Copyright
©The Author(s) 2015.
World J Pharmacol. Sep 9, 2015; 4(3): 236-264
Published online Sep 9, 2015. doi: 10.5497/wjp.v4.i3.236
Published online Sep 9, 2015. doi: 10.5497/wjp.v4.i3.236
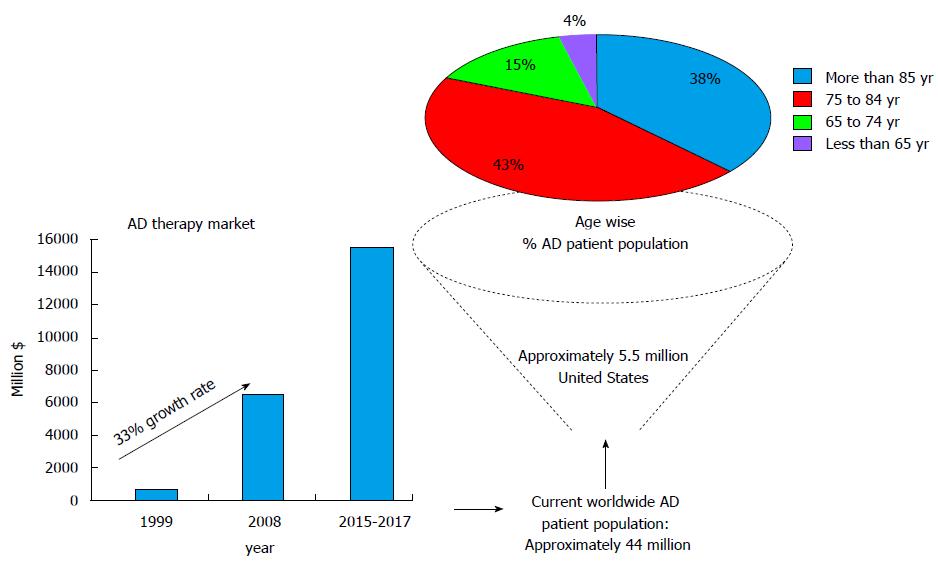
Figure 1 Schematic of Alzheimer’s disease afflicted patient population and associated therapy market data.
AD: Alzheimer’s disease.
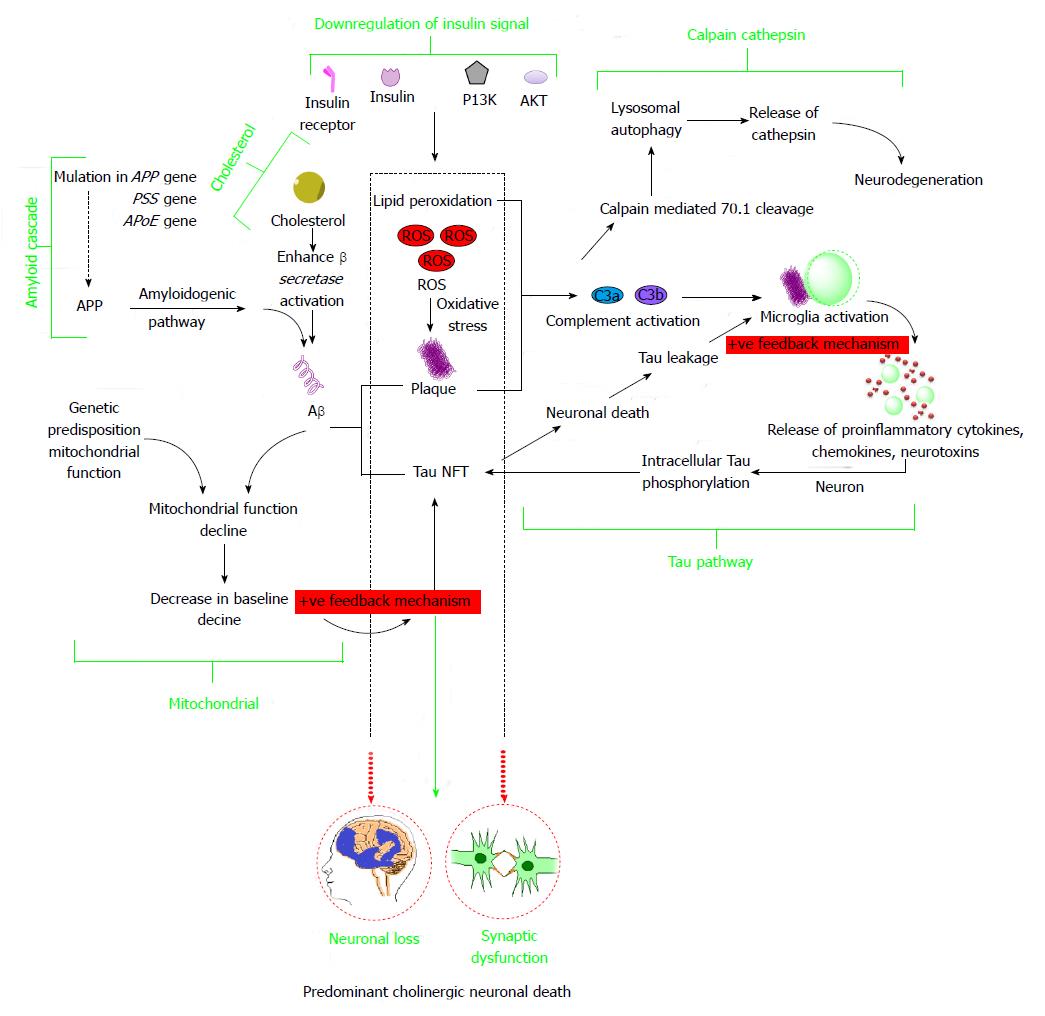
Figure 2 Schematic representation indicating interlinks among important Alzheimer’s