INTRODUCTION
It is widely accepted that breastfeeding is the best way of ensuring a good start in an infant’s life as it not only has a favourable nutrient content but also provides passive immunity and various growth hormones to the breastfed infant[1-4]. However, there is always concern regarding the transfer of medications taken by the mother to the infant via breast milk. To understand the safety of medicines in a nursing mother, it is important to elucidate the mechanism of such drug transfer.
The major determinant of the transferability of drugs from mother to baby via breast milk is usually calculated using parameters such as the physicochemical properties of the drug and the composition of the milk[4]. However, research has shown that active transport via efflux transporters may have a significant role in the transfer of drugs from the maternal plasma to breast fed infant. It has been shown that breast cancer resistance protein (BCRP) (also known as ABCG2), belonging to the ATP-binding cassette (ABC) superfamily of transporters is strongly induced during lactation[5]. There are also many other transporters belonging to the ABC family such as P-glycoprotein (P-gp) (also known as ABCB1 or MDR1) which like BCRP have some toxicological significance in lactation. Other transporters belonging to the solute carrier family such as the organic cation and anion transporters, peptide and nucleoside transporters also found in the mammary gland play an important role in the active transport of many nutrients, endogenous substances and xenobiotics[6].
The protective role of P-gp in the blood brain barrier is well established. It inhibits a wide variety of substrates from entering the central nervous system[7]. The main focus of research has so far been on the role of these transporters in the gastrointestinal tract and the blood brain barrier (BBB) affecting bioavailability of drugs, pharmacoresistance leading to ineffective drug treatment. Modulation of these efflux transporters can have an impact on drug absorption, disposition and consequently therapeutic outcome. However, we know that P-gp is also widely expressed in many other human tissues including the liver, kidneys, testes, placenta and the mammary epithelial cells[8]. It is also well known that these ABC transporters play a crucial role in the protective mechanism during embryogenesis in the placenta which is continued during lactation providing foetal protection against naturally occurring toxins[9]. Our area of interest is the role of these transporters in the lactating human mammary epithelial cells (HMEC) where they could potentially influence transfer of drugs from mother to their breastfed baby via breast milk. However, this area remains poorly researched and there is lack of controlled studies that can provide conclusive evidence. On the basis of other organ systems, co-administration of medications that are substrates or inhibitors of these transporters in a nursing mother could have significant drug-drug interactions which may lead to adverse effects in their breastfed infant. Several in vitro and animal studies have been conducted which address this area of concern. However, the applicability of these in vitro findings may not always be conclusive and are often contradictory due to differences in experimental design and use of species other than humans[10-12]. Most animal species such as rats and mice used in the laboratory have two genes (Mdr1a and Mdr1b) that code for P-gp, which further complicates issues regarding induction, expression and drug-drug interactions[11].
TRANSFER OF DRUGS FROM MATERNAL PLASMA TO THE BREASTFED INFANT
The transfer of drugs from maternal plasma to breast milk occurs via passive and active mechanisms[9,13]. The critical determinants of passive transfer include drug protein binding, drug ionisation and fat partitioning[4,13]. These factors can be used to predict milk to plasma (M:P) ratio where passive diffusion is thought to predominate. Other pharmacokinetic parameters such as half-life of the drug, protein binding, water and lipid solubility, route of drug administration, bioavailability, dissociation constant, volume of distribution, molecular size and ionisation potential can further help to determine the transfer of drugs from mother’s plasma into the breast milk[14]. Drugs with the shortest plasma half-life, highest protein binding and lowest lipid solubility usually have the lowest ductal milk transport. The dose of a drug that an infant receives during breastfeeding depends on the amount excreted into the breast milk, the daily volume of the milk ingested and the average plasma concentration of the mother. Thus, M:P ratio has large inter-subject variability[15]. Although the transfer of most drugs into breast milk can be explained by passive diffusion theories, a review of the literature shows that there are several drugs where the actual measured M:P ratio is significantly greater than predicted[16-19]. Nitrofurantoin, acyclovir and cimetidine are some drugs which exhibited a significantly higher observed M:P ratio than predicted[5,7,16,18,20]. In one study nitrofurantoin had an observed M:P ratio of 6 as opposed to the predicted 0.28[17]. It has also been shown that several members of the ABC drug efflux transporters significantly affect the pharmacokinetic disposition of drugs such as the quinolones, thereby increasing their secretion into breast milk[17,19].
