BACTERIAL ADHESION AND PATHOGENICITY
The ability of bacteria to attach and grow on almost any surface has been known for decades. The importance of adhesion in the colonisation of specific substrates, and its role in the pathogenesis of bacterial infections and in the maintenance of the carrier state has been studied widely in recent years[1]. Neisseria meningitidis (N. meningitidis) is only found in humans, suggesting that its ability to cause disease is likely an casual side effect of its life cycle. Globally, the carrier rate of N. meningitidis ranges from 10%-35% among healthy adults[2]; the mean carrier rate in children is 5.9%, peaking at 10.3% in children aged under 3 years[3]. This situation has been associated with the genetic characteristics of the circulating strains of N. meningitidis, the immune pressure exerted by vaccination programmes and the hygienic and social conditions within a community. Compared with the rates of colonisation, meningococcal disease is less common, its development being affected by interacting factors such as the virulence of the bacterium, host defence mechanisms, the age of the host and the history of previous viral infections[2]. The best-defined virulence factor of N. meningitidis is its polysaccharide capsule that indicates its serogroup. Although 13 serogroups of N. meningitidis have been described (A, B, C, D, 29E, H, I, K, L, Y, W-135, X and Z), invasive meningococcal disease is most frequently caused by serogroups A, B, C, Y and W-135, to which 10% mortality has been attributed.
The adhesion of bacteria to epithelial surfaces is an initial step in the colonisation of microbial habitats, and it ensures the survival of N. meningitidis in its ecological niche[4]. Adherence can be defined as a phenomenon resulting from the interaction between two surfaces, with the participation of physical, chemical and biological factors, with contact between the bacterium and the cell being necessary for adherence to take place. The adhesion occurs in several steps: (1) N. meningitidis attaches to the surface of target cells to form small colonies. This step is essentially a pilus-mediated process; and (2) after N. meningitidis has been attached, it comes into close contact with surface of the target cells (intimate adhesion). The adhesive interaction is present both in commensal bacteria and in pathogens and so for N. meningitidis to adhere, it has developed proprietary adhesive mechanisms that allow it to compete with flora of the same ecological niche[5]. Meningococcal pili are of type IV and are composed of pilin subunits that are encoded by pilE gene. Other homologous proteins, PilC1 and PilC2, are also involved in pilus assembly and adhesion. PilC1-containing may involve interaction with CD46, a human trans-membrane glycoprotein involved in complement regulation. The expression of pilC1 is induced following the contact of N. meningitidis with viable target cells. Both pili and capsule are downregulated upon contact with target cells. This downregulation seems to be associated with intimate adhesion of N. meningitidis to target cells[5,6].
The “adhesion process” can be defined in terms of the adhesion affinity of bacteria to epithelial surfaces, as has been described in the Michaelis-Menten equations. The maximum point of adhesion (and affinity) can be determined graphically by the Lineawever-Burk equations, using simple experimental models[7]. This first phase would be a reversible process in which Van der Waals and electrostatic forces are responsible for a wide range of interactions, including chemical bonding, dipolar interaction and hydrophobicity. Surface molecules as N-acetylneuraminic acid may alter the initial adhesion strength, reducing electrostatic repulsive forces or increasing attractive ones[8]. The cell surfaces of both prokaryotic and eukaryotic cells are negatively charged. Electrostatic repulsive forces between the cell and the bacteria can be overcome by long and short-range attractive forces, and so the specific binding of fimbriae with cell surface receptors must overcome the repulsive forces between the two surfaces. According to Smyth et al[9], the reduction of the surface bacteria potential by the intervention of hydrophobic adhesins probably facilitates adhesion, with the hydrophobic forces being a first step in the interaction of the organism with mucosal surfaces. Surface hydrophobicity is a non-specific adhesion factor which is important to the adhesion and growth of microorganisms on epithelial surfaces. The hydrophobic-hydrophilic environment of the bacterial surface is modulated by hydrophobic or hydrophilic agents (increasing or reducing hydrophobicity, respectively), which may co-exist on the surface of the outer membrane[5]. Generally, the strains that formed biofilms show high-level cell surface hydrophobicity. Many studies have examined the contribution of surface hydrophobicity to bacterial adhesion, with particular attention to Salmonella[10,11], Escherichia coli (E. coli), N. gonorrhoeae[12,13] and N. meningitidis[14,15].
