Revised: October 15, 2011
Accepted: December 20, 2011
Published online: February 9, 2012
The primary objective of this article is to analyze the role of tobacco smoke compounds able to damage the cardiovascular system and, in particular, to interfere with blood pressure. They are products of tobacco plant leaves, like nicotine, thiocyanate and aromatic amines, and a chemical derived from cigarette combustion, carbon monoxide. Of the other thousands of chemicals, there is no clear evidence of cardiovascular damage. Nicotine and its major metabolite, cotinine, usually increase blood pressure by a direct action and an action stimulating neuro-humoral metabolites of the body as well as sympathetic stimulation. An indirect mechanism of damage exerted by elevated carboxyhemoglobin concentrations is mediated by carbon monoxide, which, mainly induces arterial wall damage and, consequently, late rising in blood pressure by a toxic direct action on endothelial and blood cells. Thiocyanate, in turn, reinforces the hypoxic effects determined by carbon monoxide. Aromatic amines, depending on their chemical structure, may exert toxic effects on the cardiovascular system although they have little effect on blood pressure. A rise in blood pressure determined by smoking compounds is a consequence of both their direct toxicity and the characteristics of their chemical chains that are strongly reactive with a large number of molecules for their spatial shape. In addition, a rise in blood pressure has been documented in individuals smoking a cigarette, acutely and chronically, with irreversible artery wall alterations several years after beginning smoking. Since cigarette smoking has a worldwide diffusion, the evidence of this topic meets the interest of both the scientific community and those individuals aiming to control smoking.
- Citation: Leone A. How and why chemicals from tobacco smoke can induce a rise in blood pressure. World J Pharmacol 2012; 1(1): 10-20
- URL: https://www.wjgnet.com/2220-3192/full/v1/i1/10.htm
- DOI: https://dx.doi.org/10.5497/wjp.v1.i1.10
Growing evidence indicates that over 4000 chemical compounds are usually concentrated and condensed into tobacco mixture[1]. A large majority of these have carcinogenic effects but there are many with cardiovascular toxicity, which depends on several factors, partly related to tobacco smoke and partly due to the environment and lifestyle of individuals exposed to smoking.
The harmful health effects of tobacco smoke adversely target the cardiovascular system and there is also evidence that death rates are uniformly higher among smokers than non-smokers in both sexes and whatever the age at the death. In addition, reports[2,3] indicate that the excess mortality in smokers mainly affects smokers aged from 45 to 54 years more than those younger or older in age. It is well known that older age is related to arterial hypertension[4,5] and, consequently, there is evidence that smoking usually precedes elevated blood pressure. Moreover, the strong relationship which links smoking exposure to type of response of heart and blood vessels[6-10] may present a variety of patterns and different severe manifestations.
In spite of the great number of findings which show the adverse role of smoking compounds on blood pressure without doubt, current opinions on that are not yet unanimous. There is a discrepancy in opinions that may be attributed to the lack of reproducible data, particularly in epidemiological studies. On the other hand, experimental findings conducted on both humans and animals give evidence of reproducible results for cardiovascular events and events related to hypertension. In addition, high blood pressure has consistently been found to be a strongly predictive factor for coronary artery disease and stroke[4-6]. Undoubtedly, careful control of major cardiovascular risk factors[10], including hypertension, which should be unconditionally lowered worldwide, is able to reduce significantly the rate of stroke and coronary events.
The purpose of this review is to describe the mechanisms by which the chemicals of tobacco smoke can cause changes in arterial blood pressure, as well as explaining the reasons for selecting this subject by an analysis of chemical and pharmacological properties of tobacco smoke toxins which adversely affect the cardiovascular system, in an attempt to clarify the real potential of the topic in regard to the scientific community and health professionals concerned.
A chemical chain of a substance often influences and determines its mechanism of action. In chemistry, a chemical chain is a series of linked atoms that form a molecule, usually of the organic but also the inorganic type. Different properties and reactions characterize the chemical chains according to structural shape. There are closed-ring chains where the atoms in a molecule form a closed loop, which, consequently, is often a stable and scarcely reactive chemical; long-chains with relatively long bindings of atoms in the same molecule; and open chains ending with an open binding which may interact actively with atoms of various molecules. In addition, chemical chains spatially build the geometrical aggregation of constituent elements of a molecule that take part in isomeric substance composition. Often, more than one reaction is possible given the same starting materials. The reactions may differ in their stoichiometry according to the number and atomic concentrations of the molecules, as well as the substrates that are prevailing. So, a chemical atom may react actively with another to give a specific compound if a different atom is not present, while the same atom may react differently in the presence of other atoms, choosing to form a compound which differs from that of the chemical reaction performed in the absence of other atoms. There is evidence that the knowledge of these properties of chemical binding will contribute to better understanding the damaging mechanism of tobacco compounds, including primarily nicotine and its metabolites[11] and carbon monoxide. These substances may react differently according to their affinity towards environmental substrates and, consequently, determine various levels or type of individual responses.
Nicotine is a natural alkaloid[12] obtained from the dried leaves and stems of tobacco plants. Chemically, the alkaloid has a basic charge which is responsible for the mechanisms of chemical reaction. Nicotine concentration in the tobacco plant ranges from 0.5% to 8%.
Nicotine has always been identified as the most powerful toxin of cigarette smoking since its harmful action, either functionally or structurally, can be widely demonstrated at a relative low concentration in both clinical and experimental findings involving all body organs. Nicotine biosynthesis takes place in the roots of the tobacco plant. Then, it accumulates in the leaves, the particular shape of which is a basic factor for the harvesting and extraction of the alkaloid. After manufacturing, each cigarette may reach nicotine concentrations from 1.5 to 2.5 mg. However, not all of the smoked substance enters the blood, which is, in any case, damaged by absorbed nicotine[13].