disease pathogenetic hypotheses that majorly include amyloid cascade hypothesis, taupathy, mitochondrial dysfunction hypothesis, cholesterol induced mechanism, insulin signal downregulation, calpain-cathepsin hypothesis.
APP: Amyloid precursor protein; PS: Presinilin; APoE: Apolipoprotein E; PI3K: Phosphoinositide-3-kinase; AKT: Protein kinase B; ROS: Reactive oxygen species.
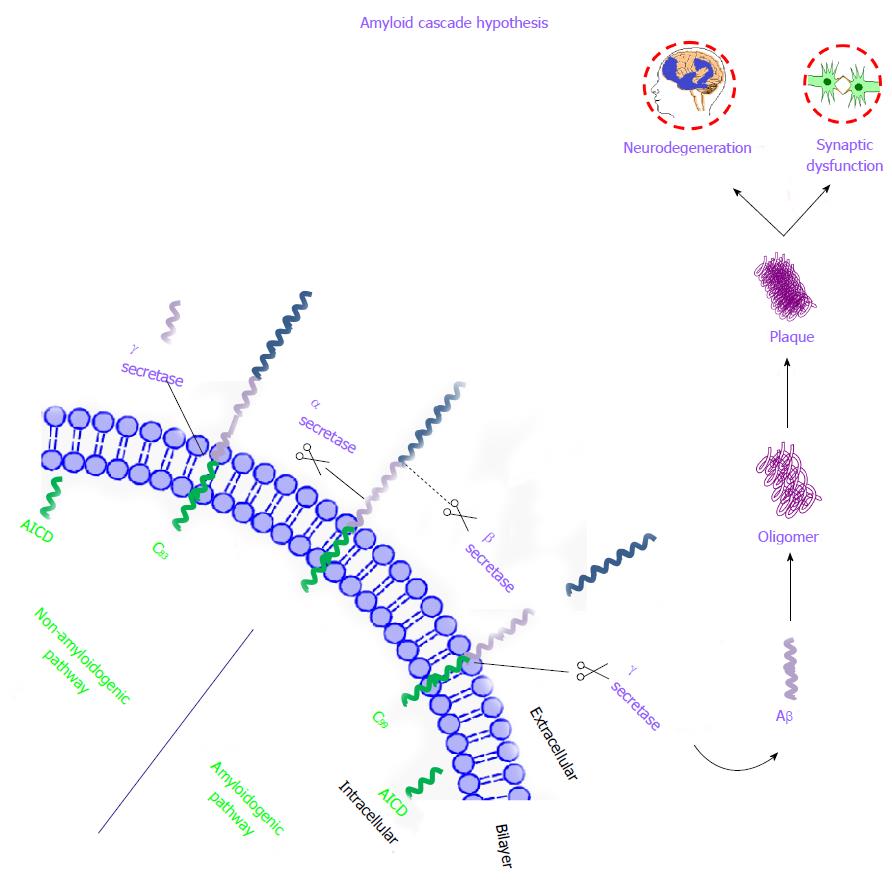
Figure 3 Schematic representation of amyloid cascade hypothesis.