ROLE OF EFFLUX TRANSPORTERS IN THE TRANSFER OF DRUGS FROM MATERNAL PLASMA TO THE BREAST FED INFANT
The extent of the involvement of these ABC transporters in the transfer of many nutrients including essential vitamins and drugs into the breast milk has been recently considered[21]. There are several efflux transporters in human mammary epithelial cells that line the alveoli within the mammary gland[8,22,23]. This leads us to believe that there may be a more substantial role of these transporter proteins in the transfer of many compounds from maternal plasma to the breast milk than currently perceived. Alcorn et al[8] have shown that there is some of variability between the level of RNA expression of various transporters in the HMEC from lactating vs non lactating breast tissue, indicating a graded expression change during induction of the lactation process that could lead to significant changes in substrate transport during lactation. Using immunocytochemical analysis and functional studies in primary human mammary epithelial cells culture, our group have demonstrated the presence of MDR1 (ABCB1), MDR3 (ABCB4) and MRP1 (ABCC1) in these cells[21].
Gilchrist and colleagues showed that there is a stage dependent change in the expression of transporters in rat mammary gland and isolated mammary epithelial organoids[24]. Using quantitative reverse transcription polymerase chain reaction, they demonstrated that the various solute carrier and ABC transporters showed a changing pattern in the different stages of lactation. Ling et al[25] studied the M:P ratio of cefepime, an actively transported drug at four and ten days post-partum in rats and found a significant reduction in the amount of cefepime excreted at these two time points. This leads us to believe that as lactation progresses from stages of mammogenesis to lactogenesis to galactopoiesis, changes in the expression of efflux transporters along with changing hormones may influence the transfer of endogenous and exogenous substances from mother to baby via breast milk. However, currently there are no studies in humans to confirm this. Our laboratory is presently investigating whether the expression of efflux transporters in humans follow a stage dependent pattern as seen in animal studies.
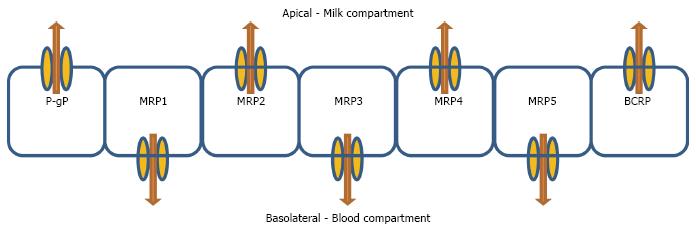
The expression of MRP1 (ABCC1) and MDR1 (ABCB1) are significantly lower in the lactating HMEC as compared to non-lactating HMEC[8] whereas that of BCRP is significantly higher[5]. It is important to note that there is a substantial overlap in the substrate specificities of these transporters[6]. These findings highlight the importance of possible drug-drug interactions between various transporter substrates and/or inhibitors when co-administered at different stages of lactation. In addition, it is important to take into account the localization of these transporters, such that their presence in the apical surface (MDR1 and BCRP) may pump drugs into milk and further place the suckling infant at risk of xenobiotic exposure[20]. Alternatively, if these transporters are located in the basolateral membrane of the cell (MRP1), then the substrate will be pumped out of the milk and into the mother’s blood, thereby reducing infant exposure.