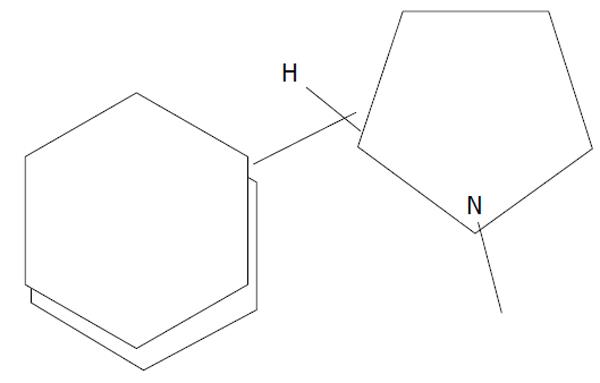
The chemical formula of nicotine is C10H14N2, equivalent to 3 (1-Methyl-2-pyrrolidinyl) pyridine. Its spatial shape (Figure 1) shows two chains, one closed chain and one open chain with an N reactive atom. This structure is basic to understanding some chemical properties, like nicotine addiction and toxicity, since it permits identification of two types of spatial aggregation of those constituents taking part in nicotine composition[13]: nicotineS (-) isomer and nicotineS (+) isomer. NicotineS (-) isomer is the main nicotine isomer[11], tasted as pleasant in cigarette by smokers but not by non-smokers, whereas the nicotineS (+) isomer is unpleasant for both smokers and non-smokers. This chemical composition helps to explain the reason for the bad taste for the smoker who starts smoking, even if it changes rapidly when pleasant nicotineS (-) isomer utilizes its effect. There is evidence that tobacco manufacturing industries try to reinforce the response linked to nicotineS (-) isomer.
Nicotine is a hygroscopic liquid miscible in its basic charge with water. This property makes the alkaloid diffusible on the whole tobacco leaf so that harvesting tobacco leaves fills up with the greatest amount of nicotine. Its chemical formula of a nitrogenous base permits an interaction between nicotine and acid compounds, forming salts usually soluble in water and, consequently diffusible, some of these characterized by high toxicity.
Absorption of nicotine by individuals occurs very quickly through several tissues of the body. The oral cavity absorbs from 4% to 45% of the total dose[14]. In addition, as nicotine enters the body[15], it reaches the blood, acting on specific receptors. Nicotine-acetylcholine receptors that feel the level of blood nicotine concentration are deputed to permit the biochemical action of the alkaloid. In the presence of low concentrations of nicotine, the activity of nicotine-acetylcholine receptors increases, whereas high concentrations inhibit receptor activity. Therefore, different pharmacological responses to nicotine may be seen, from stimulant to depressant effects, on those structures involved in alkaloid activity.
Nicotine effects are mediated through the interaction with the sympathetic system, catecholamine release, endothelial function and metabolic profile of the individuals[1,16-20].
Nicotine exerts direct and repeated effects on the sympathetic system which are, initially, transient and stimulant, but, in the long run, they become of depressant type with severe functional and structural changes in those organs involved with the nicotine action. For the heart and blood vessels, there is evidence that nicotine triggers cardiovascular responses through sympathetic stimulation and direct and mediated catecholamine release[17,20]. In addition, nicotine specifically stimulates release of norepinephrine from the hypothalamus and antidiuretic hormone from the pituitary gland[21], as well as chemoreceptors in the carotid arteries[22], triggering different reflexes, which may lead to multiple adverse responses.
Liver, kidney, lung and oral mucosa are those body organs involved in regulating nicotine metabolism which damages the cardiovascular system and endocrine glands.
Metabolites of nicotine, the main one of which is cotinine, need to be highlighted since they permit analysis of follow-up studies on selected populations of smokers, more for their toxicity which is similar to that of nicotine. Nevertheless, differences exist between nicotine and its metabolites regarding the mechanism of action. Nicotine usually acts more rapidly and therefore may be dosed earlier in biological liquids. On the other hand, chronic toxicity is better estimated by dosing cotinine in biological liquids. Large-scale trials assessing the results of antismoking campaigns dose urinary cotinine. Indeed, it is less expensive than nicotine[23,24]. Similar to nicotine, cotinine is also mainly metabolized in the liver.
Carbon monoxide is a gas with the highest toxicity, depending mainly on concentrations in the environment and body organs. This chemical is not contained in the fresh leaf of tobacco but produced by a chemical reaction, characterized by decomposition in the burned cone of a cigarette between environmental oxygen and the paper of the smoked cigarette. Carbon monoxide derived from a single smoked cigarette reaches small concentrations which can acutely induce functional but transient responses, particularly in the lungs and cardiovascular system, whereas dated chronic smoking often causes irreversible alterations. However, carbon monoxide alone from tobacco smoke may be considered, chronically, a potentially silent killer even if different levels of carboxyhemoglobin are reached after acute smoke exposure[25].
Carbon monoxide is a colorless, odorless and tasteless gas with high and potentially lethal toxicity. The blood level of the gas regulates toxic responses.
The chemical formula of the gas is CO, a diatomic molecule with binding of two atoms, carbon and oxygen[13]. Spatially, carbon monoxide outlines a diatomic linear chain which permits an easy dissociation and, consequently, re-composition in atoms of carbon and oxygen able to react again together or with other atoms originating from several different compounds. In addition, new linking with substances with major chemical affinity, such as hemoglobin, may occur. Usually, carbon monoxide is less dense than air and, therefore, is capable of spreading out more quickly. It is also soluble in water and burns in air, producing carbon dioxide, a metabolite largely diffused in the environment and the end-point of catabolic reactions associated with intracellular respiratory metabolism.
However, a little amount of carbon monoxide in a concentration not harmful for life arises spontaneously into the body through a chemical reaction involving heme and an enzyme named heme-oxygenase. Heme is an iron component of hemoglobin, a molecule strongly reactive with carbon monoxide.
The toxicity of carbon monoxide produced by cigarette smoking recognizes several complex reactions consisting of oxygen removal from hemoglobin and its replacement with carbon monoxide, formation of carboxyhemoglobin, tissue hypoxia and impairment of cellular metabolism. These mechanisms are the result of carboxyhemoglobin production, but carbon monoxide also acts directly, depending on its chemical molecule, to morphologically damage the heart and blood vessels[26-31].
Thiocyanate is the third chemical of tobacco smoke that exerts adverse cardiovascular effects with consequent increased damage caused by nicotine and carbon monoxide.
The substance is largely diffused in nature in plants of the genus Brassica (cabbages) and is also a component of biological liquids like blood, saliva and urine. In addition, it reacts at different steps of body metabolism[32-34], including mainly iodine metabolism. Thiocyanate develops in the vapour phase of tobacco smoke, acting particularly as a compound able to reinforce chronic damage derived from tobacco smoke.
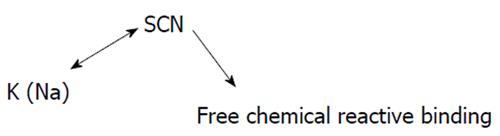
Thiocyanate is a colorless, odorless crystalline powder with a chemical formula that may vary according to the salt linked with its structure. Spatially, the basic chemical structure of thiocyanate is shown in Figure 2. It shows that there can be variability in the type of salt which bonds the thiocyanate main chain, usually K or Na, and is free binding, able to react with several other molecules[35].