Three enzymes α, β, and γ secretase play a crucial role in the proteolytic cleavage of APP. The first step, extracellular fragment of APP is cleaved by α-secretase (non-amyloidogenic and predominant pathway under normal condition) or β-secretase (amyloidogenic pathway predominant under AD) leading to 83 or 99 amino acid peptide residues respectively that remain attached as a trans membrane fragment. These fragments are ultimately cleaved by γ-secretase which leads to formation of toxic Aβ(1-42) fragments in case of amyloidogenic pathway and initiates the extracellular Aβ plaque formation. APP: Amyloid precursor protein; AD: Alzheimer’s disease.
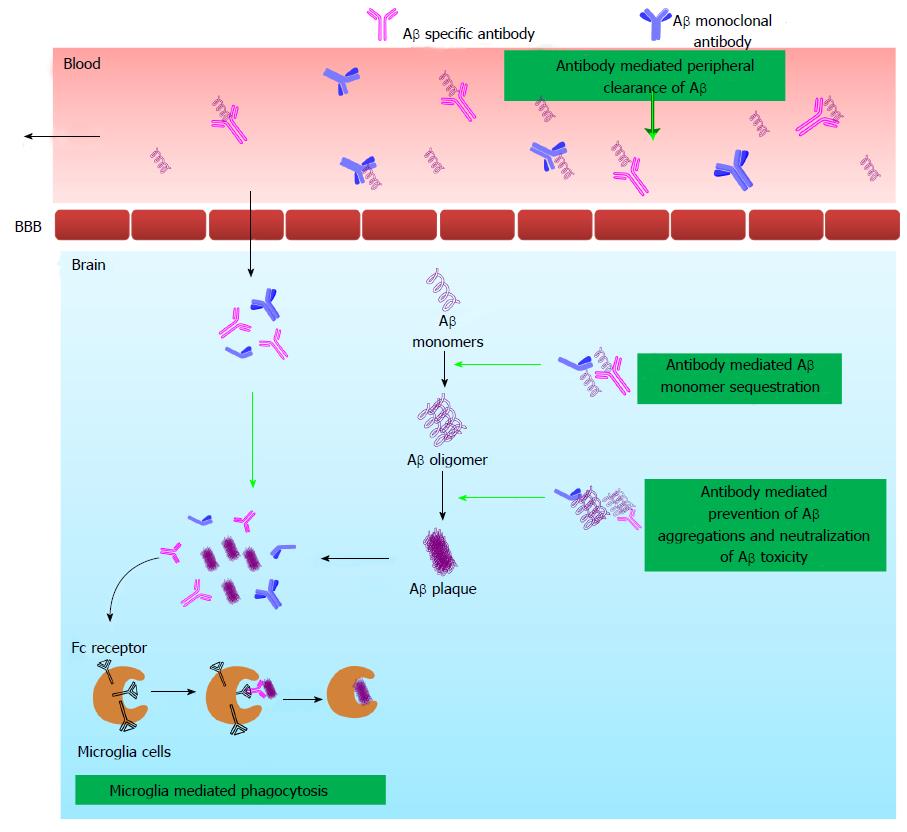
Figure 4 Schematic diagram explaining different mechanistic pathways hypothesized for immunotherapy to exert Amyloid β clearance from brain and plasma.
