Published online Dec 9, 2014. doi: 10.5497/wjp.v3.i4.97
Revised: October 30, 2014
Accepted: November 7, 2014
Published online: December 9, 2014
Processing time: 166 Days and 18.3 Hours
Antivirulence therapy inhibits bacterial virulence factors, thus preventing the development of infection without affecting bacterial growth. The development of new antibiotics is complicated by the increasing incidence of antibiotic resistance in pathogenic bacteria. Antivirulence therapy is a promising alternative to traditional antibiotic therapy for the treatment of infectious disease, either alone or in combination with antibiotic treatment. In this review, we consider patents concerning inhibition of several bacterial virulence factors: adhesion/colonization, secretion systems, cellular signalling systems and antimicrobial resistance mechanisms. Finally, we emphasize the importance of analyzing new targets and/or molecules in this field and of considering possible resistance mechanisms.
Core tip: Antimicrobial resistance in nosocomial pathogens has increased dramatically in recent years. The development of new molecules, therapies and/or new combinations for the eradication of these pathogens is therefore imperative. A new line of research in this area is called “Antivirulence Therapy”.
- Citation: López M, Barbosa B, Gato E, Bou G, Tomás M. Patents on antivirulence therapies. World J Pharmacol 2014; 3(4): 97-109
- URL: https://www.wjgnet.com/2220-3192/full/v3/i4/97.htm
- DOI: https://dx.doi.org/10.5497/wjp.v3.i4.97
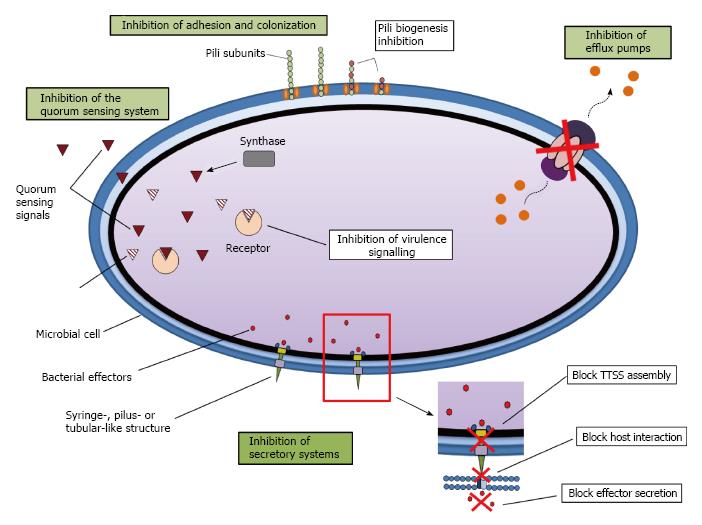
Microbial virulence is the ability of a microbe to cause disease. Antivirulence therapies are constituted on inhibition of bacterial virulence and do not influence bacterial growth. Bacteria appreciate their environment and, once in the host they respond by starting a plan determined for the activation of virulence factors. Hence, antivirulence strategies have the ability to interfere in the recognition of the host signals that alarm the bacteria localized in the place of infection and/or that activate specific virulence factors implicated to development of the infection. If the development of virulence factors is prevented, the bacteria will be less able to colonize. Moreover, this tactic will not directly kill bacteria, so initially the evolutionary pressure for the development of resistant strains would be lower than with conventional antibiotics[1]. Inhibition of the following systems enables interruption of the process of bacterial infection: toxin production, adhesion and colonization, bacterial secretory systems, cell-to-cell signalling pathways, and antibiotic resistance mechanisms, such as efflux pumps (multidrug resistance) (Figure 1)[2]. In this review, we provide details of patents concerning the inhibition of each of these mechanisms, except for toxin production, which is specific to certain pathogens such as Bacillus anthracis (which causes anthrax) and Clostridium spp. (which causes gangrene)[1].
Microorganisms adhere to host cells in order to colonize the host and begin infection. The majority of the bacteria own a determined host interval and will only infect hosts that express specific receptors for bacterial adhesion traits on their cell surface. Besides, once inside the host, bacteria will only infect the cells (tissue) that have the adequate receptor. Attachment of bacteria to a host cell is a complicated process managed by adhesin on the bacteria and the receptor on the cell. However, adherence is frequently only the first step in the infection course, that besides implicates internalization, deeper tissue penetration and likely systemic spread. Bacteria have different kinds of elements for adhere to the host surface, including- but not only-pili, fimbriae and in some cases flagella[3]. Adhesion can be inhibited by the following strategies: (1) prevention of adhesion complex assembly [as in the pili of uropathogenic Escherichia coli (E. coli)], for which the compounds bicyclic 2-pyridones (pilicides)[4] and Virstatin[5] have been developed; and (2) prevention of elongation and formation of a functional pilus.
A search carried out in patent databases[6,7] revealed a total of 26 patent applications related to strategies that interfere with adhesion and colonization mechanisms (Table 1). These include the use of probiotics such as Lactobacillus reuteri, Bifidobacterium infantis, Bifidobacterium lactis, Lactobacillus acidophilus and Lactobacillus casei, for inhibition of Candida colonization, and also Enterococcus faecium LJS-01, which displays a strong capacity to adhere to intestinal epithelial cells and also good antimicrobial activity. The following proteins have also been described: decorin binding protein, which prevents colonization by Borrelia; collagen binding protein, isolated from Staphylococcus aureus; C3 binding polypeptide, isolated from Streptococcus agalactiae; novel fluorinated linker compounds; and Zn-releasing calcium phosphate. Finally, the following targets have been identified for vaccine development: capsular polysaccharide, EtpA flagellin and pyruvate-ferredoxin oxidireductase adhesin protein.