The type IV pili of N. meningitidis are crucial determinants of the adhesion of these pathogens to epithelial and endothelial cells[16]. Under natural conditions, pili are the only means by which encapsulated N. meningitidis can adhere to human mucosal surfaces[17,18].
SURFACE MODULATION AND INTERACTION WITH THE HOST
When N. meningitidis adheres to epithelial cells, it becomes resistant to the bactericidal effect of the antimicrobial peptide LL-37, which is the first line of defence of innate immunity. The decreased binding of LL-37 to the adhered bacteria can result from its degradation by proteases released at the site of infection. Furthermore, N. meningitidis induces the formation in the nasopharynx of cholesterol-rich membrane microdomains, which are essential to the antimicrobial resistance induced by bacterial adhesion[2].
To avoid immune detection, the surface components of N. meningitidis may be modified. The structural and antigenic modification of surface molecules can involve changes in gene alleles. Studies have reported the import of genetic material from other bacteria or via intragenomic recombination[2]. N. meningitidis may be encapsulated or unencapsulated. N. meningitidis isolated from blood or cerebrospinal fluid is invariably encapsulated. The existence of the capsule enables it to withstand the effects of antibodies and complements and to resist serum opsonic activity. Some lipopolysaccharide structures help N. meningitidis to evade the immune response, and are more frequently observed in N. meningitidis isolated from blood than in healthy nasopharyngeal carriers. Both the capsule and some lipopolysaccharide immunotypes (L1, L8 and L10) of N. meningitidis may influence bacterial adhesion and invasive capacity. It has been found the inhibitory role of capsule in biofilm formation[14]. The capsule genes are located in a single chromosomal locus (cps) divided into three regions. The capsular polysaccharides B, C, W-135 and Y contain sialic acid, which contributes to make the lipopolysaccharides of the capsule less visible to the immune system, since sialic acid is a common component of the host cell surfaces. Moreover, the serogroup B capsule contains a homopolymer that is structurally identical to the neural adhesion molecule, which is responsible for the poor immune response generated by serogroup B in humans. However, the genetic similarities of the loci of serogroups B, C, W and Y (not A) favour the horizontal exchange of fragments of the capsule between different serogroups[2].
N. meningitidis expresses and secretes various surface molecules that bind to epithelial molecules, and some of these proteins include lactoferrin and the proteins bound to the transferrin that enable the meningococcus to acquire iron from the environment. Iron is a crucial element for bacterial growth in the surface colonisation stage and during the production of disease[19,20], although some adherent properties, such as hydrophobicity and adherence to inert surfaces like nitrocellulose remain unchanged after incubation in culture media supplemented with Fe[21]. Other authors have described nine porin complexes formed by different combinations of the meningococcal porin protein (Por) A, PorB and RmpM proteins[22]. N. meningitidis expresses two types of outer membrane proteins (Opa and Opc) which give an opaque appearance to colonies in agar. Opa and Opc are of a similar size (27-31 kDa). Most Opa molecules recognise one or more members of the family of carcinoembryonic antigen-related cell adhesion molecules (CEACAM). The CEACAM1 receptor is found in epithelial and endothelial cells, while other family members such as CEACAM3 and CEACAM6 are expressed in neutrophils. CEACAM receptor density in the epithelial cells is modulated by the secretion of inflammatory cytokines, such that a high expression of CEACAM receptors takes place in response to inflammation, which could influence the development of meningococcal disease. Furthermore, some Opa proteins can interact with the heparan sulphate proteoglycan that is present in most epithelial cells[2,23].