Immunotherapy exerts its activity by active and passive ways. In active immunization, the Aβ peptide or fragment is injected into the host which in turn stimulates cellular and humoral immune response to generate anti-Aβ antibody. In passive immunization, directly Aβ peptide specific antibody is injected into the host. The generated anti-Aβ antibodies provoke anti-AD activity by one of the or combination of following ways: microglia-mediated phagocytosis, antibody mediated Aβ monomer sequestration, antibody mediated prevention of Aβ aggregation and neutralization of Aβ toxicity and antibody mediated peripheral clearance of Aβ. Aβ: Amyloid β; BBB: Blood brain barrier. AD: Alzheimer’s disease.
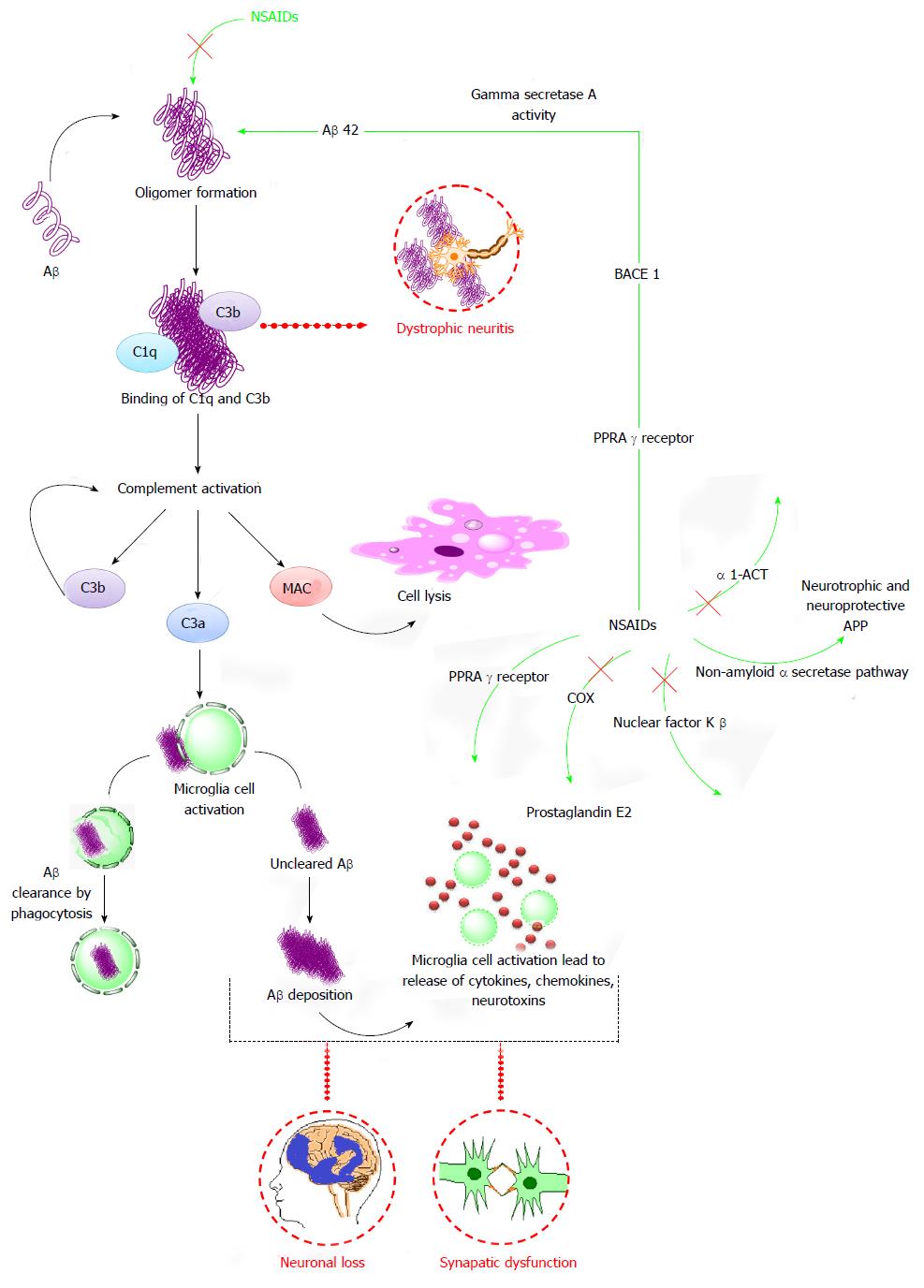
Figure 5 Diagram representation elaborating various mechanistic pathways by which anti-Alzheimer’s disease activity is executed by non-steroidal anti-inflammatory drugs.