| Patent title | Description | Applicationdate | Inventors | Publicationnumber |
| Capsular polysaccharide adhesion antigen preparation, purification and use | General method for preparing pure capsular exopolysaccharide adhesins strains of adhesin coagulase-negative staphylococci to produce vaccines | 1994 | Pier Gerald B | US5980910 (A) |
| Method for inhibiting microbial adhesion on surfaces | A method for inhibiting microbial adhesion on surfaces in contact with an aqueous system is disclosed and involves adding a treatment comprising an alkyl sulfosuccinate surfactant to the system | 1995 | Wright J Barry; Michalopoulos Daniel L | US5512186 |
| Method and apparatus for preventing adhesion and colonization of bacteria in medical devices | Activation of compounding photochemicals for preventing and eliminating adherence and colonization of bacteria | 1996 | Prescott Marvin A | WO9806340 (A1) |
| Decorin binding protein compositions and methods of use | DNA segments encoding these proteins and anti-(decorin binding protein) antibodies for use in the prevention of Borrelia colonization in an animal | 1996 | Guo Betty P; Hoeoek Magnus; Hanson Mark | WO9727301 (A1) |
| Collagen binding protein compositions and methods of use | Disclosed are the cna gene and cna-derived nucleic acid segments from Staphylococcus aur. Also disclosed are Col Binding Protein (CBP) for use in the treatment of pathological infections, and in particular, for use in the prevention of bacterial adhesion to Col | 1997 | Hoeek Magnus; Patti Joseph M; House-Pompeo Karen; Sthanam Narayana; Symersky Jindrich | US6288214 (B1) |
| Surfactants for reducing bacterial adhesion onto surfaces | Inhibiting microbial colonization (ethylene oxide units) of a surface in contact with an aqueous system | 1997 | Donlan Rodney M; Elliot David L; Kapp Nancy J; Wiatr Christopher L; Rey Paula | US6039965 (A) |
| Composition of treatment of Candidiasis | Inhibition of adhesion of Candida colonization by using probiotics (Lactobacillus reuteri, Bifidobacterium infantis, Bifidobacterium lactis, Lactobacillus acidophilus not viable and non-viable Lactobacillus casei) | 1998 | Dohnlek Margaret H; Wagner Robert Doug; Balish Edward; Hilty Milo D | WO9917788 (A1) |
| Antimicrobial adhesion surface | The invention provides an implantable medical device with a hydrophilic coating to limit in vivo colonization of bacteria and fungi | 1999 | Zhong Samuel P | US6468649 (B1) |
| Anti-bacterial compounds directed against pilus biogenesis, adhesion and activity; co-crystals of pilus subunits and methods of use thereof | The invention relates to novel compounds that mimic a chaperone G1 beta-strand or an amino terminal motif of a pilus subunit | 2000 | Hultgren Scott J; Sauer Frederic G; Waksman Gabriel; Fuetterer Klaus; Choudhury Devapriya; Knight Stefan D; Barnhart Michelle | US7041465 (B1) |
| C3 binding polypeptide of Streptococcus agalactiae group b-Streptococcus | The invention involves the identification of a human complement C3 binding polypeptide and the nucleic acid that encodes the polypeptide from Streptococcus agalactiae | 2000 | Smith Beverly L; Ferrieri Patricia | US6582950 (B1) |
| Compounds directed against pilus biogenesis and activity in pathogenic bacteria, methods and compositions for synthesis thereof | Novel fluorinated linker compounds and methods of synthesis are provided. Methods for using the fluorinated linker compounds in methods of solid-phase synthesis of the N-substituted amino acid compounds are also disclosed (inhibiting or preventing the formation of a pilus chaperone-subunit complex) | 2000 | Kilhlberg Jan; Larsson Andreas; Svensson Anette; Fex Tomas; Hultgren Scott J; Pinkner Jerry | WO2001020995 |
| DbpA compositions and methods of use | The DBP gene and decorin protein compositions of Borrelia burgdorferi are disclosed The DBP protein and antigenic epitopes derived from them are contemplated for use in preventing bacterial adhesion to decorin | 2000 | Guo Betty P; Hook Magnus | US6312907 |
| Composition and method for controlling microbial adhesion and biofilm formation of surfaces | The invention describes how coating of surfaces with an extract, particularly a fish extract, can significantly reduce microbial adhesion, attachment, colonization and biofilm formation on surfaces | 2003 | Gram Lone Kirsten; Vogel Birtefonnesbech; Bagge-Ravn Dorthe | WO03092382 (A1) |
| Packaged antimicrobial medical device and method of preparing same | An antimicrobial suture assembly (halogenated hydroxyl ethers, acyloxydiphenyl ethers, and combinations thereof) to substantially inhibit bacterial colonization | 2004 | Scalzo Howard; Fischer Jerome A; Rothenburger Stephen | US2004220614 (A1) |
| Sealing material | A sealing material is presented (fluoropolymer layer, a reinforcing layer and an adhesive) to hinder growth and colonization of bacteria | 2004 | Patel Malay; Napolitano Michael; Hanrahan James R; Chu Chaokang | US2005250398 (A1) |
| Zn-releasing calcium phosphate (Zn-CaP) compounds for antimicrobial coating on orthodontic appliances and dental implants | Compositions of Zn-releasing calcium phosphate (Zn-CaP) compounds for use as anti-bacterial coatings for orthodontic brackets and dental implants | 2006 | Legeros Racquel Z; Legeros John P; Park Jae Hyun; Mijares Dindo | WO2007022211 (A2) |
| Composition for the administration of biologically active principles in gynaecological and rectal conditions and uses thereof | The invention relates to a composition for the administration of biologically active substances in gynaecological and rectal conditions, as well as the uses of said composition | 2007 | Strozzi Gianpaolo; Mogna Luca | US2010092440 (A1) |
| Enhanced treatments to kill or debilitate pathogenic microorganisms of a mammalian body | The novel treatments involve the use of anti-adhesive polysaccharide molecules to abolish or reduce the adhesion of Helicobacter pylori | 2008 | Nifantiev Nikolay; Wieland Gerhard D | US2011245198 (A1) |
| Non-leaching surface-active film compositions for microbial adhesion prevention | Coating (surfactant) useful to prevent bacterial colonization on a variety of surface including surfaces of medical devices | 2008 | Gruening Rainer; Qu Xin; Merritt Karen; Chen Paul N; Falevich Vitaly | MX2008009326 (A) |
| Prevention and treatment of Gram-negative, flagellated bacterial infections | EtpA which binds to the conserved region of the flagellin protein located at the tip of the flagella in Gram-negative bacteria (development vaccine) | 2008 | Fleckenstein James M | US2011206694 (A1) |
| Method for coating medical device1 | Method for coating a medical device to prevent bacterial adhesion, colonization and device-associated infection (isocyanate-terminated polymer) | 2010 | Stopek Joshua | JP2011019902 (A) |
| Novel Enterococcus faecium LJS-01 and its use as a probiotic1 | Enterococcus faecium LJS-01 shows good antimicrobial activity and strong capacity to adhere to intestinal epithelial cells | 2010 | Lin Chuen-FU; Wu Cheng-Nan; Lu Cheng-Hsiung; Hsu Wei-Li; Chiou Ming-Tang | TW201143631 (A) |
| Method for detecting colonization characteristic of lactobacillus in gastrointestinal tract on basis of green fluorescent protein1 | The invention relates to a method for detecting the colonization characteristic of lactobacillus in the gastrointestinal tract on the basis of green fluorescent protein | 2011 | Yanping Wang; Jingrui Wang; Jinju Wang | CN102604877 (A) |
| Prevention of bacterial adhesion1 | Prevention of adhesion of microorganisms on hard surfaces by the semi-permanent modification thereof during the cleaning process. A cleaning agent that contains surface-active polymers is used to prevent the bacterial colonization of hard surfaces | 2011 | Veith Birgit; Weide Mirko; Corbellini Francesca; Giesen Brigitte; Stumpe Stefan; Breves Roland; Barreleiro Paula; Karten Stefan; Bockmuehl Dirkl; Meier Frank | WO2012010700 (A1) |
| Pyruvate-ferredoxin oxidoreductase (PFO) adhesive protein as a target for inhibiting the adherence of Trichomonas vaginalis and as a diagnosis and vaccinal target for trichomoniasis1 | Novel function of PFO upon participating in the cytoadherence of the Trichomonas vaginalis parasite to the hosting cell. The present invention enables development of vaccines for preventing the adhesion (and therefore the colonization) of parasites to the vaginal mucosa | 2011 | Verastegui Rossana Arroyo | MX2011011361 (A) |
| Vacuum assisted percutaneous appliance1 | This device is stabilized by fibroblast in-growth and inhibits bacterial colonization | 2012 | Kantrowitz Allen B; Mortin Chris; Wadsworth JR Daniel C | US2013006186 (A1) |
Many bacteria have a specialized excretory system that resembles a syringe through which bacterial toxins (effector proteins) are injected into the host cell. These systems work by imitating host proteins, thus altering the signalling pathways and enabling development of the disease[8]. Three different secretion systems are implicated in the translocation of bacterial effectors into host cells, III, IV and VI[9].
