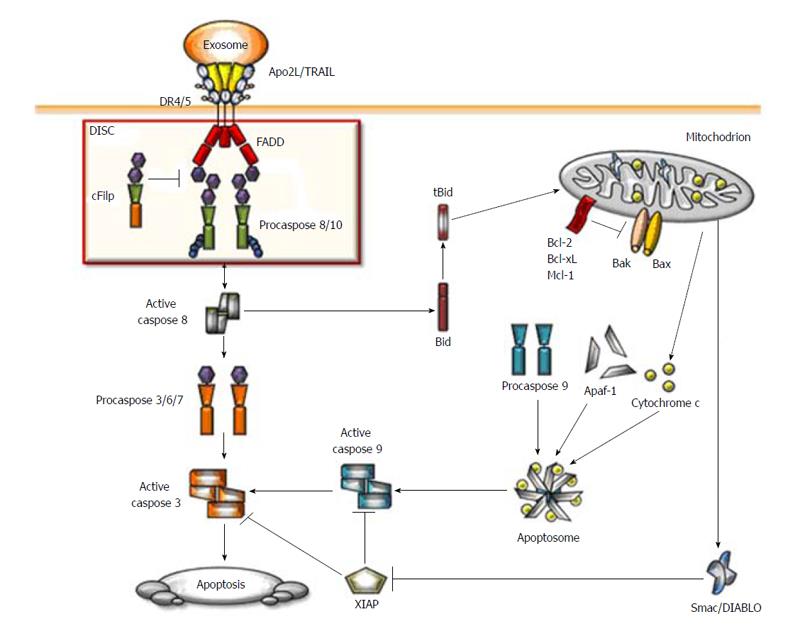
Figure 1 Schematic representation of the Apo2 ligand/ tumor necrosis factor related apoptosis-inducing ligand apoptotic signaling pathway.
When Apo2 ligand/ tumor necrosis factor related apoptosis-inducing ligand (Apo2L/TRAIL) binds to their respective receptors induced their trimerisation and formation of the death-inducing signaling complex (DISC). The adaptor protein fas-associated protein with death domain (FADD) is recruited to the DISC through its death domain (DD) which interacts with DD of the receptors. Subsequently, procaspases-8 and -10 are recruited to the protein complex where they interact with FADD via the death effector domains. Cellular FLICE inhibitory protein (cFLIP) can compete with caspase-8 for the binding to FADD and high levels of cFLIP can inhibit caspase-8 activation at the DISC. DISC-activated caspases-8 and -10 trigger a caspase cascade by cleavage of caspase-3 therby activating effector caspases. In type I cells, activation of the extrinsic pathway is sufficient to induce Apo2L/TRAIL-induced apoptosis whereas in type II cells, Bid cleavage is required for apoptosis induction by Apo2L/TRAIL. Caspase-8 cleaves Bid into tBid which initiates the mitochondrial pathway leading to release of cytochrome c and second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI (Smac/DIABLO) from the mitochondria. After release from mitochondria, cytochorme c, together with Apaf-1 forms the apoptosome, which leads to activation of caspase-9. Smac/DIABLO counteracts the inhibitory function of X-linked inhibitor of apoptosis thereby allowing for full activation of caspases-3 and -9, ultimately leading to cell death.
- Citation: Martinez-Lostao L, Anel A. Role of Apo2L/TRAIL in immunity: Applications to rheumatoid arthritis. World J Rheumatol 2012; 2(1): 1-11
- URL: https://www.wjgnet.com/2220-3214/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5499/wjr.v2.i1.1