The main metabolites of thiocyanate formed in the body have high toxicity, primarily hydrogen cyanide. However, the metabolite concentration in the vapor phase of smoking usually does not reach values acutely harmful for the individual’s life. When cyanide enters the blood, it forms a stable complex with enzymatic chains, particularly with those cytocrome oxidases involved in the synthesis of adenosine triphosphate (ATP). The result of the chemical reaction is a reduced ATP synthesis which causes problems of intracellular respiratory chains and different degrees of hypoxia. Therefore, thiocyanate increases the hypoxia due to carbon monoxide and nicotine from smoking. There is evidence that a deep interaction exists among the main chemical compounds of smoke to cause damage of heart and blood vessels[36].
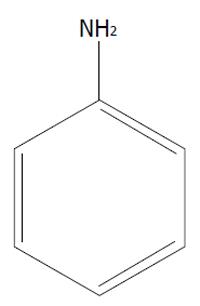
Aromatic amines are chemical compounds with a chemical formula (Figure 3) containing one or more closed benzene rings added to aromatic constituents like NH2, NH or other nitrogen groups. These classes of chemicals, largely concentrated and diffused into the environment from burned tobacco, exert mainly carcinogenic effects[1,37], even if morphological alterations of heart and blood vessels have been described. However, their toxic effect on blood pressure is still to be demonstrated.
There are four main factors that regulate blood pressure; in their turn, they are influenced by a large number of physiological and pathological responses that determine the level of blood pressure. Tables 1 and 2 summarize the main influencing events.
| Factors | Controlled parameters |
| Cardiac inotropism | Heart rate, cardiac output |
| Arterial resistance | Arterial stiffness, arterial vasoconstriction, arterial dilation, endothelial function, anatomical structure of the arteries |
| Blood volume | Salt uptake |
| Blood viscosity | Red blood cell numbers |
| Factors | Influenced parameter |
| Dietary factors | |
| Salt intake-sodium, potassium | |
| Calcium, magnesium | Blood flow and volume |
| Vegetarian diet, alcohol | |
| Bio-humoral and hormonal factors | |
| Angiotensin | |
| Vasopressin | Arterial wall |
| Vasodilators | Blood flow and volume |
| Vasoconstrictors | Arterial resistance |
| Catecholamines | |
| Neural factors | |
| Central nervous system | |
| Sympathetic system | Sympathetic stimulation |
| Catecholamine release | |
| Genetic and metabolic factors | Glucose and lipid metabolism |
| Genetic code | |
| Lifestyle | Preventive measures |
Blood pressure regulation is under the control of cardiac inotropism, arterial resistance, blood volume and viscosity or thickness of circulating fluid. These factors cause blood pressure levels as a result of a meeting or engagement between different types of lifestyle and a genetic code, characteristics of a single individual or his family. The result may be a normal blood pressure level or changes in blood pressure, even in the absence of identifiable triggering causes. This concept of a different distribution of blood pressure values within populations as well as establishing the appearance of complications related to abnormal blood pressure is a basic point of preventive antihypertensive measures[38].
Cardiac inotropism, usually evaluated by assessment of the amount of blood pumped out in every single beat by the heart, regulates cardiac output, which is the result of the heart rate multiplied by the number of each cardiac systole. As one can easily deduce, this regulating parameter of blood pressure may often be influenced by several factors and, therefore, it is difficult to maintain stable levels.
Arterial resistance plays a strong role in the control of blood pressure. There is evidence that increased arterial stiffness[39], which is a parameter strongly influenced by smoking compounds, causes adverse effects on arterial elasticity. Stefanidis and co-workers[39] investigated aortic elasticity in 48 male patients, most of whom had coronary heart disease. By means of a sonometric catheter, they measured the diameter of the aorta while simultaneously determining arterial blood pressure at the same location. Increased aortic stiffness, strongly influenced by exposure to passive smoking, with consequent reduction in aortic elasticity, augmented left ventricular afterload and impaired left ventricular function. Vasoconstriction in systemic arteries, caused by tobacco smoke[40,41], in the presence of a stiffer aorta necessarily also worsens myocardial ischemia in individuals suffering from ischemic heart disease and among those with hypertension whose aortic elasticity is already compromised. Endothelial function is a basic parameter able to control blood pressure, functionally or morphologically. An excellent paper by Deedwania[42] specifically analyses the role and significance of endothelium, stressing the importance of this structure as a new target for cardiovascular therapeutics. It has been well established that vascular endothelium has a pivotal role in maintaining vascular tone. In addition, endothelial dysfunction is an early marker of impending atherosclerosis, also induced by smoke compounds and strongly related to hypertension[43-46]. By its chemical compounds, endothelium modulates artery dilation under normal conditions.
The patent arterial lumen, with its cylinder shape that progressively reduces the caliber along the entire length from the origin to the end, determines a different degree of resistance to blood flow. Blood flow resistance is lower at the origin of the arterial vessel[47] while increasing significantly at the end.
Finally, blood flow and viscosity change their power in the control of blood pressure according to a large number of factors[1], depending on their capacities of influencing the cardiovascular system.
The above parameters are significantly changed by a large number of factors that stimulate or reduce their activity. The main factors which influence the parameters regulating blood pressure may be classified into five groups: dietary factors, bio-humoral and hormonal factors, neural factors, genetic and metabolic factors and factors associated with lifestyle. Table 2 analyzes the most important of them.
Salt intake strongly influences blood pressure by involving several mechanisms like blood flow and volume, kidney function and acid-basic balance[48-51]. Smoking compounds exert little or no effects directly on these parameters, although indirectly their effect is mediated by those changes induced by neuro-humoral stimulation[52].
Among bio-humoral factors, angiotensin and renin-angiotensin-aldactone are primarily associated with development of hypertension, either through isolated or combined activity with other structures, like the sympathetic system and catecholamine release that are stimulated by the renin system.
Angiotensin[53-56] is one of the major stressing substances at any vascular district, increasing vascular tone and consequently arterial resistance. Plasma angiotensin levels are increased by several hormones, like plasma corticosteroids, estrogens, thyroid hormone concentrations as well as its metabolite Angiotensin II, which is the most potent pressor known. Among the angiotensin family, which has several compounds like angiotensin I, II, III and IV, differing in both type and number of constituents of amino-acids, angiotensin II is of great importance to vascular tone control, particularly for coronary circulation. Studies[57-61] concluded that angiotensin II is one of the most potent substances for increasing vascular tone and arterial resistance by a direct action on the arterial wall mediated by angiotensin II receptors. In addition, a significant increase in myocardial oxygen consumption exists because of increased heart rate, systemic blood pressure and left ventricular wall stress. There is clear evidence that a close and complex interaction links angiotensin II to the responses of other factors that modulate vascular tone, whether they exert vasoconstrictor or vasodilator effects[62]. Sympathetic stimulation also correlates its effects with angiotensin II, strongly reinforcing their power[63].