The type III secretion system (TTSS) comprises some proteins that form a spire-like construction through which the bacteria inject the effector proteins from the bacterial cytoplasm to the cytoplasm of eukaryotic host cells. These secreted effector proteins often modify signal transduction in the host cells to improve microbial survival, invasion or attachment[10,11]. The type IV secretory system is utilized to transfer bacterial DNA or bacterial effector proteins to eukaryotic cells. This system also forms a duct between the bacterial and eukaryotic cell cytoplasm. It is a pilus-like structure rather than a spire construction[9]. Type VI secretion systems form tubular construction; however, exactly how these systems assemble and give effector proteins into the eukaryotic host cells stays in great measure unknown[12].
Although a lot of various types of TTSS are known, there are a restricted number of manners of inhibiting them: (1) prevention of assembly of the TTSS; (2) inhibition of interplay with the eukaryotic host cells; and (3) inhibition of secretion of the effector proteins.
Three components that are capable of inhibiting bacterial secretion systems have been reported[13]: (1) inhibitors of the type III secretion systems such as acylated hydrazones of salicylaldehydes in Chlamydia and Shigella infections; (2) 2-amino-5-arylidene thiazolidinone in Salmonella, Pseudomonas and Yersinia infections; and (3) dirylacrylonitrile, which inhibits sortase A and has shown in vitro activity against S. aureus.
A search of the patent database (up to April 2014) revealed 22 patents involving inhibition of the proteins related to secretion systems (Table 2). All of these are based on methods that describe how to identify inhibitors and target proteins of bacterial secretion systems. Two proteins groups are associated with these secretion systems: Inc and HpaB group proteins.
| Patent title | Description | Applicationdate | Inventors | Publicationnumber |
| Method for screening for inhibitors and activators of type III secretion machinery in Gram-negative bacteria | This invention relates to mutant strains of Gram-negative bacteria that constitutively secrete proteins via the type III secretion machinery and to methods of identifying molecules that are able to activate or inhibit secretion in wild-type strains of Gram-negative bacteria | 2001 | Demers Brigitte; Sansonetti Philippe; Parsot Claude | US6696249 (B1) |
| Method of detecting substance inhibiting type III secretion mechanism of bacterium and the function of secretory protein thereof | A method whereby a substance specifically inhibiting the type III secretion mechanism and the function of a type III secretory protein secreted therefrom can be detected in large amounts within a short period of time without depending on any animal infection experiments | 2001 | Omura Satoshi; Abe Akio | KR1020020086208 |
| Secreted Chlamydia polypeptides and method for identifying such polypeptides by their secretion by a type III secretion pathway of a Gram-negative bacteria | The present invention uses a heterologous secretion system, namely a type III system, to investigate whether some Chlamydia proteins, especially Inc proteins and other proteins exhibiting a similar hydropathy profile, might be secreted and demonstrates that these hybrid proteins are secreted by the type III secretion system of Shigella flexneri | 2003 | Subtil Agathe; Parsot Claude; Dautry-Varsat Alice | US2004131624 (A1) |
| Bacterial system for protein transport in eukaryotic cells | Development of a system for the targeted transport of proteins into eukaryotic cells by using a type III secretion system and bacteria strains that are mutated in hpaB or homogenous genes. The inventive bacterial system is used to transport bacterial proteins into eukaryotic cells, in order to influence or modify cellular processes such as gene expression, growth, development and defence/resistance mechanisms | 2005 | Bonas Ulla; Buettner Daniela | WO2005085417 (A2) |
| Methods of identifying modulators of bacterial type III protein secretion system | Provides methods for identifying inhibitors or activators of bacterial type III protein secretion system by using a recombinant beta-lactamase that can be secreted by a type III protein secretion system. The assay could be easily adapted to a high throughput mode to allow daily screening of several tens of thousands compounds | 2005 | Goldschmidt Raul; Loeloff Michael | WO2005113791 (A2) |
| Pharmaceutical composition for the treatment of bacterial infections and sepsis | The invention involves a pharmaceutical composition comprising at least one glycogen synthase kinase 3 β; inhibitor, at least one Rho-kinase inhibitor, and an optional adequate pharmaceutical carrier for producing a drug for the preventive or therapeutic treatment of bacterial infectious diseases by synergistically increasing synthesis and secretion of type II. A secretory phospholipase A2 into the bloodstream so as to boost the body's inherent resistance to infections | 2005 | Menschikowski Mario; Hagelgans Albert; Siegert Gabriele | WO2005120475 |
| Pyridone compounds as inhibitors of bacterial type III protein secretion systems | Provides compounds that inhibit type III protein secretion useful for the treatment and prevention of bacterial infections, particularly those caused by Gram-negative bacteria, and methods for their use | 2005 | Li Xiaobing | US2005256137 (A1) |
| Methods for stimulating an immune response using bacterial antigen delivery system | Provides methods for stimulating and/or increasing an immune response against tumor antigens through the use of the type III secretion system of bacteria. The invention also relates to the preparation of antigen presenting cells from peripheral blood mononuclear cells by using bacteria with a type III secretion system | 2006 | Old Lloyd J; Ritter Gerd; Nishikawa Hiroyoshi; Gnjatic Sacha; Galan Jorge E | US2009324651 (A1) |
| Screening system for inhibitors and activators of type III secretion machinery in Gram-negative bacteria | Provides a screening system (comprising inhibitors and activators of type III secretion machinery) that directly transfers pathogenic proteins of Gram-negative bacteria into a host cell to identify substances capable of activating or inhibiting the secretion of type III protein secretion system | 2006 | Hwang In Gyu; Moon Jae Sun; Kim Sung Uk | KR20080051240 (A) |
| Application of bovine lactoferrin for preparing a medicinal agent for inhibition of bacteria growth | The invention refers to a new application of bovine lactoferrin for preparing a medicinal agent for inhibiting bacteria growth. The bovine lactoferrin inhibits the growth of bacterial pathogens expressing the type III secretory system | 2007 | Makmakhon Robert Dzh; Kliari Tomas; Ochoa Tereza | RU2007140789 (A) |
| Bacterial secretion system and uses | - | 2007 | Gey Van Pittius Nicolaas Claudius; Warren Robin Mark; Van Helden Paul David | ZA200706520 (A) |
| Biopolymer and protein production using type III secretion systems of Gram-negative bacteria | Provides proteins, polynucleotide, expression cassette, vector and bacterium compositions for obtaining proteins of interest by expression of same in Gram-negative bacteria with a type III secretion system. Also provides uses for the proteins obtained in the manufacture of isolated proteins and pharmaceutical compositions | 2007 | Voigt Christopher Ashby; Widmaier Daniel Matthew | WO2008019183 (A2) |