The active efflux transporters usually help in preventing accumulation of drugs into the tissues as they work against a concentration gradient and push drugs from the tissues back into the blood[7,8,24]. There have also been reports of transporter proteins being involved in the transfer of essential nutrients and vitamins to the breast fed infant which are mostly located in the basolateral side[7,22,26]. However, the extent to which they affect drug transfer in the mammary gland is not fully known[6]. Similar to P-gp, BCRP has a protective role at the blood side of many organ systems by facilitating the extrusion of toxins, xenobiotics and drugs out of the capillaries prior to interstitial accumulation with an important role being in the blood-placental barrier, where they protect the foetus from endogenous and exogenous toxins[27]. A current vexing question is how relevant is the role of efflux transporters at other blood-tissue barriers such as in HMECs of a lactating female who is breastfeeding her infant compared to the plethora of studies examining gastrointestinal or BBB transport. Can P-gp or BCRP modulation by an inhibitor drug that the mother consumes cause less drug to be transferred to the breastfed infant via milk? Also if P-gp is located on the apical membrane of the HMEC, the evidence that RNA expression of P-gp is lower in the lactating HMEC[8] could have a relatively protective effect on the breastfed infant, by virtue of less P-gp to excrete drug into the milk (Figure 1).
Figure 1 Schematic representation of expected localisation of drug efflux transporters in the human mammary epithelial cells.
P-gp: P-glycoprotein; BCRP: Breast cancer resistance protein; MRP: Multidrug resistance protein.
The expression of BCRP in pregnant mice was found to be strongly induced during late pregnancy and lactation, which is the opposite to that of P-gp[5]. Lindner and colleagues reported that the expression of BCRP in the mammary glands of several species of animals including sheep, goats and cows were significantly increased (up to 10 fold) during pregnancy and lactation[28]. Both P-gp and BCRP are located in the apical membrane of alveolar epithelial cells of the mammary gland and actively transport their substrates into breast milk as confirmed by animal studies[5,20]. BCRP has a significant role in accumulation of drugs and xenotoxins in breast milk which could be either beneficial or detrimental to the breastfed infant’s health depending on the drug administered[29]. A BCRP substrate that is toxic can accumulate in milk and result in adverse effects in the infant whereas the accumulation of a drug such as aciclovir could be beneficial in reducing transmission of milk borne viruses from mother to baby. There is some evidence that the role of P-gp in the lactating HMEC is relatively insignificant in the transfer of medications which could possibly be due to its down regulation in lactation. Animal studies have shown that the transfer of nelfinavir, a known P-gp substrate is not affected by P-gp in the mammary gland[28]. However, many other studies have shown that the role of BCRP is much more significant, given that BCRP is strongly induced during pregnancy and lactation[5,16,18,30].
As there is a significant overlap between P-gp, BCRP, other efflux transporters and CYP3A4 substrates[6], it is crucial that each drug or the combination of drugs is considered with respect to these transporters and metabolism pathways in addition to the usual pharmacokinetic parameters to ensure minimum inadvertent exposure to a breastfed infant.
SPECIFIC DRUGS
Antidepressants - selective serotonin reuptake inhibitors
Post-natal depression is considered to be a significant problem in women of child bearing age with approximately 14% of all women affected by this condition at some stage[31]. Psychotherapy is considered quite useful in the management of post natal depression but due to a lack of adequate service provision in the community, it is often necessary to treat women with pharmacotherapeutic agents[31]. Selective serotonin reuptake inhibitors (SSRI) are considered the mainstay of postnatal depression due to their perceived low transferability into breast milk and safety profile unlike tricyclic antidepressants that may have a higher breast milk exposure leading to reduced usage[31]. The recommended SSRIs for post-natal depression are sertraline and paroxetine as they have been widely studied and are not associated with many adverse effects[32]. Fluoxetine and citalopram have measurable plasma levels in some infants[33,34]. However, these levels were usually low. Some women may choose to continue using one of the other SSRIs that they have been stabilised on before and during pregnancy. Nonetheless, most SSRIs are considered to be fairly safe in breastfeeding.