A large number of substances with vasoconstrictor effects, like vasopressin or some prostaglandins and vasodilator effects like nitric oxide or relaxing factors, directly or indirectly regulate vascular tone causing increased or lowered blood pressure, respectively[64-70].
Some observations on the effects of the sympathetic nervous system and catecholamine release are as follows.
The role of these structures for blood pressure control seems to be limited to a short time, whereas the kidneys have a long-time control[71].
Indeed, the major hypothesis for the development of rising blood pressure involves the kidney with an abnormal excretory function[72]. However, cigarette smoking poorly influences this mechanism of blood pressure control.
Increased sympathetic nervous system activity has been identified as a factor able to adversely influence renal excretory function and smoking has been recognized as exerting a strong action on the sympathetic system[1,73-75].
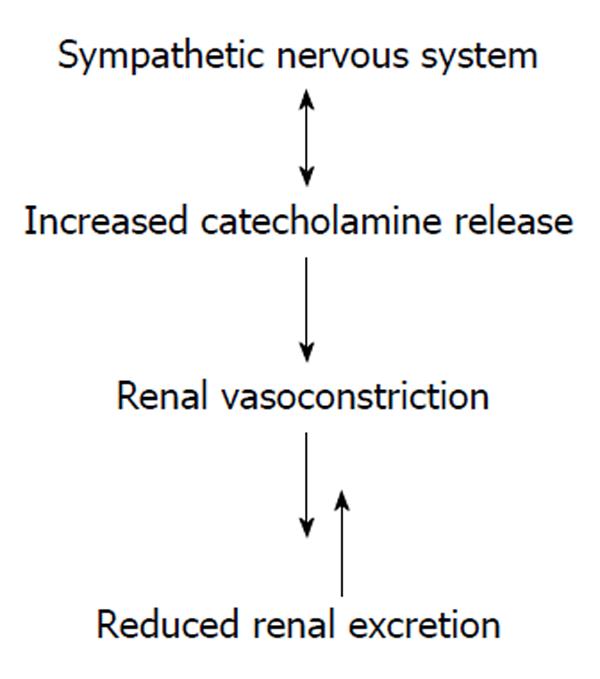
In turn, sympathetic activity triggers a wide number of endocrine responses capable of inducing a short-term increase in blood pressure followed by a long-term increase[76-78]. In addition, there is evidence that the interaction between sympathetic stimulation and endocrine metabolism influences renal function, determining renal vasoconstriction and, therefore, reduced renal excretion (Figure 4).
The increased sympathetic activity determines a major renal tubular absorption of sodium and, therefore, sodium retention. In addition, decreased renal blood flow and glomerular filtration rate as a consequence of vasoconstriction, increases renal vascular resistance and, finally, stimulation of the renin-angiotensin-aldosterone system with an increased renin release. That leads to major angiotensin production. The primary result of these combined actions is a decrease in renal excretory function.
The sympathetic nervous system also stimulates aortic baroreceptors capable of inducing an increase in blood pressure.
Catecholamines induce a sympathetic response[75-79]. There are several catecholamines: epinephrine primarily modulates those responses linked with acute stress because of its prompt availability. On the other hand, norepinephrine acts primarily as a neurotransmitter of sympathetic postganglionic neurons. In practice, epinephrine induces acutely elevated blood pressure that, chronically, norepinephrine release contributes to maintain. Therefore, it is hard to establish whether rising blood pressure depends on one or the other hormone, explaining how the interpretation of the regulation of blood pressure linked to two hormones would be very complex. However, plasma concentrations of epinephrine and norepinephrine usually reflect adrenal medullary secretion and sympathetic nerve activity, respectively. Several factors related to exercise, mental stress, electrolyte balance, smoking and age[77,78] can stimulate the feed-back mechanism of catecholamine production, metabolism, uptake and excretion.
A third catecholamine, dopamine, the immediate metabolic precursor of norepinephrine, exerts sympathetic activity[79].
The effects of catecholamine release, primarily epinephrine, are very quick if compared with hormonal activity of other endocrine glands. Therefore, changes in target organ function may occur acutely after exposure to a standard stimulus, such as smoking a cigarette or passive exposure[80-82].
The effects of catecholamine release depend strictly on the type of catecholamine adrenoreceptor involved. Two types of adrenoreceptors play a strong role in modulating catecholamine response: α-adrenoreceptors and β-adrenoreceptors. Β-stimulation causes an increase in heart rate and myocardial contractility, whereas α-stimulation is responsible for vasoconstriction. Norepinephrine induces a rise in both systolic and diastolic blood pressure as a consequence of increased vascular tone[79], while epinephrine usually acutely raises only systolic blood pressure. Finally, catecholamine release induces changes in lipid and glucose metabolism[1,10] with evident impact on blood pressure levels. There is clear evidence that tobacco smoke directly affects the metabolic profile of glucose and lipids[10,83,84].
In conclusion, a large number of factors control or are involved in regulating blood pressure, determining changes in baseline values that are the result of deep and complex interactions. External and modifiable stimuli, including cigarette smoking, play a strong role in maintaining blood pressure changes.
Two groups of individuals need to be identified to better interpret how and why cigarette smoking influences blood pressure: active smokers and passive smokers. Past smokers may be included into one of the two groups, depending on when they quit smoking.
Active smokers display blood pressure values which vary widely according to a great number of individual, racial, social and lifestyle factors[85]. In addition, changes in blood pressure characterize the same smoker whether he was smoking a cigarette or not[8]. There is evidence that while a smoker smokes a cigarette, more elevated values than baseline measures in systolic blood pressure and heart rate are usually observed. This fact should prove that a smoking individual triggers transient but effective sympathetic responses, which acutely raise blood pressure levels. There is evidence that smoking is a chemical toxicosis[86] able to cause both acute and chronic detrimental effects and, similar to toxic diseases, exerts a double mechanism of acute damage which may be superimposed onto previous chronic damage caused by smoking itself.