| Use of the Pseudomonas syringae effector protein HopU1 related to its ability to ADP-ribosylate eukaryotic RNA binding proteins | The invention provides novel methods for modulation of the innate immune response of a plant to infection caused by Pseudomonas syringae, which injects effector proteins into host cells via a type III protein secretion system. Also provides methods for enhancing or suppressing the innate immune response of the plant | 2007 | Alfano James R; Fu Zheng Qing; Elthon Thomas E | WO2008042026 (A2) |
| Method and means for preventing and inhibiting type III secretion in infections caused by Gram-negative bacteria | Discloses a means of decreasing bacterial virulence in a mammal or in a plant by inhibition of the type III secretion system at concentrations that do not prevent or substantially reduce bacterial growth. Also disclosed are a therapeutic method and a pharmaceutical composition | 2008 | Elofsson Mikael | US2010099674 (A1) |
| Carboplatin compound inhibiting secretion system of phytopathogenic Gram-negative bacteria and biocontrol agent of plant diseases with this compound | Provides an agent for preventing plant diseases, containing carboplatin compounds, to selectively suppress secretion system related to plant pathogenicity | 2009 | - | KR20110048335 (A) |
| Inhibition of quorum sensing-mediated processes in bacteria | Provides methods for identifying molecules that can be used to positively and negatively manipulate quorum-sensing-mediated communication to control bacterial behavior. Methods of inhibiting quorum sensing-mediated activity in Gram-negative bacteria are provided wherein the activity is pathogenicity, bioluminescence, siderophore production, type III secretion, or metalloprotease production | 2009 | Bassler Bonnie; Swem Lee | US2011123586 (A1) |
| Type III secretion inhibitors, analogs and uses thereof | The invention relates to compounds and compositions useful for inhibiting type III secretion systems in pathogenic bacteria, such as Yersinia pestis, and uses of such inhibitors in the treatment and prevention of disease | 2009 | Goguen Jon; Pan Ning; Lee Kyungae | US2011034463 (A1) |
| 5-substituted-2-imino-thiazolidinone compounds and their use as inhibitors of bacterial infection1 | Provides a method for inhibiting Gram-negative bacterial pathogenesis, a method of screening for compounds that inhibit type III secretion in Gram-negative bacteria, and compounds that inhibit type III secretion in Gram-negative bacteria | 2010 | Felise Heather B; Miller Samuel I; Kline Toni | US2011039849 (A1) |
| Methods for Identifying Inhibitors of the type III Secretion System1 | Provides a method for determining whether a test compound can inhibit the function of the type III secretion system. The method identifies drug candidates that are highly specific anti-bacterial agents for treating diseases caused by Gram-negative bacteria with a T3SS | 2010 | Marlovits Thomas C; Radics Julia; Schmied Wolfgang | US2013130283 (A1) |
| Attenuated Salmonella inducible secretory expression oral vaccine presentation system and application there of1 | The invention comprises an attenuated salmonella inducible secretory expression oral vaccine presentation system containing an antigen expression carrier. The system is controlled by a promoter induced by a microenvironment in an antigen presenting cell and excreted by induction of a bacteria excretion signal, and it uses the attenuated salmonella as the host of the antigen expression carrier | 2011 | Zichun Hua; Guo Chen | CN102335421 (A) |
| Bacterial mediated delivery of nuclear protein into pluripotent and differentiated cells1 | A modified Pseudomonas aeruginosa type III secretion system has been developed that efficiently delivers selected proteins into a host cell | 2011 | Jin Shouguang; Bichsel Candace | WO2012012605 (A2) |
| Inhibitors of bacterial type III secretion system1 | Discloses organic compounds showing the ability to inhibit effector toxin secretion or translocation mediated by bacterial type III secretion systems. These inhibitor compounds are useful for combating infections by Gram-negative bacteria with such type III secretion systems | 2012 | Moir Donald T.; Aiello Daniel; Peet Norton P; Williams John D; Torhan Matthew | US2014142134 (A1) |
Cell-cell communication, or quorum sensing (QS), is a widespread phenomenon in bacteria that is used to coordinate gene expression between local populations[13]. Bacterial populations can use QS communication to coordinate the execution of important biological functions, many of which are involved in pathogen virulence, e.g., biofilm formation, extracellular polysaccharide production, host colonization, motility, bioluminescence, transfer of plasmids by conjugation, and biosynthesis of antibiotics and siderophores.
All QS systems utilize small, secreted signalling molecules known as autoinducers (AIs): (1) AI-1 molecules are N-acyl-homoserine lactones (AHLs); (2) AI-2 molecules are heterocyclic furanosyl-borates; (3) AI-3 signals are catecholamines and finally; and (4) AI-4 signals are cyclic peptides. Some other QS signals go beyond these classes, e.g., Pseudomonas quinolone signal and diffusible signal factor. New molecules will undoubtedly be discovered as the study of QS expands to species of bacteria yet to be investigated.
Targeting bacterial virulence (quorum quenchers, Figure 1) is an alternative focusing to antimicrobial therapy that offers a hopeful opportunity to inhibit pathogenesis and its consequences without producing immediately the death the target bacterium. Bacterial virulence factors have been shown to be potential targets for drug design and therapeutic intervention for Gram-negative pathogens[1]. Numerous quorum sensing inhibitors have been reported in the literature[1,2].
In 2012, Romero et al[14] published an article about patents concerning quorum quenching (QQ) (i.e., the mechanisms that cause the inactivation of QS communication systems)[15].
A search of patent databases (up to August 2011) revealed a total of 45 applications related to strategies for interfering with QS systems as a method of fighting microbial infections. Following the bias in the literature, the vast majority of the patented technologies based on the inhibition of QS mechanisms target AHL signals, whereas only 5 out of 45 patent cases are based on the inhibition of AI-2 signals and only 4 are based on the inhibition of peptide-based QS signals from Gram-positive bacteria. In this review, a search of more recent reports (up to April 2014) was conducted, revealing 32 patents concerning the inhibition of QS systems (Table 3). QQ occurs in Lysobacter enzymogenes, Shewanella piezotolerans, Bacillus pumillus, Tenacibaculum discolor (cect 7426) and novel alpha-proteobacteria. The molecules involved in QQ distinguish inhibitors of the AI-2 signals (triazol derivates, furan compounds and phosphorylated, branched dihydroxy-pentane-dione) and inhibitors of AHLs [(oxododecanoyl)-L-homoserine lactone and bicyclic furanones with low toxicity]. Furanones, which are naturally occurring compounds, appear to be the most widely studied QQ compounds. These compounds are toxic to Artemia and rotifers, which will limit their use in humans[15]. However, the use of C-30, a synthetic furanone, at non toxic concentrations, significantly reduced the pathogenicity of Vibrio anguillarum in rainbow trout[16]. Other patented compounds involved in QQ include honaucin A, 2-methylthiopyrrolidines, lovastatin and hydroxytirosol. Finally, one enzyme (OLB-26) is known to be involved in QQ.