Paroxetine and fluoxetine are thought to be P-gp substrates[35]. Citalopram and its enantiomer, escitalopram have also been found to be substrates of P-gp in animal studies as demonstrated by Weiss et al[36]. The implications and relevance for this evidence in lactation remains elusive. However, in vivo it has been shown that the peak plasma concentration and area under the curve of paroxetine, is significantly increased by itraconazole (a P-gp and CYP3A4 inhibitor)[37]. Again, this could mean that a P-gp substrate which normally has low transferability into breast milk may potentially transfer in significantly higher amounts if given with a P-gp inhibitor.
Furthermore, it has been found that sertraline, a P-gp substrate and inhibitor, can modulate P-gp both in vivo and in vitro at the BBB and blood testes barrier sites[38]. This finding suggests that concurrent administration of other P-gp substrates with sertraline potentially increase CNS penetration of that substrate[39]. Again, the significance and implication of this finding in lactation needs to be investigated further as another study found modulation of P-gp by sertraline was site specific with different tissues reacting to sertraline in different ways[40]. It is important to investigate whether this finding has any potential for causing interactions in the lactating HMEC leading to adverse effects in the breastfed infant as this tissue was not directly investigated. A different study by Bhuiyan et al[41] found that a single dose of sertraline does not affect the pharmacokinetic profile of fexofenadine, another P-gp substrate but paroxetine and fluvoxamine do. There was no data with regards to BCRP substrate specificity in the selective serotonin reuptake inhibitors.
Of other new antidepressants, duloxetine, which is a serotonin noradrenaline reuptake inhibitor (SNRI), not used often in pregnancy and breastfeeding due to lack of safety data in this population, was found to cause no immediate adverse effects in an exclusively breastfed 32 d old infant[42]. The measured M:P ratio and the relative infant dose for this drug were also found to be very low[43]. Venlafaxine, another SNRI was found to induce BCRP in brain tissue and is thought to be a P-gp substrate whereas desvenlafaxine, the active metabolite of venlafaxine was found to have no effect on BCRP induction or P-gp modulation[44,45].
Another case study on sertraline reported signs of serotonergic overstimulation in a preterm baby whose mother had therapeutic levels of both the drug and its metabolite, desmethylsertraline. The symptoms disappeared on discontinuation of breastfeeding[46]. This adverse reaction was attributed to immaturity in the development of the infant’s clearance mechanisms and lack of development of the BBB. Interestingly, the plasma sertraline and desmethylsertraline levels of the infant were significantly below the threshold levels considered to cause symptoms. There was no record of other medications that may have been used by the mother acutely or long term during the postpartum period. Hence, it raises the question of whether another drug(s) administered acutely, able to modulate P-gp activity, could have resulted in the adverse effects experienced by the breastfed baby. The lack of a full medication record makes it difficult to draw conclusions regarding the drug-drug interactions.
Current guidelines place paroxetine and sertraline amongst the recommended antidepressant drugs for use in lactation. It is imperative that infants of mothers taking these drugs are regularly monitored for adverse effects especially when the mothers are also treated acutely or chronically with another pharmacologic agent that could modulate active transporters as these drugs have the potential to do[35,47].
Antipsychotics
About one third of pregnant women with psychotic illness use antipsychotics at least once during pregnancy or whilst breastfeeding[48]. About 10% of women of child bearing age have a postpartum psychiatric disorder, with a significant number warranting the use of an antipsychotic medication[49]. Although the second generation (atypical) antipsychotics are considered the best treatment option for schizophrenia, female patients who are pregnant or breastfeeding are often excluded from this treatment option due to safety concerns.
Several atypical antipsychotics are substrates for P-gp in therapeutic concentrations. These include amisulpride, aripiprazole, olanzapine, risperidone, quetiapine and paliperidone, with quetiapine and risperidone having high affinity for P-gp[50]. Friedman et al[50] found olanzapine and quetiapine to be P-gp substrates whereas another in vitro study by Müller et al[47] contradicted this finding and identified that quetiapine, haloperidol, olanzapine and clozapine are not P-gp substrates. As the first study assessed P-gp activity by measuring ATPase activity whereas the second study was an inhibition study carried out using Caco-2 cell monolayers, these different methods of P-gp substrate identification may be responsible for their different conclusions. Our studies using Caco-2 monolayers support the latter work, demonstrating the lack of P-gp mediated efflux associated with quetiapine and olanzapine. Hence, it is important to exercise caution when interpreting results from different cell lines and membrane systems[35]. In vivo studies using knockout and wild type mice may be more reliable and may provide a clearer picture of functional consequence[51]. However, it is worth noting that these rodents may have more than one gene coding for P-gp, hence may differ from human P-gp structurally.