Findings[87,88] documented that cigarette smoking in males was inversely related to systolic blood pressure, with a reduction of 1.3 mmHg in 1.1% of light smokers, 3.8 mmHg in 3.1% of moderate smokers and 4.6 mmHg in 3.7% of heavy smokers when smokers were compared with non-smokers. On the other hand, diastolic blood pressure did not seem to undergo these changes. Both western and oriental populations participated in these studies so that the observed response did not relate to racial factors and, moreover, reduction probably characterized mainly young smokers since not enough time elapsed from starting smoking. Consequently, vascular damage did not clearly appear. Epidemiological surveys[89-91] would confirm these results, identifying a lowering of blood pressure in smokers compared to non-smokers, although the observations concern particularly young smokers and adolescents, frequently with loss in body weight. Therefore, unanimous opinions do not exist about that assessment. There is evidence, however, that chronic older smokers usually display elevated blood pressure[88,92,93]. The role of active smoking on blood pressure is still being debated but evidence indicates that older smokers display systolic blood pressure values significantly higher than those experienced by systolic hypertension of old age[94].
The different opinions about the behavior of blood pressure in smokers may be explained by the phenomenon of masking the damage as the result of the combined action of nicotine and carbon monoxide on the vessel wall, as suggested by Leone[85] and Landini et al[95]. Nicotine, after an early and transient vasoconstriction with a consequent increase of systolic blood pressure and heart rate, has vaso-paralytic effects followed by a decrease of these two parameters. At the same time, carbon monoxide exerts its pathological action of a structural type on the arterial wall resulting, within several years, in irreversibly anatomical damage of the arteries with a steady increase in blood pressure. This is routinely observed in older individuals who have been heavy smokers.
Hypertension exacerbates the cardiovascular risk thus previously thought to be linked only to cigarette smoke, with an obvious increased incidence of stroke and coronary artery disease which sometimes can pre-exist the hypertension. Ex-smokers show a progressive reduction in blood pressure levels only in the event that carbon monoxide has not arrived to determine irreversible damage to the arterial wall. At this level, the damage[1,17,88] is also not tied to functional responses evoked by the vasoconstriction due to activation of the sympathetic nervous system, altered sensitivity of the nicotine-receptors, circulating catecholamines and vasoconstrictor endothelium-mediators, including primarily endothelin.
Therefore, an acute rise in blood pressure observed while an individual is smoking a cigarette is due primarily to nicotine as a consequence of sympathetic stimulation, while carbon monoxide chronically damages arterial wall, causing morphological lesions that, in time, become irreversible and induce a change in arterial blood pressure with the appearance of hypertension[88].
There is evidence that inter individual variability in blood pressure response in active smokers depends on a wide number of factors, which exert their effects, time by time, prevailing one on the other according to anatomical and functional health status.
Passive smoking causes blood pressure changes which are a result of two main factors: type of exposure and its duration.
Acute exposure usually causes a transient increase in systolic blood pressure[37] due to adrenergic and sympathetic stimulation, as a heart rate increase also demonstrates. These changes accompany transient endothelial dysfunction that is strongly related to passive smoking exposure, even in healthy people and it is the door to atherosclerotic lesions[43,96-98]. In addition, it is assumed that endothelial dysfunction has a strong association with hypertension[99]. Acute exposure to passive smoking does not initially cause morphological alterations of the arterial wall. They are a result of direct action of carbon monoxide on endothelial cells and platelets and will appear later, becoming responsible for the rise in blood pressure. However, when structural lesions induced by smoking appear, they do not differ from those caused by other factors that cause hypertensive disease or its complications, as well as those from pre-existing atherosclerotic diseases. Therefore, anatomically, one can observe the lesions of the atherosclerosis and its complications, even in cases of rising blood pressure.
In summary, blood pressure in passive smokers shows transient and acute increases followed by chronic lowering in its values and, again, late increase when irreversible morphological alterations of the arterial wall appear.
The chemical structure of smoking compounds influences the functional and pathological response of hypertensive individuals, although clinical implications depend primarily on the degree and severity of cardiovascular damage.
Substantial evidence[95,100,101] indicates that hypertension associated with pleasure-loving habits, including cigarette smoking and alcohol consumption, tend to maintain or increase blood pressure and are also predictive of hypertension in normotensive individuals. Therefore, an incorrect lifestyle associated with hypertension certainly helps to damage body organs with a more elevated risk of complications.
Among the body organs, heart and blood vessels, brain, kidney, eyes and genitals primarily feel the adverse effects caused by association of smoking and hypertension.
Clinically, symptoms related to reduced coronary and cerebral perfusion dependent on vascular narrowing, often a high degree of narrowing of the coronary, cerebral and carotid artery can be demonstrated in hypertensive smokers with a rate significantly higher than those observed in hypertensive non-smokers and former smokers[95]. Therefore, ischemic chest pain, arrhythmias and late clinical signs due to congestive heart failure can be documented in hypertensive smokers[102-104].
Clinically, the brain alterations consist of transient ischemic attack, stroke and cognitive disorders, characterized by varying degree of memory disturbances to vascular dementia[105,106].
The kidney normally responds with reduced glomerular filtration responsible for oliguria due to arteriolosclerotic narrowing of resistance arteries[107]. Extreme complications of renal disorders can develop to chronic renal failure which, in a few cases, requires dialysis[108].
This colourful mosaic of alterations that can be isolated or variously combined with each other, lead to a range of clinical, diagnostic and therapeutic implications in patients affected by the above pathological pictures, making it difficult to control. It follows that a correct approach to the pathological aspects that relate smoking and hypertension is far from being achieved and, therefore, all the innovative factors depending on findings that may help to clarify this phenomenon should be accepted and carefully evaluated. In this context, the most recent data on a possible mechanism of damage related to the chemical structure of compounds in cigarette smoking should be taken into account.
Eyes, sexual function and often calcium metabolism in bone, followed by osteoporosis or enhanced pre-existing osteoporosis in both women and men[109], may be altered as a result of the combined action of smoking and hypertension with a development of serious clinical implications requiring a close physician-patient interaction.
Studies conducted to translate obtained data in different findings to all populations worldwide do not always come to the expected results. That also characterizes large scale trials[110-114]. Therefore, evident discrepancies exist between research and clinical practice, so patients do not always get appropriate treatment for their disease. For example, it would seem useless to choose one drug over another if the goal is to achieve a reduction in blood pressure which can be achieved with a different lifestyle and therapy.
Table 3 analyses the main clinical picture which may result from the combined action of smoking and hypertension.
| Body organ | Clinical picture |
| Heart | Coronary artery disease |
| Heart enlargement | |
| Congestive heart failure | |
| Brain | Transient ischemic attack |
| Stroke | |
| Cognitive decline | |
| Kidney | Arteriolosclerosis |
| Chronic renal failure | |
| Eyes | Artery vessel alterations |
| Genital system | Sexual dysfunction |
| Bone | Osteoporosis |
These observations suggest without doubt that a routine assessment of smoking habits in patients suffering from hypertension is warranted in an attempt to avoid or reduce the rate of serious pathological events.