| Patent title | Description | Application date | Inventors | Publication number |
| Anti-inflammatory and quorum sensing inhibition compounds and methods of making and using them | This invention generally relates to novel compositions based on a structure designated as “Honaucin A”, including Honaucin A variants and analogs, and pharmaceutical compositions, liposomes and nanoparticles comprising them, and methods of making and using them | 2011 | Gerwick William H; Gerwick Lena; Choi Huykjae; Villa Francisco A; Smith Jennifer; Rowley David C | WO2011153502 (A2) |
| Composition for oral use | A method for suppressing dental caries by regulating biofilm formation by the bacteria that cause dental caries instead of controlling these bacteria | 2011 | Tsugane Takanori; Saeki Yoji | EP2620160 |
| Conjugates of acyl homoserine lactone and catalase a from Pseudomonas aeruginosa | The present invention relates to the acyl homoserine lactone N-3-(oxododecanoyl)-L-homoserine lactone or butyryl L-homoserine lactone and Pseudomona aeruginosa KatA protein, or an antigenic portion conjugate thereof, used to treat Pseudomona aeruginosa infections by limiting biofilm formation and inhibiting a range of quorum-sensing dependent virulence factors | 2011 | Kyd Jennelle M; Cooley Margaret | WO/2012/083382 |
| Enzyme bag containing quorum quenching enzyme immobilized silica for inhibiting biofilm formation and membrane bioreactor system for water treatment system using the bag | This invention relates to an enzyme bag containing silica-immobilized enzyme for inhibiting biofilm formation. A membrane bioreactor system for water treatment using the bag is provided for stable implementation of filtering operations over a long period of time by improving the performance of operational processes | 2011 | Lee Chung Hak; Yang Cheon Seok; Lee Jung Kee; Han Jong Yun; Lee Chung Hak; Yang Cheon Seoket; Lee Jung Kee; Han Jong Yun | KR20120134724 (A) |
| Fluidizable carrier with biofilm formation-inhibiting microorganisms immobilized therein and membrane water treatment apparatus using the same | This invention relates to a biofilm formation-inhibiting microorganism immobilized fluidizable carrier in which a biofilm formation-inhibiting microorganism is fixed therein and a membrane water treatment apparatus including the same are provided to increase a membrane cleaning period | 2011 | Lee Chung Hak; Kim Sang Ryoung; Lee Jung Kee | KR20130034935 (A) |
| Methods of disrupting quorum sensing to affect microbial population cell density | The invention relates to the modulation of quorum sensing mechanisms in a microorganism for the purpose of exploiting the fermentation capabilities of the microorganism | 2011 | Marrs Barry; Swalla Brian M | US2011124522 (A1) |
| Quorum-sensing signal molecular preparation and application thereof in tobacco waste treatment | The invention relates to the field of environmental biotechnology, in particular to preparation of a quorum sensing signal molecule and application in processing tobacco waste | 2011 | Meizhen Wang; Hongzhen He; Huajun Feng; Xin Zheng; Dongsheng Shen; Zhenmei LV; Hang Min | CN102392051 (A) |
| Synthetic analogs of bacterial quorum sensors | The invention relates to synthetic analogs of bacterial quorum sensing molecules, and methods of use of these | 2011 | Iyer Rashi; Ganguly Kumkum; Silks Louis A | US2012071430 (A1) |
| System and method for reversing the antibiotic tolerance of bacterial persister cells | The present invention relates to antibiotics and, more particularly, to a system and method for decreasing the tolerance of bacterial persister cells to antibiotics | 2011 | Ren Dacheng; Pan Jiachuan | EP2603576 |
| Triazole compounds as well as preparation method and application thereof | The present invention relates to triazole derivatives, the preparation method and as the autoinducer-2 (AI-2) quorum sensing inhibitors, belonging to anti-AI-2 quorum sensing type drug technology | 2011 | Minyong Li; Lvpei Du; Peng Zhu | CN102219753 (A) |
| Use of a novel alpha-proteobacteria for quorum quenching | The invention (concerning the fields of biology, molecular biology, and aquaculture) specifically relates to a new a-proteobacteria capable of degrading/V-acyl-homoserine lactones (AHLs) for control of bacterial infectious diseases and prevention of biofilm formation | 2011 | Otero Casal Ana María; Romero Bernardez Manuel | WO2011154585 (A1) |
| 2-methylthiopyrrolidines and their use for modulating bacterial quorum sensing | Formula (I) compounds are disclosed and their use in inhibiting quorum sensing in bacteria is reported | 2012 | Malladi Venkata L; Schneper Lisa; Sobczak Adam J; Mathee Kalai; Wnuk Stanislaw F | WO/2012/174511 |
| Compositions for regulating or modulating quorum sensing in bacteria, methods of using the compounds, and methods of regulating or modulating quorum sensing in bacteria | The report encompasses compounds and compositions that are useful as specific AI-2 antagonists for the control of bacterial quorum sensing and methods for inhibiting or attenuating microbial virulence, biofilm formation and drug resistance | 2012 | Wang Binghe; Ni Nantin; Wamg Junfeng; Lu Chung-Dar; Chou Han-Ting; Li Minyong; Zheng Shilong; Cheng Yunfeg; Peng Hanjing | EP2529793 (A2) |
| Construction and application of unmarked Lysobacter enzymogenes engineering strain capable of preventing plant bacteriosis | The invention (within the field of microbial genetic engineering), specifically relates to a plant bacterial disease that can prevent dissolving enzyme production strains of Bacillus unmarked engineering construction and application | 2012 | Liu Fengquan; Qian Guoliang | CN102943061 (A) |
| Furan compound and preparation method and application of furan compound | The invention relates to furan derivatives, the method of preparation and as the AI-2 quorum sensing inhibitors, are anti-AI-2 type of quorum sensing | 2012 | Minyong Li; Peng Zhu | CN102603683 (A) |
| Method for increasing output of microbial lovastatin based on quorum sensing mechanism | The invention relates to the pharmaceutical raw material fermentation industry, in particular to a method of increasing microbial production of lovastatin | 2012 | Li Haoming | CN102925509 (A) |
| Method for quickly identifying food-borne pathogen bacterial biofilm formation inhibitor | The invention relates to the field of food microbiology control technology, in particular to the rapid identification of an inhibitor of foodborne bacterial biofilm formation | 2012 | Wenyan Zhang; Hongmei Zhang; Zhihua Tao; Wenyuan Zhou | CN102706821 (A) |
| Phosphorylated and branched dihydroxy-pentane-ione analogs as quorum sensing inhibitors in bacteria | The invention provides compositions and methods for modulating quorum sensing in microbes and can be used in prophylactic methods or therapy for bacterial infections and for reduction of biofilms. The compounds are AI-2 analogs and as such have structures similar to 4,5-dihydroxy-2,3-pentanedione that can act as agonists/antagonists of quorum sensing | 2012 | Sintim Herman; Bentley William E; Roy Yarnika; Smith Jacqueline | US2012294900 (A1) |
| Preparation method of imprinted polymer of bacterial quorum sensing signal molecule AI-1 | The present invention relates to bacterial quorum sensing signal molecules A1-1 imprinted polymer preparation | 2012 | Xin Li; Ling Wang | CN102604010 (A) |
| Probiotics for biological control against Vibrio sp. | The invention relates to probiotics for biological control against Vibrio sp., and in particular, to a newly isolated bacillus strain that degrades quorum-sensing signal molecules of the pathogenic bacteria Vibrio sp., and inhibits biofilm formation | 2012 | Yang Si Yong; Woo Seo Hyung; Kang In Hye; Im Hyun Jung | WO2012105805 |
| Quorum sensing inhibitor against a pathogenic microorganism, and an antibacterial composition using the same | A quorum sensing inhibitor and an antibacterial composition using the same are provided to suppress quorum sensing between bacteria, and to prevent or treat infection or diseases | 2012 | Undescribed | KR101243696 |
| Shewanella piezotolerans 34# and application thereof to algae inhibition | The invention relates to the field of biotechnology, in particular to a marine bacterium Shewanella and the inhibition of algal growth | 2012 | Zhou Jin; Yin Peng | CN103173383 (A) |