Several antipsychotics including clozapine, quetiapine, paliperidone and chlorpromazine also exert some inhibitory effects on BCRP[52]. Risperidone has major inhibitory effects on BCRP, making it a potential contributor of adverse effects with co-administered with BCRP modulators such as commonly prescribed pantoprazole and omeprazole[52,53]. Aripiprazole, an atypical antipsychotic was found to have an inhibitory effect on BCRP[54]. Most antipsychotics are thought to act as inhibitors of P-gp/BCRP and therefore can influence plasma and brain concentrations of other substrates[55].
A prospective controlled observational study of olanzapine use in 30 pregnant women who were taking olanzapine during pregnancy and whilst breastfeeding found that no adverse effects were imputable to the use of olanzapine by the mother. However, the rate of breastfeeding was significantly lower in the treated group. Also three out of thirty babies (10%) experienced withdrawal symptoms after birth and interestingly all three mums were on multiple medications including zuclopenthixol, lithium and paroxetine[56]. A case report of a lactating patient who was taking olanzapine after a psychotic episode reported low infant plasma levels of olanzapine and no adverse effects in the breastfed baby[57]. Another case study reported no adverse effects in the infant of a woman who was initiated on olanzapine during her third trimester and continued breastfeeding six months post-partum[49]. There is no mention of concurrent therapy if any. A case report of risperidone by Lutz et al[58] showed that risperidone, a P-gp substrate, and its metabolite 9-hydroxyrisperidone were moderately transferred into breast milk. A dose (concentration) of less than 10% of that received by the mother (on a mg/kg basis) has been suggested and is widely accepted as a “safe” dose (or concentration) in the infant[45]. Although, the amount transferred (4.3% of maternal dose) was below the notional 10% threshold, the mother was encouraged not to breastfeed due to concerns for the safety of her infant. Again, no information was provided on whether the mother was on any other concurrent medications. Certainly, we would prefer clinical studies to state that there were no other medications in the study rather than just omitting this crucial information. Often when the focus is on one particular drug and snapshot studies such as M:P ratio are being investigated, other medications do not make it into the clinical notes. Given our presumption that in psychiatry multiple medications are often used concurrently, this makes subsequent contextual analysis very difficult. Furthermore, a series of case reports by Illet’s group[59] confirmed the findings from the first report that risperidone on its own is not transferred into breast milk in levels high enough to be considered a clinical issue for the safety of the breastfed infant.
Ziprasidone, an atypical antipsychotic was found to be excreted in very low concentrations in breast milk from a treated patient while no adverse effects were observed in the infant of a different patient with psychotic depression treated with citalopram and ziprasidone[60,61]. It is not known whether ziprasidone is a P-gp substrate or BCRP modulator at this time[55]. Further research and more long term studies are warranted to ensure the safety of the newer antipsychotics on infant growth and development.