In conclusion, a rise in blood pressure in smokers, although there is no clear causal relationship between these two factors, depends on four main factors: the toxic effect of smoking compounds on the arterial wall, sympathetic stimulation, adrenergic stimulation and the spatial shape of chemical chains that constitute smoking compounds able to damage heart and blood vessels.
The toxic effect is primarily due to carbon monoxide that deeply alters arterial wall cells. Sympathetic and adrenergic stimulation mainly activated by nicotine and its metabolites trigger all those responses that characterize atherosclerotic progression.
Finally, the observations that chemical chains of smoking compounds, particularly nicotine, are more strongly reactive according to their spatial shape provide study material on a subject not yet well investigated but potentially of positive impact. In my opinion, changing the molecular reactivity of smoking compounds towards the production of less toxic substances could open unexpected positive results for a better control of damage from smoking.
Peer reviewer: George Panagis, PhD, Associate Professor, Department of Psychology, Laboratory of Behavioral Neuroscience, University of Crete, University Campus at Gallos, 74100 Rethymno, Crete, Greece
S- Editor Wang JL L- Editor Roemmele A E- Editor Zheng XM
| 1. | Leone A. Biochemical markers of cardiovascular damage from tobacco smoke. Curr Pharm Des. 2005;11:2199-2208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 2. | Department of Health, Education and Welfare. Smoking and health: a report of the Surgeon General (DHEW Publication No. PHS-79-50066). Washington, DC: US Government Printing Office 1979; . |
| 3. | Royal College of Physicians. Smoking or health. London: Pitman Medical 1977; . |
| 4. | EDWARDS F, McKEOWN T, WHITFIELD AG. Arterial pressure in men over sixty. Clin Sci. 1959;18:289-300. [PubMed] |
| 5. | MASTER AM, LASSER RP, JAFFE HL. Blood pressure in white people over 65 years of age. Ann Intern Med. 1958;48:284-299. [PubMed] |
| 6. | Leone A. Cardiovascular damage from smoking: a fact or belief? Int J Cardiol. 1993;38:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Wells AJ. Passive smoking as a cause of heart disease. J Am Coll Cardiol. 1994;24:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 105] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Glantz SA, Parmley WW. Passive smoking and heart disease. JAMA. 1995;273:1047-1053. [RCA] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 226] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Leone A. Cigarette smoking and health of the heart. J R Soc Health. 1995;115:354-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Leone A. Relationship between cigarette smoking and other coronary risk factors in atherosclerosis: risk of cardiovascular disease and preventive measures. Curr Pharm Des. 2003;9:2417-2423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Hummel T, Hummel C, Pauli E, Kobal G. Olfactory discrimination of nicotine-enantiomers by smokers and non-smokers. Chem Senses. 1992;17:13-21. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 12. | Kice JL, Marvell EN. Modern principles of organic chemistry: An Introduction. 3rd ed. New York: Macmillan 1967; . |
| 13. | Leone A. Biochemistry of smoking compounds. Coronary circulation in nonsmokers and smokers. New York: Nova Science Pub Inc 2008; 79-100. |
| 14. | Armitage AK, Turner DM. Absorption of nicotine in cigarette and cigar smoke through the oral mucosa. Nature. 1970;226:1231-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Armitage AK, Dollery CT, George CF, Houseman TH, Lewis PJ, Turner DM. Absorption and metabolism of nicotine from cigarettes. Br Med J. 1975;4:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 197] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83:1-12. [PubMed] |
| 17. | Benowitz NL, Jacob P, Jones RT, Rosenberg J. Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther. 1982;221:368-372. [PubMed] |
| 18. | Turner DM, Armitage AK, Briant RH, Dollery CT. Metabolism of nicotine by the isolated perfused dog lung. Xenobiotica. 1975;5:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 26] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Fielding JE, Phenow KJ. Health effects of involuntary smoking. N Engl J Med. 1988;319:1452-1460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 99] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Ball K, Turner R. Smoking and the heart. The basis for action. Lancet. 1974;2:822-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 1.3] [Reference Citation Analysis (2)] |
| 21. | Castro de Souza E, Rocha E Silva M. The release of vasopressin by nicotine: further studies on its site of action. J Physiol. 1977;265:297-311. [PubMed] |
| 22. | Cohen AJ, Roe FJ. Monograph on the pharmacology and toxicology of nicotine. London: Tobacco Advisory Council 1981; . |
| 23. | Greenberg RA, Haley NJ, Etzel RA, Loda FA. Measuring the exposure of infants to tobacco smoke. Nicotine and cotinine in urine and saliva. N Engl J Med. 1984;310:1075-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 155] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Strachan DP, Jarvis MJ, Feyerabend C. Passive smoking, salivary cotinine concentrations, and middle ear effusion in 7 year old children. BMJ. 1989;298:1549-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Leone A, Mori L, Bertanelli F, Fabiano P, Filippelli M. Indoor passive smoking: its effect on cardiac performance. Int J Cardiol. 1991;33:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Horvath SM, Raven PB, Dahms TE, Gray DJ. Maximal aerobic capacity at different levels of carboxyhemoglobin. J Appl Physiol. 1975;38:300-303. [PubMed] |
| 27. | Adams JD, Erickson HH, Stone HL. Myocardial metabolism during exposure to carbon monoxide in the conscious dog. J Appl Physiol. 1973;34:238-242. [PubMed] |
| 28. | DeBias DA, Birkhead NC, Banerjee CM, Kazal LA, Holburn RR, Greene CH, Harrer WV, Rosenfeld LM, Menduke H, Williams N. The effects of chronic exposure to carbon monoxide on the cardiovascular and hematologic systems in dogs with experimental myocardial infarction. Int Arch Arbeitsmed. 1972;29:253-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ehrich WE, Bellet S, Lewey FH. Cardiac changes from CO poisoning. Am J Med Sci. 1944;208:511-523. |
| 30. | Musselman NP, Groff WA, Yevich PP, Wilinski FT, Weeks MH, Oberst FW. Continuous exposure of laboratory animals to low concentration of carbon monoxide. Aviat Space Environ Med. 1959;30:524-529. |
| 31. | Astrup P. Some physiological and pathological effects of moderate carbon monoxide exposure. Br Med J. 1972;4:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Apple FS, Lowe MC, Googins MK, Kloss J. Serum thiocyanate concentrations in patients with normal or impaired renal function receiving nitroprusside. Clin Chem. 1996;42:1878-1879. [PubMed] |
| 33. | Jimenez de la Higuera A, Olea MF, Olea N, Jimenez F. Determination of serum thiocyanate in patients with thyroid disease using a modification of the Aldridge method. J Anal Toxicol. 1994;18:58-59. [PubMed] |