The aggregation and deposition of Aβ plaques provoke the activation of microglia, astrocytes and complement system (C1q, C3b, C3a, MAC, cytokines and chemokines). The Aβ plaques which evades the clearance process forms deposit and provokes microglial cell activation and release of cytokines, chemokines and neurotoxins which consequently results in neuronal loss and synaptic dysfunction. NSAIDs are reported to act by multiple ways to elicit anti-AD activity which includes suppresion of oligomer formation, PPRAγ, COX, NFKβ, α1-ACT and non-amyloid α secretase pathway. Aβ: Amyloid β; APP: Amyloid precursor protein; MAC: Membrane attack complex; PPARγ: Peroxisome proliferator-activated receptor-γ; COX: Cycloxygenase; α1-ACT: α1-antichymotrypsin; BACE1: Beta-secretase 1.
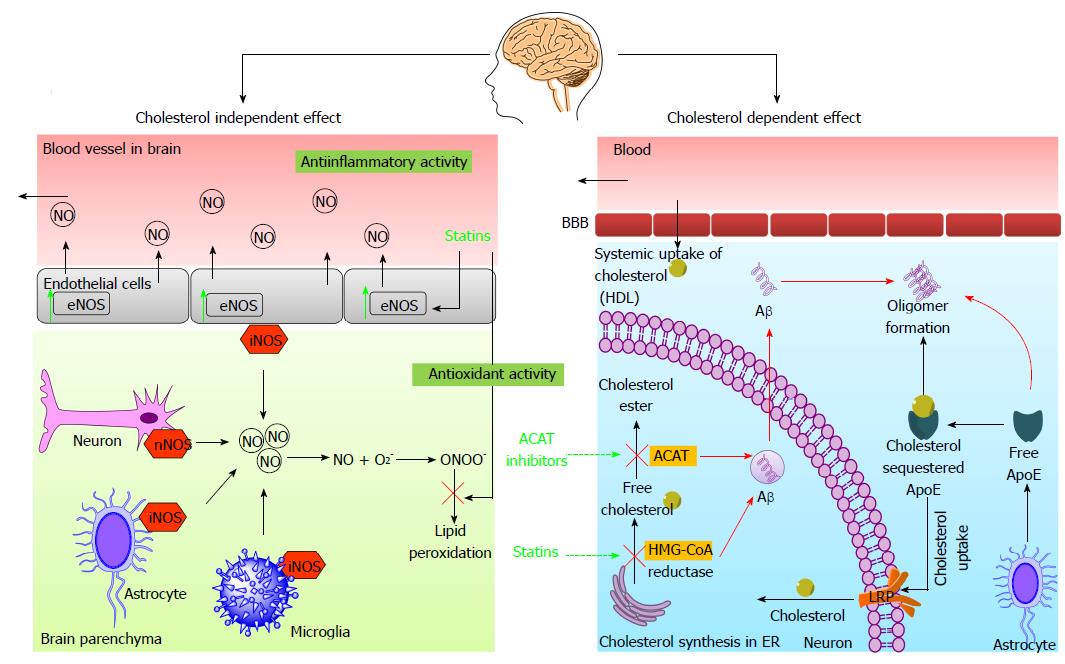
Figure 6 Diagrammatic illustration of anti-amyloid activity of 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors via cholesterol independent and cholesterol dependent mechanistic pathways.