| Simple method for testing disease resistance of pathogenic bacteria quorum-quenching gene prokaryotic expression product | The invention relates to a prokaryotic expression product of disease resistance testing methods, in particular to test pathogen populations prokaryotic expression product quenching effect of a simple disease, and belongs to the field of gene function identification techniques | 2012 | Ouyang Lejun; Huang Zhenchi; Zeng Fuhua; Li Limei; Li Heng | CN102972220 (A) |
| Use of quorum sensing inhibitors and biofilm dispersing agents for controlling biofilm-associated implantable medical device related infections | The invention generally relates to implantable medical devices and, more specifically, to the use of quorum sensing inhibitors and/or biofilm dispersing agents to control biofilm-associated infections related to the use of implantable medical devices | 2012 | Samade Richard; Dinesh Prashant; Nabutovsky Yelena; Bornzin Gene A; Poore John W; Karicherla Annapurna; Dalal Nirav | US2014005605 (A1) |
| Bacillus pumillus microbial preparation with quorum sensing system inhibiting effect | The invention relates to Bacillus pumillus with a quorum sensing system inhibiting effect. The invention has the advantages that Chromobacterium violaceum is used for screening out a bacterial strain F3-1. It can be used to produce a microbial preparation capable of preventing and treating aquatic bacterial diseases | 2013 | Song Zengfu; Fan Bin; Chen Biao | CN103525723 (A) |
| Bicyclic furanones with low toxicity for microbial control | The invention relates to a class of bicyclic brominated furanone structures with reduced toxicity and high activity for inhibiting biofilm formation and quorum sensing by microbes | 2013 | Luk Yan-Yeung; Yang Sijie | US2013197077 (A1) |
| Method for detecting quorum sensing quenching bacterial strain | The invention relates to a method for detecting a quorum sensing quenching bacterial strain. The method comprises the addition of a bacterial strain to be detected in a PIPES (1,4-piperazinediethanesulfonic acid) buffer solution of pH 6 | 2013 | Zhang Xiaohua; Tang Kaihao; Shi Xiaochong; Zhang Yunhui | CN103215342 (A) |
| Quorum-quenching enzyme OLB-26, and coding gene and application thereof | The invention relates to a quorum sensing quenching enzyme 0LB-26 and its coding gene and application | 2013 | Zhou Zhigang; Zhang Meichao; Yang Yalin; Xu Li; He Suxu; Li Qing; Yu Qiang | CN103275949 (A) |
| Targeted enzymatic degradation of quorum-sensing peptides | The present invention generally relates to the fields of microbiology and wound care. More particularly, it concerns methods and compositions for inhibiting biofilms in wounds and on medical devices | 2013 | Alarcon Rodolfo; Mcnulty Amy K | US20130253382 |
| Use of ellagitannins as inhibitors of bacterial quorum sensing | Materials and methods for the inhibition of bacterial QS are described. Methods of treating bacterial infections by administration of one or more ellagitannins in amounts effective for inhibiting bacterial QS are also provided | 2013 | Athee Kalai; Adonizio Allison L; Ausubel Frederick; Clardy Jon; Bennett Bradley; Downum Kelsey | US2013317094 (A1) |
| Use of hydroxytirosol and derivatives thereof as quorum quenchers | The quorum quenching activity of formula (I) or (II) compounds, such as hydroxytyrosol (HT), hydroxytyrosol acetate (HTA), 3,4-dihydroxyphenylacetic acid (DOPAC) and derivatives thereof are described | 2013 | Au Ón Calles David; Allende Prieto Ana; Fábregas Casal Jaime; Gómez-Acebo Gullón Eduardo | WO2014060581 (A1) |
| Use of the cect 7426 strain for generating quorum quenching of the autoinducer-2 signal (ai-2) | The invention relates to the use of a bacterial strain of the species Tenacibaculum discolor in the control of infectious diseases and for inhibiting biofilm formation caused by bacteria, through the inhibition of quorum sensing signals type AI -2. The invention applies to the field of molecular biology | 2013 | Otero Casal, Ana María; Romero Bernárdez Manuel | WO2014057151 (A1) |
Multidrug resistance (MDR) efflux pumps have multiple functions in natural microbial ecosystems. In clinical environments, MDR pumps are implicated in the following: (1) resistance to antimicrobial compounds localized on mucosal surfaces (colonization factor)[17,18]; (2) efflux virulence factors[19]; (3) QS-regulated expression of virulence traits[20]; and (4) antibiotic resistance, which is a code element in patients under treatment[21]. All of these roles are important for the survival, colonization and pathogenic outcome of virulent bacteria in clinical environments. In nonclinical environments, MDR pumps may be associated to resistance to heavy metals[22] and organic solvents[23] (plant colonization factor). Bacterial efflux pumps are classified into five families according to their composition, number of transmembrane spanning regions, energy sources and substrates: the resistance-nodulation-division (RND) family, the major facilitator superfamily, the adenosine triphosphate-binding cassette superfamily, the small multidrug resistance family, and the multidrug and toxic compound extrusion family[17,18].
Several MDR pump inhibitors were published[24]. We consider two examples of RND inhibitors pumps: (1) 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-β-naphthylamide. These act as inhibitors of RND efflux pumps and virulence traits in Vibrio cholerae, such as the cholera toxin and the toxin-coregulated pilus[25], and were suggested as a suitable tool for the treatment of cholera infections; and (2) Of 12 trifluoromethyl ketone compounds tested, 6 proved to be effective inhibitors of the quorum-sensing response by Chromobacterium violaceum 026, as well as inhibitors of the RND efflux pumps of CV026 and E. coli. This result is of clinical applicability and may be used for the prevention of QS responses of infecting bacteria[26].
We found 22 patents related to the inhibition of the MDR efflux in patent databases up to April 2014 (Table 4). Most of these are screening methods for microbial efflux pump inhibitors. Moreover, pump inhibitors are described as potentiators of the action of antiseptics, disinfectants and antimicrobial agents such as tigecycline. Finally, polyamine molecules are inhibitors of bacterial efflux pumps that could be used in combination with other drugs such as antibiotics, as well as pharmaceutical compositions thereof.
| Patent title | Description | Application date | Inventors | Publication number |
| Method for screening for non-tetracycline efflux pump inhibitors | Provides screening methods for inhibitors of microbial efflux pumps and pharmaceutical compositions containing such efflux pump inhibitors as well as methods for treating microbial infections by use of these compositions | 1995 | Trias; Joaquim Chamberland; Suzanne Hecker; Scott J Lee; Ving J | US5989832 A |
| Efflux pump inhibitors | Provides screening methods for inhibitors of microbial efflux pumps and pharmaceutical compositions containing such efflux pump inhibitors. Also provides methods for treating microbial infections using those compositions | 1996 | Trias Joaquim; Hecker Scott J; Chamberland Suzanne; Lee Ving J | CA2217865 |
| Methods and compositions for reducing bacterial tolerance of disinfectants and organic solvents | Methods and compositions useful for manipulating bacterial resistance to non-antibiotic antibacterial compositions, disinfectants and organic solvents, and for rendering bacterial cells susceptible to non-antibiotic antibacterial compositions | 1997 | Levy Stuart B | WO9917607 (A2) |
| Methods and compositions for reducing bacterial tolerance to antibacterials, disinfectants and organic solvents | Methods and compositions useful for manipulating bacterial resistance to non-antibiotic antibacterial compositions, disinfectants and organic solvents, and methods for rendering bacterial cells susceptible to non-antibiotic antibacterial compositions | 1997 | Levy Stuart B | US6068972 (A) |
| Inhibitors of cellular efflux pumps of microbes | Describes compounds that are inhibitors of efflux pump in bacteria. Also describes methods of preparing and using such compounds and the pharmaceutical compositions that include such compounds | 2001 | De Souza Noel John; Patel Mahesh Vithalbhai; Gupte Shrikant V; Upadhyay Dilip J; Shukla Milind Chintaman; Chaturvedi Nishith C; Bhawsar Satish B; Nair Sheela Chandresekharan; Jafri Mohammed A; Khorakiwala Habil Fakhruddin | US2002177559 (A1) |