| 34. | Olea F, Parras P. Determination of serum levels of dietary thiocyanate. J Anal Toxicol. 1992;16:258-260. [PubMed] |
| 35. | Man S, Potáček M, Nečas M, Žák Z, Dostál J. Molecular and crystal structures of three berberine derivates. Molecules. 2001;6:433-441. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Leone A. Biochemical markers of passive smoking. Passive Smoking and Cardiovascular Pathology: Mechanisms and Physiopathological Basis of Damage. New York: Nova Science Pub Inc 2007; 19-37. |
| 37. | Leone A, Giannini D, Bellotto C, Balbarini A. Passive smoking and coronary heart disease. Curr Vasc Pharmacol. 2004;2:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Robertson JIS. Hypertension: primary and secondary prevention. Preventive Cardiology. London: Butterworths 1983; 62-85. |
| 39. | Stefanadis C, Vlachopoulos C, Tsiamis E, Diamantopoulos L, Toutouzas K, Giatrakos N, Vaina S, Tsekoura D, Toutouzas P. Unfavorable effects of passive smoking on aortic function in men. Ann Intern Med. 1998;128:426-434. [PubMed] |
| 40. | Moreyra AE, Lacy CR, Wilson AC, Kumar A, Kostis JB. Arterial blood nicotine concentration and coronary vasoconstrictive effect of low-nicotine cigarette smoking. Am Heart J. 1992;124:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Quillen JE, Rossen JD, Oskarsson HJ, Minor RL, Lopez AG, Winniford MD. Acute effect of cigarette smoking on the coronary circulation: constriction of epicardial and resistance vessels. J Am Coll Cardiol. 1993;22:642-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Deedwania PC. Endothelium: a new target for cardiovascular therapeutics. J Am Coll Cardiol. 2000;35:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Davis JW, Shelton L, Watanabe IS, Arnold J. Passive smoking affects endothelium and platelets. Arch Intern Med. 1989;149:386-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Vane JR, Anggård EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1299] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 45. | Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3361] [Cited by in RCA: 3311] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 46. | Ghiadoni L, Taddei S, Virdis A, Sudano I, Di Legge V, Meola M, Di Venanzio L, Salvetti A. Endothelial function and common carotid artery wall thickening in patients with essential hypertension. Hypertension. 1998;32:25-32. [PubMed] |
| 47. | Leone A. Anatomy of the coronary arteries. Coronary circulation in nonsmokers and smokers. New York: Nova Science Pub Inc 2008; 1-20. |
| 48. | Brunner HR, Kirshman JD, Sealey JE, Laragh JH. Hypertension of renal origin: evidence for two different mechanisms. Science. 1971;174:1344-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 219] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Freis ED. Salt, volume and the prevention of hypertension. Circulation. 1976;53:589-595. [PubMed] |
| 50. | Ithakissios DS, Kubiatowicz DO, Windorski DC, Wicks JH. Immune and non-immune T4 radioassays utilizing albumin magnetic microparticles. Clin Chim Acta. 1978;84:69-84. [PubMed] |
| 51. | Gleibermann L. Blood pressure and dietary salt in human populations. Ecol Food Nutr. 1973;2:143-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 139] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Castelli WP, Kannel WB, Mcgee DL. Latest perspectives on cigarette smoking and cardiovascular disease: the framingham study. J Cardiac Rehabil. 1984;4:267-277. |
| 53. | Regoli D, Park WK, Rioux F. Pharmacology of angiotensin. Pharmacol Rev. 1974;26:69-123. [PubMed] |
| 54. | Davis JO, Freeman RH. Mechanisms regulating renin release. Physiol Rev. 1976;56:1-56. [PubMed] |
| 55. | Ferrario CM, Gildenberg PL, McCubbin JW. Cardiovascular effects of angiotensin mediated by the central nervous system. Circ Res. 1972;30:257-262. [PubMed] |
| 56. | Britton S, Di Salvo J. Effects of angiotensin I and angiotensin II on hindlimb and coronary vascular resistance. Am J Physiol. 1973;225:1226-1231. [PubMed] |
| 57. | FOWLER NO, HOLMES JC. CORONARY AND MYOCARDIAL ACTIONS OF ANGIOTENSIN. Circ Res. 1964;14:191-201. [PubMed] |
| 58. | Drímal J, Pávek K, Selecký FV. Primary and secondary effects of angiotensin on the coronary circulation. Cardiologia. 1969;54:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Cohen MV, Kirk ES. Differential response of large and small coronary arteries to nitroglycerin and angiotensin. Autoregulation and tachyphylaxis. Circ Res. 1973;33:445-453. [PubMed] |
| 60. | Catt KJ, Mendelsohn FA, Millan MA, Aguilera G. The role of angiotensin II receptors in vascular regulation. J Cardiovasc Pharmacol. 1984;6 Suppl 4:S575-S586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Gunther S, Gimbrone MA, Alexander RW. Regulation by angiotensin II of its receptors in resistance blood vessels. Nature. 1980;287:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 62. | Griendling KK, Murphy TJ, Alexander RW. Molecular biology of the renin-angiotensin system. Circulation. 1993;87:1816-1828. [PubMed] |
| 63. | Hatton R, Clough DP, Adigun SA, Conway J. Functional interaction between angiotensin and sympathetic reflexes in cats. Clin Sci (Lond). 1982;62:51-56. [PubMed] |
| 64. | Nakano J. Cardiovascular actions of vasopressin. Jpn Circ J. 1973;37:363-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Khayyal MA, Eng C, Franzen D, Breall JA, Kirk ES. Effects of vasopressin on the coronary circulation: reserve and regulation during ischemia. Am J Physiol. 1985;248:H516-H522. [PubMed] |
| 66. | Martín de Aguilera E, Vila JM, Irurzun A, Martínez MC, Martínez Cuesta MA, Lluch S. Endothelium-independent contractions of human cerebral arteries in response to vasopressin. Stroke. 1990;21:1689-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Pullan PT, Johnston CI, Anderson WP, Korner PI. Plasma vasopressin in blood pressure homeostasis and in experimental renal hypertension. Am J Physiol. 1980;239:H81-H87. [PubMed] |
| 68. | Vanhoutte PM, Mombouli JV. Vascular endothelium: vasoactive mediators. Prog Cardiovasc Dis. 1996;39:229-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 69. | Dusting GJ, Moncada S, Vane JR. Prostaglandins, their intermediates and precursors: cardiovascular actions and regulatory roles in normal and abnormal circulatory systems. Prog Cardiovasc Dis. 1979;21:405-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 117] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Needleman P, Kaley G. Cardiac and coronary prostaglandin synthesis and function. N Engl J Med. 1978;298:1122-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 71. | Cowley AW, Roman RJ. The role of the kidney in hypertension. JAMA. 1996;275:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 165] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 72. | DiBona GF. Neural control of the kidney: past, present, and future. Hypertension. 2003;41:621-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 124] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 73. | Baer L, Radichevich I. Cigarette smoking in hypertensive patients. Blood pressure and endocrine responses. Am J Med. 1985;78:564-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Heistad DD, Armstrong ML, Marcus ML, Piegors DJ, Mark AL. Augmented responses to vasoconstrictor stimuli in hypercholesterolemic and atherosclerotic monkeys. Circ Res. 1984;54:711-718. [PubMed] |