Cholesterol independent pathway: Statins cause NO mediated anti-inflammatory activity and facilitate the clearance of systemic Aβ by stimulating endothelial nitric oxide synthase. The statins with the virtue of its antioxidant effect cause reduction in lipid peroxidation which is escalated due to elevated levels of NO in AD brain. Cholesterol dependent pathway: The systemic cholesterol enters the brain in form of HDL. The astrocytes originated ApoE facilitates the uptake of extracellular free cholesterol and release into the neuronal cells via LRP. This act reduces free cholesterol and curtails the Aβ genesis whereas un-sequestered free ApoE aggravates the Aβ formation. Statins inhibits the HMG-CoA reductase and subsequently reverse the elevated intracellular cholesterol levels and blocks the Aβ formation. Also, ACAT inhibition leads to reduction in Aβ levels by an unknown mechanism. HDL: High density lipoprotein; BBB: Blood brain barrier; Aβ: Amyloid β; ApoE: Apolipoprotein E; LRP: LDL receptor-related protein; HMG-CoA reductase: 3-hydroxy-3-methyl-glutaryl-CoA; ER: Endoplasmic reticulum; NO: Nitric oxide; eNOS: Endothelial nitric oxide synthase; nNOS: Neuronal nitric oxide synthase; iNOS: Inducible nitric oxide synthase; ACAT: Acetyl-coenzyme a acetyltransferase. AD: Alzheimer’s disease.
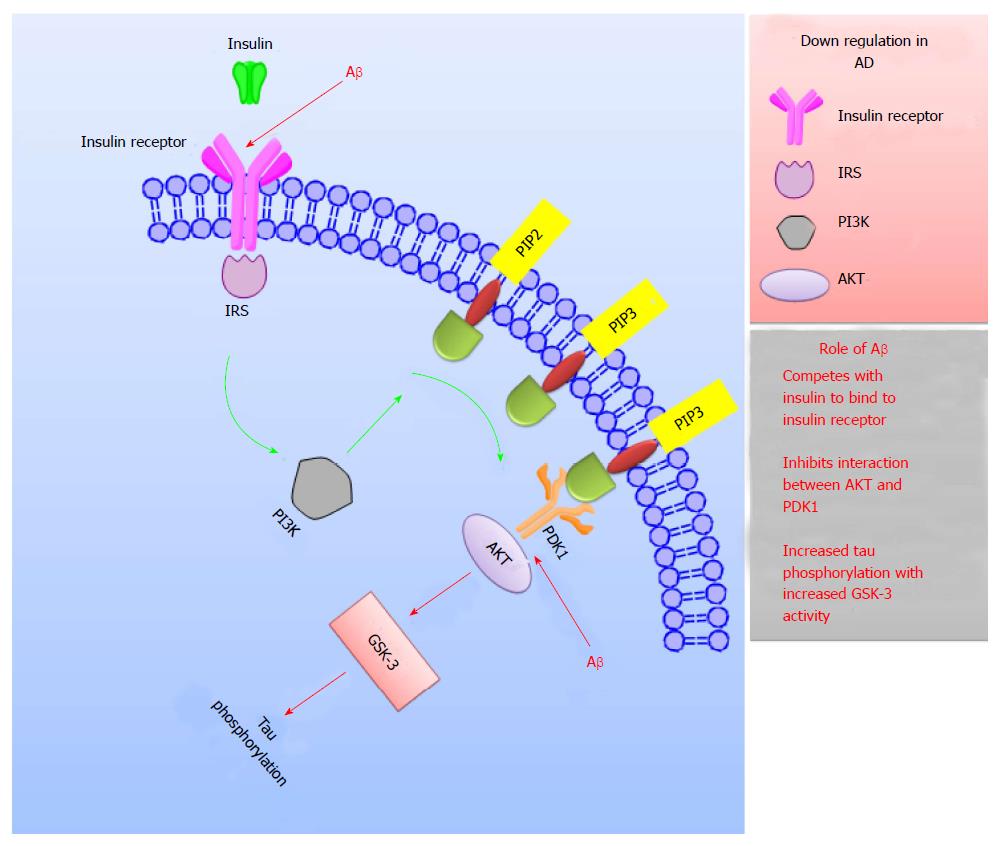
Figure 7 Schematic representation explaining the role of insulin in Alzheimer’s disease treatment.