| Drug discovery and increased potency of antiseptics and disinfectants based on high extracellular pH, the disablement of cellular efflux pumps, and the unexpected synergism therebetween | Methods for increasing the therapeutic potency of amphipathic compounds, e.g., antiseptics and disinfectants, and the exploitation of these discoveries in the screening of small molecules, and libraries thereof, for biological activity in prokaryotes and eukaryotes | 2002 | Lewis Kim; Hsieh Peichung | US2003118541 (A1) |
| Methods to study and mechanisms of biofilm-related antibiotic resistance | Discloses various genes that encode proteins shown to play a role in microbial resistance of an organism in a biofilm and homologs thereof. Describes methods of identifying a compound that modulates microbial resistance of an organism in a biofilm and also genes encoding proteins that play a role in biofilm resistance | 2002 | O'Toole George A; Mah Thien-Fah | US2003166030 (A1) |
| Minicell-based gene therapy | Provides compositions and methods for the production of achromosomal and anucleate cells useful for applications such as diagnostic and therapeutic uses, as well as research tools and agents for drug discovery | 2002 | Sabbadini Roger A; Berkley Neil; Surber Mark W | US2003199088 (A1) |
| Potentiators of antibacterial activity | The invention relates to compounds that potentiate the activity of antibacterials, compositions useful in treating bacterial infection, and related methods. Also relates to a method of inhibiting bacterial efflux of an antibiotic, thereby increasing its efficacy | 2004 | Grossman Trudy H | US20040887719 20040709 |
| Substituted polyamines as inhibitors of bacterial efflux pumps | Methods of treating bacterial infections, including those caused by multidrug resistant bacteria, by using polyamine efflux pump inhibiting compounds, optionally in combination with other drugs such as antibiotics. The pharmaceutical compositions of the polyamines are also reported | 2004 | Nelson Mark L; Alekshun Michael N | US2004204378 (A1) |
| Essential and important genes of Pseudomonas aeruginosa and the use thereof to design or identify antibacterial agents | Database of candidate essential genes in Pseudomonas aeruginosa, and other important genes that, when mutated, produce a growth attenuated phenotype. The invention includes methods for confirming the need for or importance of candidate genes, methods for using those genes to screen for new antibacterial drugs, the antibacterial agents identified using the disclosed methods, and also methods of using the same for treating and preventing Pseudomonas infection | 2005 | Bruce Kim F; Warrener Paul; Hou Kevin | US2007196829 (A1) |
| Method for increasing the susceptibility of peptide deformylase inhibitors by using efflux pump inhibitors | Provides methods and compositions for increasing the susceptibility of PDF inhibitors against Gram-negative organisms by using efflux pump inhibitors | 2005 | Dean Charles Richard; Ryder Neil Stewart | CA2569681 |
| Products and methods for in vivo secretion of monatin | Provides products and methods for the in vivo production of monatin sweetener. The products include microorganisms that are genetically modified to secrete or to improve secretion of monatin, to produce monatin, and to both secrete/ improve secretion and produce monatin | 2006 | Laplaza Jose; Anderson James C; Desouza Mervyn L; Hicks Paula M; Kollmann Sherry R | WO2006113897 |
| Rhamnose-inducible expression systems and methods | Describes rhamnose-inducible expression constructs which may include at least one rhamnose-inducible regulatory element expressing a regulatory protein and one promoter that is inducible by the regulatory protein. An open reading frame expressing a protein of interest may be placed under control of the promoter. Also describes optimized Shine-Dalgarno sequences for use with the promoter | 2006 | Surber Mark W | US2007122881 (A1) |
| Enhancement of tigecycline potency using efflux pump inhibitors | Discloses Efflux Pump Inhibitor (EPI) compounds that can be co-administered with antimicrobial agents for the treatment of infections caused by drug resistant pathogens and methods of treatment and pharmaceutical compositions for co-administering tigecylcine with an EPI | 2007 | Glinka Tomasz; Bostian Keith; Lomovskaya Olga; Surber Mark; Sun Dongxu | US20070832626 20070801 |
| Method or agent for inhibiting the function of efflux pump of Pseudomonas aeruginosa | Discloses a method comprised of modifying any amino acid residue selected from 100th to 109th and 311th to 320th amino acid residues in an amino acid sequence for mature OprM protein, as well as an agent for inhibiting the function of an efflux pump of Pseudomonas aeruginosa with good efficiency. Further disclosed is a method for screening the agent | 2007 | Yoshihara Eisaku; Inoko Hidatoshi | CA2641988 |
| Method or agent for inhibiting the function of efflux pump of Pseudomonas aeruginosa | Provides a method for inhibiting the function of the drug efflux pump of Pseudomonas aeruginosa, comprising modification of any amino acid residue selected from 100th to 109th or 311th to 320th amino acid residues in the amino acid sequence of mature OprM protein. Also reports an agent with such inhibitory effect, as well as a screening method | 2007 | Yoshihara Eisaku; Inoko Hidatoshi | WO2007/091395 |
| Microbial production of aromatic acids | Method for the microbial production of aromatic acids from a fermentable carbon substrate using a host cell capable of producing said aromatic acid, and comprising an efflux pump for said aromatic acid. A preferred host cell comprises a member of the proton-dependent resistance/nodulation/cell division (RND) family of efflux pumps | 2007 | Wery Jan | US2007259409 (A1) |
| Near-infrared electromagnetic modification of cellular steady-state membrane potentials | Discloses systems and methods for applying near-infrared optical energies and dosimetries to alter the bioenergetic steady-state trans-membrane and mitochondrial potentials (DeltaPsi-steady) of all irradiated cells through an optical depolarization effect. This membrane depolarization provides the ability to potentiate antimicrobial, antifungal and/or antineoplastic drugs against only targeted undesirable cells | 2007 | Bornstein Eric | US2008139992 |
| In vivo gene sensors | Describes methods and compositions for the detection of target genes that permit the selective expression of an effector gene in those cells expressing the target gene, thus selectively targeting these cells for treatment or elimination. The methods and compositions described may also permit the selective expression of an agent such as a therapeutic gene product, in a targeted population of cells | 2009 | Collins James J; Lu Timothy Kuan-Ta | WO2009/137136 |
| Methods of reducing microbial resistance to drugs | Provides methods of treating infection, screening for inhibitors of AcrAB-like efflux pumps, and enhancing antimicrobial activity of drugs. Pharmaceutical composition comprising an inhibitor of an AcrAB-like efflux pump and an antimicrobial agent are also provided | 2009 | Oethinger Margaret; Levy Stuart B | US2009298873 |
| Minicells displaying antibodies or derivatives thereof and comprising biologically active compounds1 | Minicells are used to deliver biologically active compounds, including polypeptides, nucleic acids, small molecules, drug molecules, and chemotherapeutic agents. In some cases, the minicell displays ligands or binding moieties that target the minicell to a desired host cell | 2011 | Sabbadini Roger A; Berkley Neil; Surber Mark W; Klepper Robert | US20070725196 20070316 |
Although antivirulence therapies are novel in the field of treatment of infectious diseases, several studies involving clinical strains have demonstrated the development of mechanisms of resistance, especially to Quorum Quenching compounds[27]. Vibrio cholerae strains that are resistant to virstatin have also been described; the mechanisms whereby these strains colonize ther hosts are independent of the elaboration of the toxin co-regulated pilus[28].
The first evidence that cells develop resistance to QQ compounds has been reported by Maeda et al[29] (published ahead of print in 2011). These authors worked with a concentration of brominated furanone C-30 [the gold standard for QQ compounds, and which is a synthetic brominated furanone 4-bromo-5-(bromomethylene)- 2(5H)-furanone] that did not influence growth in rich medium (i.e., it did not inhibit growth) and utilized both transposon mutagenesis and spontaneous mutants to detect resistant bacteria.