| 75. | Watts DT. The effects of nicotine and smoking on the secretion of epinephrine. Ann NY Acad Sci. 1960;90:74-80. [RCA] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Axelrod J. The metabolism, storage, and release of catecholamines. Recent Prog Horm Res. 1965;21:597-622. [PubMed] |
| 77. | Chalmers JP, West MJ. The nervous system in the pathogenesis of hypertension. Handbook of hypertension. Clinical aspects of essential hypertension. Amsterdam: Elsevier Science Ltd 1983; 64-96. |
| 78. | Calaresu FR, Yardley CP. Medullary basal sympathetic tone. Annu Rev Physiol. 1988;50:511-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 157] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 79. | Leone A. Humoral and metabolic regulation of coronary circulation. Coronary circulation in nonsmokers and smokers. New York: Nova Science Pub Inc 2008; 43-60. |
| 80. | Schievelbein H, Richter F. The influence of passive smoking on the cardiovascular system. Prev Med. 1984;13:626-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 81. | Glantz SA. Air pollution as a cause of heart disease. Time for action. J Am Coll Cardiol. 2002;39:943-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Pope CA, Eatough DJ, Gold DR, Pang Y, Nielsen KR, Nath P, Verrier RL, Kanner RE. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect. 2001;109:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 83. | Craig WY, Palomaki GE, Haddow JE. Cigarette smoking and serum lipid and lipoprotein concentrations: an analysis of published data. BMJ. 1989;298:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 536] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 84. | Pedersen TR. Lowering cholesterol with drugs and diet. N Engl J Med. 1995;333:1350-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 85. | Leone A. Does Smoking Act as a Friend or Enemy of Blood Pressure? Let Release Pandora's Box. Cardiol Res Pract. 2011;2011:264894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 86. | Leone A, Landini L, Leone A. What is tobacco smoke? Sociocultural dimensions of the association with cardiovascular risk. Curr Pharm Des. 2010;16:2510-2517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 87. | Hughes K, Leong WP, Sothy SP, Lun KC, Yeo PP. Relationships between cigarette smoking, blood pressure and serum lipids in the Singapore general population. Int J Epidemiol. 1993;22:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Leone A, Lopez M, Picerno G. [Role of smoking in determining coronary heart disease. Hypothesis on the possible mechanism of myocardial damage]. Minerva Cardioangiol. 1984;32:435-439. [PubMed] |
| 89. | Gordon T, Kannel WB. Multiple risk functions for predicting coronary heart disease: the concept, accuracy, and application. Am Heart J. 1982;103:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Karvonen M, Orma E, Keys A, Fidanza F, Brozek J. Cigarette smoking, serum-cholesterol, blood-pressure, and body fatness; observations in Finland. Lancet. 1959;1:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Ballantyne D, Devine BL, Fife R. Interrelation of age, obesity, cigarette smoking, and blood pressure in hypertensive patients. Br Med J. 1978;1:880-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 92. | Trap-Jensen J. Effects of smoking on the heart and peripheral circulation. Am Heart J. 1988;115:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Su C. Actions of nicotine and smoking on circulation. Pharmacol Ther. 1982;17:129-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 94. | Leone A. Interactive effect of combined exposure to active and passive smoking on cardiovascular system. Recent Pat Cardiovasc Drug Discov. 2011;6:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 95. | Landini L, Leone A. Smoking and hypertension: effects on clinical, biochemical and pathological variables due to isolated or combined action on cardiovascular system. Curr Pharm Des. 2011;17:2987-3001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149-2155. [PubMed] |
| 97. | Desideri G, Ferri C. Endothelial activation. Sliding door to atherosclerosis. Curr Pharm Des. 2005;11:2163-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684-2698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 620] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 99. | Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1763] [Cited by in RCA: 1697] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 100. | Leone A. Smoking and hypertension: independent or additive effects to determining vascular damage? Curr Vasc Pharmacol. 2011;9:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 101. | Miller PM, Anton RF, Egan BM, Basile J, Nguyen SA. Excessive alcohol consumption and hypertension: clinical implications of current research. J Clin Hypertens (Greenwich). 2005;7:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Aronow WS. Effect of passive smoking on angina pectoris. N Engl J Med. 1978;299:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 108] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Marius-Nunez AL. Myocardial infarction with normal coronary arteries after acute exposure to carbon monoxide. Chest. 1990;97:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Leone A. Passive smoking causes cardiac alterations in post-MI subjects. Int J Smoking Cessation. 1996;3:42-43. |
| 105. | Reinprecht F, Elmståhl S, Janzon L, André-Petersson L. Hypertension and changes of cognitive function in 81-year-old men: a 13-year follow-up of the population study "Men born in 1914", Sweden. J Hypertens. 2003;21:57-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Bonita R, Duncan J, Truelsen T, Jackson RT, Beaglehole R. Passive smoking as well as active smoking increases the risk of acute stroke. Tob Control. 1999;8:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 107. | Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 108. | Fabbian F, Cantelli S, Molino C, Pala M, Longhini C. Dialysis initiation and survival in patients with refractory congestive heart failure. Int J Artif Organs. 2009;32:492-495. [PubMed] |
| 109. | Seeman E, Melton LJ, O'Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med. 1983;75:977-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 405] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 110. | ALLHAT officers and coordinators for the ALLHAT collaborative research group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4118] [Cited by in RCA: 3714] [Article Influence: 161.5] [Reference Citation Analysis (0)] |
| 111. | Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6011] [Cited by in RCA: 5642] [Article Influence: 225.7] [Reference Citation Analysis (0)] |
| 112. | Nadar S, Lim HS, Lip GY. Implications of the LIFE trial. Expert Opin Investig Drugs. 2003;12:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 113. | Progress collaborative group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2266] [Cited by in RCA: 2049] [Article Influence: 85.4] [Reference Citation Analysis (0)] |