Interaction of IRS with insulin receptor initiates the event of insulin signaling. This act triggers a cascade of activities which subsequently results in expression of PIP3 on the plasma membrane. This further leads to the activation of PDK-1 which interacts with AKT to generate the transmission of the insulin signal. In AD, the crucial components for insulin signaling like insulin receptors, IRS, PI3K and AKT; are down regulated. Also, Aβ competes with insulin to bind to insulin receptors, inhibits the PI3K-AKT interaction and enhances the GSK-3 activity which subsequently increases tau phosphorylation leading to impaired signaling in AD. IRS: Insulin receptor substrate; PI3K: Phosphoinositide-3-kinase; PIP: Phosphatidylinositol phosphate; PDK1: Phosphoinositide-dependent kinase-1; AKT: Protein kinase B; GSK-3: Glycogen synthase kinase-3; AD: Alzheimer’s disease.
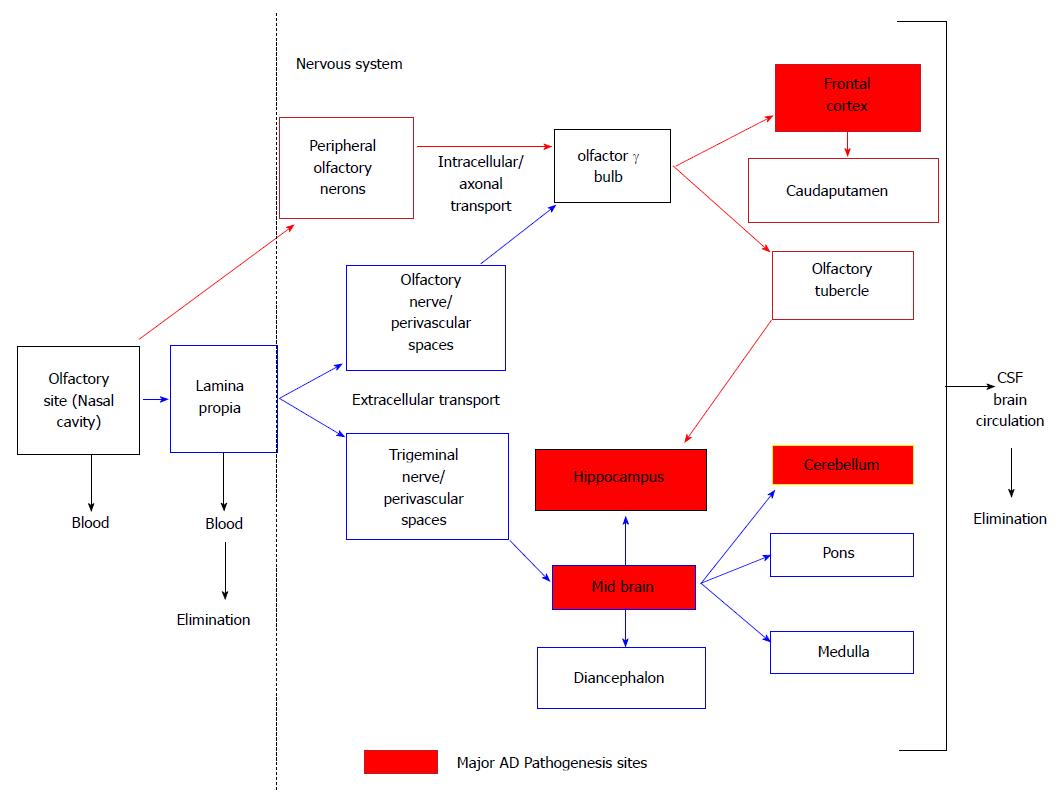
Figure 8 Schematic representation of nose to brain uptake by olfactory pathway circumventing blood brain barrier that allows entry via peripheral olfactory neurons and lamina propria in the central nervous system.
Modified from[172]. CSF: Cerebrospinal fluid.
- Citation: Desai P, Shete H, Adnaik R, Disouza J, Patravale V. Therapeutic targets and delivery challenges for Alzheimer’s disease. World J Pharmacol 2015; 4(3): 236-264
- URL: https://www.wjgnet.com/2220-3192/full/v4/i3/236.htm
- DOI: https://dx.doi.org/10.5497/wjp.v4.i3.236