Over the past 50 years, our understanding of the importance of serogroup B (MenB) disease per se, the social impact of fear caused by the devastating effects of the disease. The difficulty of inducing an effective immune response against the MenB capsular polysaccharide has lead to the search in vaccines for this serogroup based on outer membrane proteins (OMP). Public health interventions in Cuba, Norway and New Zealand have demonstrated that these protein-based vaccines can prevent MenB disease.
By combining a pangenome analysis with an extensive experimental validation to identify new potential vaccine candidates, genes coding for antigens likely to be exposed on the surface of the MenB were selected after a multistep comparative analysis of entire Neisseria genomes. Again, in the quest for vaccine candidates are successfully identified a significant number of new genes. Recent studies with meningococcal membrane proteins have centered on conserved antigens in order to obtain a universal vaccine that confers protection against a broad range of strains. There are several recent reports about the use of conserved minor OMP from N. meningitidis as immunogens[24].
The classical bioinformatic approaches, in combination with proteomic data, conventional protein purification and immunological evaluation are powerful tools for the identification of novel meningococcal antigens and open reading frames and potential vaccine components[25].
We now know that OMP based vaccines are most effective when are used against epidemics due to a homologous or clonal strain carrying the same PorA as that present in the vaccine. When used against endemic disease or outbreaks due to a number of different strains (heterologous epidemiologic situations), the level of effectiveness will generally be too low to rely on the effects of a conventional OMP vaccine alone for protection.
The general strategy in the Pajon et al[25] study, was to maximize the chance of identifying bacterial surface components by selecting not only proteins predicted by protein localization algorithms in outer membrane components of gram-negative bacteria, but also those predicte as periplasmic or inner membrane proteins. However, we must stress that while the most attention in the development of meningococcal vaccines has been devoted to major OMPs. The impact of conserved protein components in the induction of a significant immune response, and their potential as adjuvants, it must not be overlooked. The success in expressing all cloned genes came from the use of a highly optimized expression/purification platform designed precisely for this scenario, but also from the stringent selection procedure of potential vaccine candidates. Finally, five proteins are capable of inducing a functional antibody response vs N. meningitidis strain CU385: NMB0606 a potential YajC orthologue, NMB0928 the neisserial NlpB (BamC), NMB0873 a LolB orthologue, NMB1163 a protein belonging to a curli-like assembly machinery, and NMB0938 (a neisserial specific antigen) with evidence of positive selection appreciated for NMB0928[25].
The new set of vaccine candidates and the novel proposed functions will open a new wave of research in the search for the elusive neisserial vaccine. The key limitation of conventional wild-type outer membrane vesicle (wtOMV) vaccines is their lack of broad protective activity against the large diversity of MenB strains circulating globally. The experience with wtOMV vaccines also provide important information for the next generation of MenB vaccines designed to give more comprehensive protection against multiple strains.
BACTERIAL ADHESION AND OXIDATIVE STRESS
It has been shown that the N. meningitidis loci involved in defence against oxidative stress are also involved in biofilm formation and contribute to the colonisation of epithelial surfaces[26]. Incubation of N. meningitidis in vitro with antioxidant molecules increases their adherence to inert surfaces and therefore the ability to generate biofilm, and at the same time increases their surface hydrophobicity[14,21]. Similar observations regarding adherence to nitrocellulose have been demonstrated in E. coli[27]. These observations are an example of how environmental conditions can modulate in N. meningitidis the expression of molecules to make it more virulent or more adherent. In vivo, plasma antioxidant levels are lower in children who are asymptomatic carriers of N. meningitidis[3]. We have analysed the association between total antioxidant capacity in plasma and the carrier state of N. meningitidis. In the carrier state, the odds ratio for this association (total antioxidant capacity in plasma < 0.25) was 8.44 (95%CI: 1.5-48.9). These observations are in the line with Jamet et al[26], who reported that the activation in N. meningitidis of genes involved in defence against oxidative stress (lower levels of plasma antioxidants) favours the adhesion of the bacteria and nasopharyngeal carrier status. Other studies have shown that oxidative stress can be induced experimentally with cysteine depletion, can trigger growth arrest and release of outer membrane vesicles (sOMV). Outer membrane vesicles contain immunogenic proteins and contribute to in vivo survival and virulence of bacterial pathogens[28].
BACTERIAL ADHESION AND VIRULENCE
Virulence, defined as the degree of aggressiveness of a pathogen, is a highly variable condition. The degree of virulence fluctuates according to the conditions in which microorganisms and their genetic makeup are located. In general, a pathogen becomes less virulent on passing from a natural environment to an artificial culture medium; in these circumstances, it is said to be attenuated, and the same effect can be observed in unfavourable environmental conditions. The virulence of a microorganism can be reduced, either by the use of certain culture media, or by exploiting its successive passage through animals. Numerous studies support this; thus, Horská et al[29] have reported that three different bacterial strains of Pseudomonas are capable of changing the surface charge and their hydrophobicity. By contrast, an attenuated microorganism can acquire greater virulence by its prior passage through certain animal species; specifically, pneumococcal virulence is enhanced by its passage through mice[30]. N. meningitidis requires iron, and in the absence of iron alters its gene expression to increase iron acquisition[19,31]. When N. meningitidis, has grown in an iron-restricted environment, it synthesises new outer membrane proteins, which are necessary for its survival. Some of these proteins Tbp A and Tbp B are examples of meningococcal surface antigens regulated by iron, which are not expressed after culture in common laboratory media[32]. TbpA was found to possess a similar architecture to the siderophore and is highly immunogenic, allowing for prediction of potentially important ligand-binding epitopes[33].
The passage of microorganisms through the epithelial layer is not a passive phenomenon (Figure 1). Microorganisms, after overcoming the first hurdle, consisting of the surface epithelium, are exposed to various host defence mechanisms, of which the most important is the inflammatory response. In the course of this response, the blood vessels dilate, thereby increasing their permeability and allowing various serum factors (immunoglobulins and other proteins) to come into contact with the infectious agent. Moreover, the activation of fibrinogen to fibrin delays the diffusion of the microorganisms.
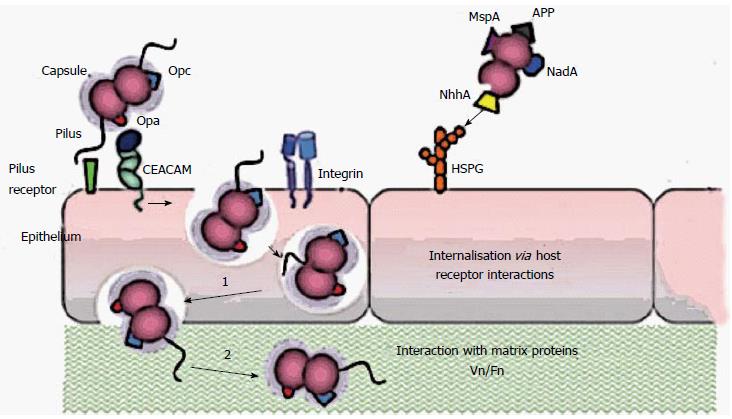
Figure 1 Schematic representation of the interaction mechanisms of Neisseria meningitidis with cellular receptors.
The first adherence phase would be a reversible process in which Van der Waals and electrostatic forces are responsible for a wide range of interactions, including chemical bonding. Finally we added a summary at the endding, dipolar interaction and hydrophobicity. Pili extending beyond the capsule are considered to mediate the primary interaction with epithelial cells. Opa proteins may bind to carcinoembryonic antigen-related cell-adhesion molecule (CEACAMs) and heparan sulphate proteoglycan (HSPGs), and Opc proteins can interact with HSPGs and, via vitronectin and fibronectin, to their integrin receptors. Engagement of CEACAMs, integrins and HSPGs can result in meningococcal internalization by epithelial cells. MSP: Meningococcal serine protease A; App: Adhesion and penetration protein; NadA: Neisserial adhesin; NhhA: Neisseria hia/hsf homologue A.
In general, type-1 somatic fimbriae are encoded by chromosomal genes and are found both in commensal bacteria and in pathogenic strains of E. coli. The adhesion factors that are most frequently associated with pathogenicity are usually coded by plasmids[34], although this may take place chromosomally. The bacterial surface appendages related to association functions are generally termed “fimbriae”, with the term “pili” being reserved for cases in which their presence is related to the exchange of genetic material among microorganisms. The pili of N. meningitidis are 6 nm in diameter and extend several micrometres into the surface of the bacterium. They are, therefore, sexual or conjunctive fimbriae. The genes for the bacterial adhesion factors that have been most thoroughly studied, such as K-88, K-99 and CFA/I, are located in plasmids. Of these, the genes for factor K-88 are known to have a total length of 75-135 Kb, and are frequently associated with genes for the fermentation of raffinose. The gene for the CFA/I factor has a size of approximately 90 Kb, and it is bound to a gene for a stable enterotoxin[35]. For adhesion factor K-88, three plasmids have been shown to be responsible for the three known antigenic variants: K-88ab, K-88ac and K-88ad. Mooi et al[36], designed experiments to determine which genes of the plasmid chain were responsible for the formation of the K-88 factor in each of the variants. For this purpose, each of the three K-88 plasmids was digested with restriction enzymes, and the fragments obtained from each one were then cloned by inclusion in the PBR-322 vector. The bacterial clones carrying each of the K-88 antigens were then identified. This procedure revealed that the expression of the K-88 factor depends of the orientation of the DNA chain responsible and on the variant in question. In the case of K-88ab, its insertion into the vector PBR-322 in a direction or another modifies the quantity of antigen expressed. The lipopolysaccharide of N. meningitidis is known as the major determinant of its virulence, and the use of monoclonal antibodies, together with structural studies, have highlighted the heterogeneity and complexity of meningococcal lipopolysaccharides, which can be divided into 12 immunotypes[37].
Studies by McGee et al[38], have underlined the importance of gonococcal fimbriae in cell colonisation and destruction in cultures of cells from the human fallopian tube. These assays show that both fimbriate and non-fimbriate gonococci bind epithelial cells, although in the former case cell destruction is produced more quickly, this process being mediated by one or more toxic factors, such as surface lipopolysaccharides. Type IV pili, which are protein structures associated with the surface, have also been associated with the adhesion of N. meningitidis to endothelial cells and the development of fulminant meningococcal disease[39,40]. The pili of E. coli, which have been studied in detail, consist of protein subunits that are thought to play an important role in the interaction with specific surface carbohydrates in eukaryotic cells, and some of them are K antigens. D-(+)-Mannose inhibits the in vitro adhesion of bacteria with type-1 fimbriae on the surface of eukaryotic cells containing mannose residue[41]. This is an indiscriminate mechanism of adhesion, as oligosaccharide chains containing mannose are very commonly present in cell surface oligoproteins, including phagocytic cells. Preincubation of bacteria with inhibitor sugars does not affect the adhesiveness, while the pretreatment of cells with carbohydrates effectively prevents adhesion. This indicates that the cell surface structures recognise the radicals of fucose and glucose in the bacterial lipopolysaccharides.
Some authors[42,43], have analysed phenotypic changes in bacteria associated with epigenetic changes. Aspects such as virulence, response to oxidative stress and the formation of biofilm have been observed among epigenetic modifications. Unfortunately, these processes and their relationship with pathogenic changes in N. meningitidis are as yet incompletely understood.
Despite the high prevalence of carriers of N. meningitidis, it only occasionally causes meningococcal disease in the context of endemic disease, in certain geographic areas or in isolated epidemic outbreaks. Some studies have reported that oxidative stress in the environment can modify the surface characteristics of N. meningitidis. Also the antigenic structure can be modified by its importing genetic material from other bacteria in its ecological niche, and some structures of lipopolysaccharides, pili and capsule change the immune response. This paper reviews current knowledge on host-environment-bacteria mechanisms and interactions, with the aim of contributing to our understanding of the pathogenic mechanisms of N. meningitidis.
P- Reviewer: Callegan MC, Weng CF S- Editor: Yu J L- Editor: A E- Editor: Wu HL