Amisulpride, another atypical antipsychotic has been used in one woman who was breastfeeding her 13 mo old infant. An unusually high M:P ratio for this drug compared to predicted values based on pharmacokinetic parameters was found[62]. This high M:P ratio was attributed to amisulpride being a P-gp substrate[47,63]. Amisulpride used in conjunction with desvenlafaxine in a partially breastfed infant yielded no higher than expected relative infant dose. Given that Amisulpride is a P-gp substrate, the combination with desvenlafaxine did not appear to alter its pharmacokinetics reaffirming the in vitro evidence that desvenlafaxine does not modulate efflux transporters[44,45]. Olanzapine is considered a weak substrate whereas data for quetiapine are contradictory with one study identifying it as a substrate and the other not a substrate[47,63]. Another in vitro study found that olanzapine and risperidone may inhibit P-gp activity. Most other drugs in this therapeutic group though, such as clozapine, haloperidol chlorpromazine and quetiapine did not inhibit P-gp[64]. Much of the studies discussed above were cell based, and projecting this data into clinical studies has been sorely lacking. Nonetheless, it is appropriate to exercise caution when using these agents especially in combination with another agent which may modulate P-gp especially in a lactating mother who is breastfeeding or exclusively breastfeeding, given that some studies support the notion that blocking P-gp (or other efflux transporters) can elevate milk concentration of these drugs.
Antiepileptic drugs
Many women with epilepsy require treatment with antiepileptic drugs (AED) during pregnancy and post-partum when they may be breastfeeding their baby. Usually women with epilepsy do not have a choice to discontinue treatment while pregnant or breastfeeding. All antiepileptic drugs are transferred across the placenta and to a lesser extent into breast milk in varying amounts. The implications of AED exposure via breast milk is still not fully understood. A large prospective study of epileptic mothers on AED prenatally showed adverse development in their children regardless of breastfeeding status[65,66]. Some other large studies also showed no damaging effects on neurodevelopment of breastfed children of women who were prescribed AED during breastfeeding[67]. Nevertheless, most antiepileptic drugs are known to be teratogenic and increase the risk of foetal malformations[68].
The role of efflux transporters such as the MRP group and P-gp in the transport of AED showed variable results[69]. Luna-Tortós et al[10] initially found that several AED were substrates of the human P-gp. Lamotrigine and phenobarbital were also found to be MRP substrates. However, in a subsequent study they indicated that AED were not substrates of the human P-gp and that the different interpretations from the two studies were attributed to the different experimental designs used[11]. Several other studies also suggested that AED were not human P-glycoprotein substrates[12,68-70]. Studies performed in animals yielded conflicting results showing that AED were indeed substrates of P-gp[71-74]. The variability in these results may be attributed to the differences between human and rat P-gp. The cell lines and experimental designs used for conducting these studies can also have a significant impact on the results[71]. Another study in mice found that levetiracetam, topiramate and phenytoin demonstrate biphasic modulation of P-gp in BBB whereby at therapeutic doses they act as inducers of efflux. Sodium valproate and lamotrigine were found not to interact with P-gp[11,73,75]. Carbamazepine, itself was not a P-gp substrate but its metabolites were[76,77]. Dickens and co-workers also identified that lamotrigine and carbamazepine were not affected by P-gp[78,79]. Several case reports show that lamotrigine was transferred into breast milk in moderate amounts and in one case, where the mother was on 850 mg/d, it led to a severe apnoeic reaction in the exclusively breast fed infant[80]. It is not specified whether the mother was on any other medications at the time that could have contributed to this adverse reaction. Another study has shown that although lamotrigine’s M:P ratio is highly variable, it is transferred into breast milk in moderate amounts[67].
In vitro studies carried out on MDCKII cells by Nakanishi et al[75] found that phenobarbital, clobazam, zonisamide, gabapentin and levetiracetam were BCRP substrates. Contrary to their findings, another group found that phenobarbital as well as other AED including phenytoin, ethosuximide, primidone, sodium valproate, carbamazepine, clonazepam, and lamotrigine did not interact with BCRP[79]. The differences in the experimental design and human and animal transporters may partly explain the variations in these results, with the animal studies appearing to favour anti-epileptic P-gp affinity while the human P-gp studies do not support this[68,77,79].
It is interesting that genetic polymorphism of P-gp is not associated with drug response in epileptic patients[80,81]. Again this concurs with the evidence that AED may not be transported by efflux transporters such as P-gp and BCRP[81]. Weiss et al[36] indicate that P-gp may not be of great significance in the transport of AED. Further studies are required to elucidate if there are any other active transport mechanisms which may have significant clinical implications in breastfeeding mothers.