The mechanism of this resistance was overexpression of the MexAB-OprM multidrug resistance operon due to mutations in the gene repressors mexR and nalC, resulting in efflux of the compound C-30. This quorum quenching compound showed a reduced capacity to decrease some QS-controlled virulence traits and phenotypes in the mexR mutant, and the pathogenicity of the mexR mutant against the nematode Caenorhabditis elegans was not decrease by the inclusion of C-30. Importantly, these authors also worked with cells from cystic fibrosis patients (Liverpool epidemic strain 12142) with mexR and nalC mutations[30] to demonstrate that, even in the absence of the QS inhibitor, cells develop resistance to quorum quenching compounds in the pathogenic state when coexisted with the pressures of antibiotic treatment; so, antimicrobial treatment can induce to resistance to QQ compounds.
Intensified efforts are needed to establish whether resistance may develop to other QQ compounds, as is the case of lactonase or acylase enzymes in connection with AHL autoinducers.
Antimicrobial resistance in nosocomial pathogens is increasing worldwide. Mortality rates of patients infected with drug-resistant pathogens have increased by approximately 50% in recent years. Hospitals have become breeding grounds for extremely resistant pathogens, exacerbating the risk associated with hospitalization. These pathogens are extremely resistant to last line antimicrobials. If the current trend continues, the beginning of a “post-antibiotic era” is predicted. Development of novel antibiotics has almost totally ceased, at least against Gram-negative bacteria, and the prospects for a reversal of this trend are bleak. It is therefore imperative to develop new molecules, therapies and/or new combinations of these for the eradication of resistant pathogens. In this review, we discuss some examples of patented molecules that act by inhibiting different bacterial virulence mechanisms (adhesion/colonization and quorum sensing mechanisms, and secretory and efflux pump systems) and which open the way to studying potential new treatments for infections caused by multiresistant bacteria.
Novel targets and molecules must be discovered for antivirulence therapies, taking into account the possible development of resistance mechanisms. Further study of combinations of these compounds with other antimicrobials for the treatment of infectious diseases is also important.
P- Reviewer: Galgoczy L, Hays J, Zhang ZM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1008] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 2. | Beceiro A, Tomás M, Bou G. Antimicrobial resistance and virulence: a successful or deleterious association in the bacterial world? Clin Microbiol Rev. 2013;26:185-230. [PubMed] |
| 3. | Proft T, Baker EN. Pili in Gram-negative and Gram-positive bacteria - structure, assembly and their role in disease. Cell Mol Life Sci. 2009;66:613-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 351] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 4. | Svensson A, Larsson A, Emtenäs H, Hedenström M, Fex T, Hultgren SJ, Pinkner JS, Almqvist F, Kihlberg J. Design and evaluation of pilicides: potential novel antibacterial agents directed against uropathogenic Escherichia coli. Chembiochem. 2001;2:915-918. [PubMed] |
| 5. | Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci USA. 2007;104:2372-2377. [PubMed] |
| 6. | European Patent Office. Espacenet: Patent search. Available from: http: //worldwide.espacenet.com. |
| 7. | United States Patent and Trademark Office. Patent Full-Text and Image Database. Available from: http: //patft.uspto.gov/netahtml/PTO/search-adv.htm. |
| 8. | Galán JE, Cossart P. Host-pathogen interactions: a diversity of themes, a variety of molecular machines. Curr Opin Microbiol. 2005;8:1-3. [PubMed] |
| 9. | Filloux A, Hachani A, Bleves S. The bacterial type VI secretion machine: yet another player for protein transport across membranes. Microbiology. 2008;154:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 274] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 10. | Keyser P, Elofsson M, Rosell S, Wolf-Watz H. Virulence blockers as alternatives to antibiotics: type III secretion inhibitors against Gram-negative bacteria. J Intern Med. 2008;264:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Stavrinides J, McCann HC, Guttman DS. Host-pathogen interplay and the evolution of bacterial effectors. Cell Microbiol. 2008;10:285-292. [PubMed] |
| 12. | Filloux A. The type VI secretion system: a tubular story. EMBO J. 2009;28:309-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269-275. [PubMed] |
| 14. | Romero M, Acuña L, Otero A. Patents on quorum quenching: interfering with bacterial communication as a strategy to fight infections. Recent Pat Biotechnol. 2012;6:2-12. [PubMed] |
| 15. | Dong YH, Wang LY, Zhang LH. Quorum-quenching microbial infections: mechanisms and implications. Philos Trans R Soc Lond B Biol Sci. 2007;362:1201-1211. [PubMed] |
| 16. | Rasch M, Buch C, Austin B, Slierendrecht WJ, Ekmann KS, Larsen JL, Johansen C, Riedel K, Eberl L, Givskov M. An inhibitor of bacterial quorum sensing reduces mortalities caused by Vibriosis in rainbow trout (Oncorhynchus mykiss, Walbaum). Syst Appl Microbiol. 2004;27:350-359. [PubMed] |
| 17. | Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infect Immun. 2003;71:5576-5582. [PubMed] |
| 18. | Martinez JL, Sánchez MB, Martínez-Solano L, Hernandez A, Garmendia L, Fajardo A, Alvarez-Ortega C. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev. 2009;33:430-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, Kamihira S, Hancock RE, Speert DP. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J Exp Med. 2002;196:109-118. [PubMed] |
| 20. | Pearson JP, Feldman M, Iglewski BH, Prince A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infection. Infect Immun. 2000;68:4331-4334. [PubMed] |
| 21. | Martínez JL, Baquero F. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev. 2002;15:647-679. [PubMed] |
| 22. | Silver S, Phung LT. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753-789. [PubMed] |
| 23. | Ramos JL, Duque E, Gallegos MT, Godoy P, Ramos-Gonzalez MI, Rojas A, Teran W, Segura A. Mechanisms of solvent tolerance in gram-negative bacteria. Annu Rev Microbiol. 2002;56:743-768. [PubMed] |
| 24. | Kourtesi C, Ball AR, Huang YY, Jachak SM, Vera DM, Khondkar P, Gibbons S, Hamblin MR, Tegos GP. Microbial efflux systems and inhibitors: approaches to drug discovery and the challenge of clinical implementation. Open Microbiol J. 2013;7:34-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 25. | Bina XR, Philippart JA, Bina JE. Effect of the efflux inhibitors 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide on antimicrobial susceptibility and virulence factor production in Vibrio cholerae. J Antimicrob Chemother. 2009;63:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Varga ZG, Armada A, Cerca P, Amaral L, Mior Ahmad Subki MA, Savka MA, Szegedi E, Kawase M, Motohashi N, Molnár J. Inhibition of quorum sensing and efflux pump system by trifluoromethyl ketone proton pump inhibitors. In Vivo. 2012;26:277-285. [PubMed] |
| 27. | García-Contreras R, Maeda T, Wood TK. Resistance to quorum-quenching compounds. Appl Environ Microbiol. 2013;79:6840-6846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Shakhnovich EA, Sturtevant D, Mekalanos JJ. Molecular mechanisms of virstatin resistance by non-O1/non-O139 strains of Vibrio cholerae. Mol Microbiol. 2007;66:1331-1341. [PubMed] |
| 29. | Maeda T, García-Contreras R, Pu M, Sheng L, Garcia LR, Tomás M, Wood TK. Quorum quenching quandary: resistance to antivirulence compounds. ISME J. 2012;6:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Tomás M, Doumith M, Warner M, Turton JF, Beceiro A, Bou G, Livermore DM, Woodford N. Efflux pumps, OprD porin, AmpC beta-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother. 2010;54:2219-2224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |