Published online Jun 20, 2025. doi: 10.5493/wjem.v15.i2.105636
Revised: March 3, 2025
Accepted: March 14, 2025
Published online: June 20, 2025
Processing time: 75 Days and 7.1 Hours
Periodontal disease is a chronic inflammatory condition characterized by perio
To compare the clinical efficacy of green tea extract gel and ornidazole gel as adjuncts to scaling and root planing (SRP) in patients with periodontitis.
Teeth with probing pocket depths (PPD) of 4-7 mm were selected. Participants’ baseline oral hygiene index-simplified, plaque index, clinical attachment loss, and PPD were recorded. The participants were randomized into two groups: One received green tea extract gel after SRP, and the other received ornidazole gel. Subgingival drug delivery was performed, and participants refrained from brushing or inter
The PPD decreased significantly from baseline to one month in both groups. However, the green tea extract gel group exhibited superior outcomes to the ornidazole gel group, with a mean difference in PPD of 0.28 ± 0.78 mm at one month (P < 0.007).
As an adjunct to SRP, green tea extract gel showed greater efficacy in improving clinical periodontal parameters than ornidazole gel.
Core Tip: Green tea extract gel, rich in epigallocatechin gallate, outperformed ornidazole gel as an adjunct to scaling and root planing in treating periodontitis, demonstrating superior reduction in probing pocket depths after one month. This study highlights green tea extract’s potential as a natural, effective alternative for localized antimicrobial therapy, offering enhanced anti-inflammatory and antimicrobial benefits for periodontal disease management. Its promising results suggest a shift toward plant-based adjuncts in periodontal care.
- Citation: Rengaraj S, Thilagar SS, Yadalam PK, Pampani P, Mani E, Ardila CM. Evaluation of the clinical efficacy of green tea extract gel as local drug delivery for periodontitis. World J Exp Med 2025; 15(2): 105636
- URL: https://www.wjgnet.com/2220-315x/full/v15/i2/105636.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i2.105636
Periodontal disease is a chronic infectious condition involving inflammatory and immune responses, leading to increased periodontal pocket depth, clinical attachment loss, and destruction of alveolar bone and cementum[1]. Its conventional treatments include mechanical debridement, which disrupts subgingival flora and creates clean, smooth, biologically compatible root surfaces[2]. Controlling supragingival plaque is essential to prevent the recolonization of subgingival areas by periodontal pathogens.
Local drug delivery (LDD) systems are primarily designed to target site-specific action in patients with periodontitis. Consequently, LDD may be a viable treatment option for patients exhibiting resistance to systemic antibiotics. Localized antimicrobial therapy has gained significant attention for its site-specific nature, offering advantages such as minimal systemic interference, reduced side effects, improved patient compliance, and greater comfort. Common agents for localized antimicrobial therapy include metronidazole, ornidazole, doxycycline, minocycline, chlorhexidine, and stannous fluoride[3].
Tea is the world’s second-most consumed beverage. One form is green tea, which accounts for 20% of global tea production[4]. Green tea is made from the leaves and buds of Camellia sinensis and is categorized as fermented or non-fermented based on its processing methods. Rich in polyphenols, the non-fermented variety is produced by drying and steaming fresh leaves to inhibit oxidation. Research has shown that green tea extract possesses antioxidant properties and is classified as a dietary supplement[5]. Green tea’s anti-inflammatory, antioxidant, and antimicrobial properties suggest its potential as a promising agent for periodontal therapy. It is rich in polyphenols, particularly flavonoids such as epicatechin (EC), EC-3-gallate (ECG), epigallocatechin (EGC), and EGCG, which is its most abundant polyphenolic compound[6,7]. These polyphenols have demonstrated health benefits, including reducing inflammation, promoting wound healing, and supporting the vitality of human periodontal ligament fibroblasts (hPDLFs) and dental pulp fibroblasts. Notably, hPDLF proliferation and viability have been shown to correlate inversely with the EGCG concentration, achieving 37% proliferation and 99% viability at low concentrations[8].
LDD systems used in periodontal therapy must target pathogens efficiently, provide high drug concentrations, avoid adverse effects, and be biodegradable, cost-effective, and easy to administer without causing allergic reactions or inflammation[4,9]. LDD has emerged as a promising adjunct to conventional nonsurgical periodontal therapy (phase I) to achieve better clinical outcomes[9]. However, traditional antibiotic-based LDD systems face limitations, including the potential development of antibiotic resistance and systemic effects due to drug diffusion beyond the target site[10]. These challenges highlight the need for innovative LDD approaches that minimize resistance risks and localized adverse effects while maintaining therapeutic efficacy.
Due to its health-promoting polyphenols, including ECG and EGCG, green tea extract gel has demonstrated therapeutic effects in periodontitis. Studies suggest that green tea catechins offer numerous health benefits, including antioxidant and antimicrobial activities[11].
Genetic studies have provided additional insight into the relationship between tea consumption and periodontal health. Single nucleotide polymorphisms linked to tea intake have been identified, with Mendelian randomization analyses suggesting a potential causal relationship between tea consumption and periodontitis risk[12]. Interestingly, while higher tea intake was associated with fewer natural teeth and an increased risk of periodontitis, no link was established with caries risk. Moreover, clinical trials have demonstrated the efficacy of green tea products. For example, green tea mouthwash significantly reduced plaque, gingival, and sulcular bleeding indices, albeit less effectively than chlorhexidine[11].
Ornidazole, a 5-nitroimidazole antimicrobial agent, has proven effective in periodontal therapy. It exhibits specific action against obligate anaerobes common in periodontal pockets, including Porphyromonas gingivalis, Fusobacterium nucleatum, Tannerella forsythia, and Peptostreptococcus species[13-15]. Its low minimum inhibitory concentration against these pathogens (0.1-1 µg/mL) and its compatibility with biodegradable delivery systems make it a robust candidate for LDD in periodontitis treatment. Controlled-release systems, which maintain high ornidazole concentrations over extended periods, are particularly advantageous due to their antimicrobial and anti-mutagenic properties[14].
To our knowledge, this study is the first to directly compare the efficacy of green tea extract gel and ornidazole gel as adjuncts to scaling and root planing (SRP) in patients with periodontitis. This pilot clinical study was designed to evaluate and compare the feasibility, clinical efficacy, and patient adherence of these two LDD agents. It also aimed to determine primary outcome measures for larger-scale trials, establishing a foundation for future definitive studies in periodontal therapy.
This pilot clinical study used a split-mouth design to evaluate and compare the efficacy of green tea extract gel and ornidazole gel in patients with periodontitis[16]. It recruited 20 patients (10 per group) aged 27-60 years diagnosed with periodontitis and presenting with bilateral probing pocket depths (PPDs) of 4-7 mm. The participants were selected based on specific inclusion and exclusion criteria[17]. This study was conducted in the Department of Periodontology and Implantology, Adhiparasakthi Dental College and Hospital, Melmaruvathur, Tamil Nadu, India. It was approved by the Institutional Review Board (2020-MDS-BrI1-SIV-05/APDCH) and adhered to the guidelines outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants.
The sample size was estimated to ensure adequate statistical power, considering factors such as expected effect size, population standard deviation, and confidence level.
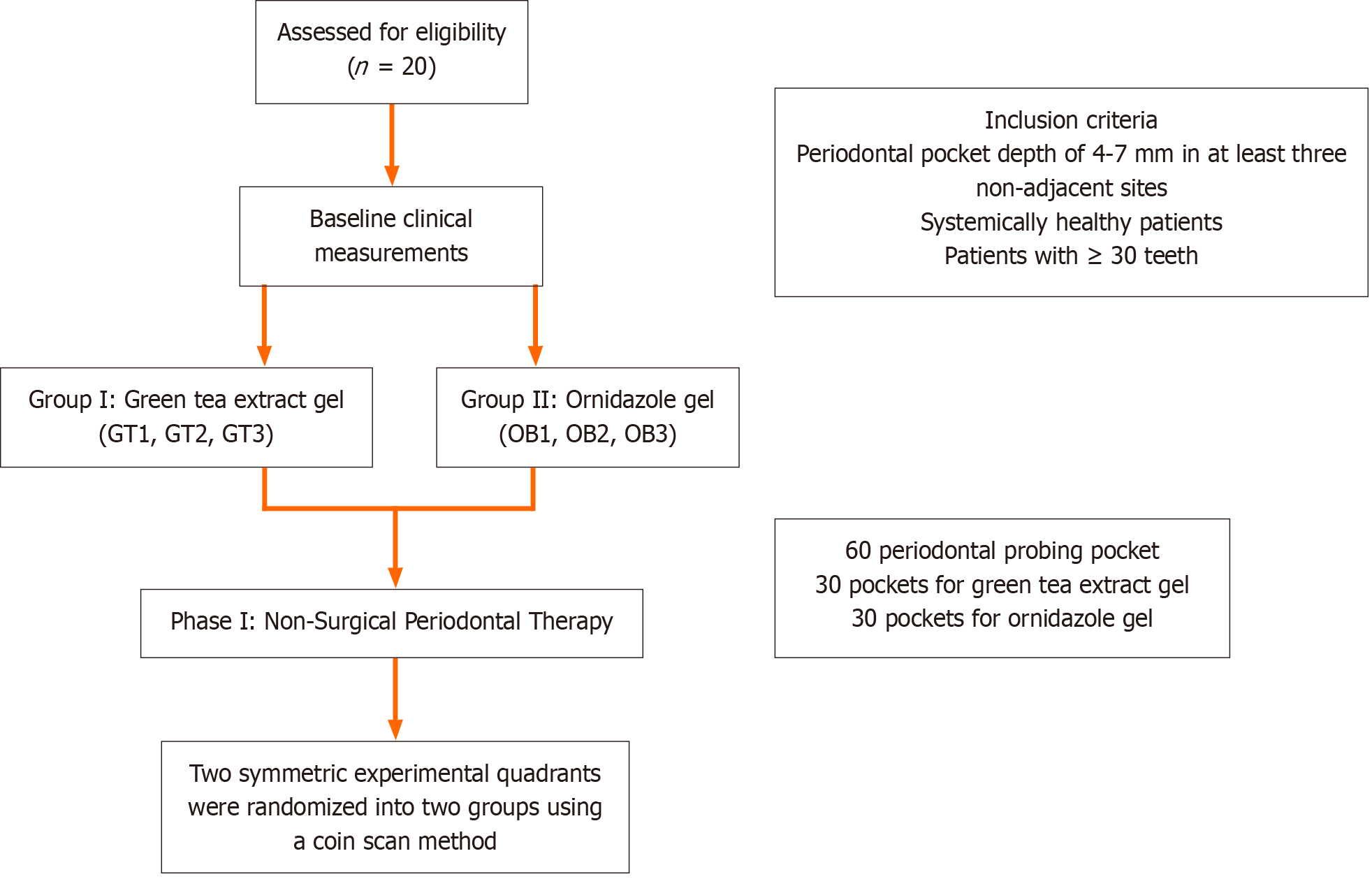
This study was structured into five phases: Initial screening, baseline clinical measurements, SRP and LDD, and maintenance with monthly re-evaluations. For clarity, the study flowchart is shown in Figure 1.
Participants who met the following inclusion criteria were enrolled in this study: (1) Periodontal pocket depth of 4-7 mm at a minimum of three non-adjacent sites; (2) Systemic health with no underlying medical conditions; (3) Cooperative and motivated to adhere to oral hygiene instructions; and (4) Presence of at least 20 teeth.
Participants who met any of the following exclusion criteria were excluded from this study: (1) Antibiotic therapy within the last month; (2) Pregnancy or lactation; (3) Tobacco use; and (4) History of prior periodontal therapy.
The participants were clinically examined using a mouth mirror, explorer, periodontal probe, tweezers, and cotton pellets under optimal lighting. After obtaining written informed consent, detailed case histories were recorded using a standardized pro forma. Due to the visible nature of the treatment gels, double blinding of participants and clinician was not possible. However, the examiner measuring clinical parameters was blinded to the treatment groups.
The following clinical parameters were assessed: (1) Oral hygiene index-simplified (OHI-S); (2) Plaque index (PI). The PI is unique among the indices used to assess plaque because it ignores the coronal extent of plaque on the tooth surface area and assesses only plaque thickness at the gingival area of the tooth. It is among the most widely used indices and has demonstrated good validity and reliability[18]; (3) PPD. The PPD refers to the depth measured from the gingival margin to the base of the clinical pocket. Probing involves stepping a calibrated Williams periodontal probe around the tooth and recording the deepest point at each of its six surfaces. A PPD reading that falls between two calibrated marks on the probe should be rounded up to the nearest millimeter; and (4) Clinical attachment level (CAL). The CAL refers to the distance between the base of the pocket and the cementoenamel junction.
The gels were formulated, each containing 1% ornidazole (commercially available) and 1% green tea extract. The green tea extract was prepared from 100 g of dried and coarsely powdered green tea leaves using a maceration process. The gels were designed to target periodontal pathogens with ornidazole at a concentration of 250 mg and green tea extract at 250-500 µg/mL. A simple gel formulation method with a Carbopol gel base was used. Both green tea extract and commercially available ornidazole were prepared in gel form to minimize bias related to the application methods. The gels were stored in a cool, dry place, protected from direct sunlight.
The green tea extract gel was prepared at Adhiparasakthi College of Pharmacy, Kanchipuram, Tamil Nadu, India, using the maceration method[17]. Green tea leaves were sourced from Ooty Green Tea Estate and processed as follows.
Extraction: 100 g of dried, coarsely powdered green tea leaves were soaked in ethanol (1: 2 ratio) for seven days with occasional stirring. Ethanol, serving as a preservative at concentrations above 20%, was used as the solvent due to its non-toxic properties at low concentrations.
Filtration and recovery: After maceration, the solution was decanted, left to stand for 24 hours, and filtered. The solvent was recovered using distillation.
Gel formulation: The extract was incorporated into a Carbopol gel base (0.5%) containing sodium benzoate (0.1%), ethylenediaminetetraacetic acid (0.1%), glycerin (1%), and triethanolamine.
The gel formulation began by adding glycerin, propylene glycol, and carbomer to deionized water while stirring for 15 minutes to eliminate globules. After allowing the mixture to rest for 60 minutes for the carbomer to dissolve completely, the pH was adjusted to 6 using triethanolamine. A preservative (propylparaben) was dissolved in propylene glycol, and the green tea extract was dissolved in methanol, and both were combined with the gel mixture. The gel base was prepared by dissolving Carbopol 934 in purified water with constant stirring, and the pH was adjusted to 6.0-6.5. This study highlights the difference between commercial green tea and green tea gel preparation, noting that while both Carbopol 934 and 940 are effective in gel formation, Carbopol 940 provides greater viscosity and smoother flow than the creamier texture of Carbopol 934, which is suited to softer applications due to its lower viscosity.
The gel was prepared by stirring Carbopol in distilled water at 800 rpm for 1 hour, followed by the gradual addition of glycerin and neutralization with triethanolamine until a transparent gel was achieved.
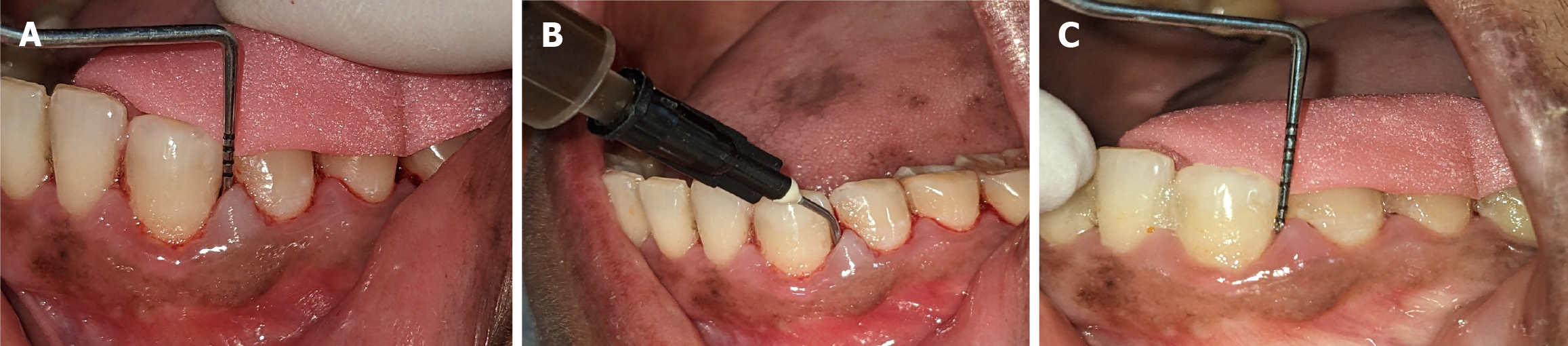
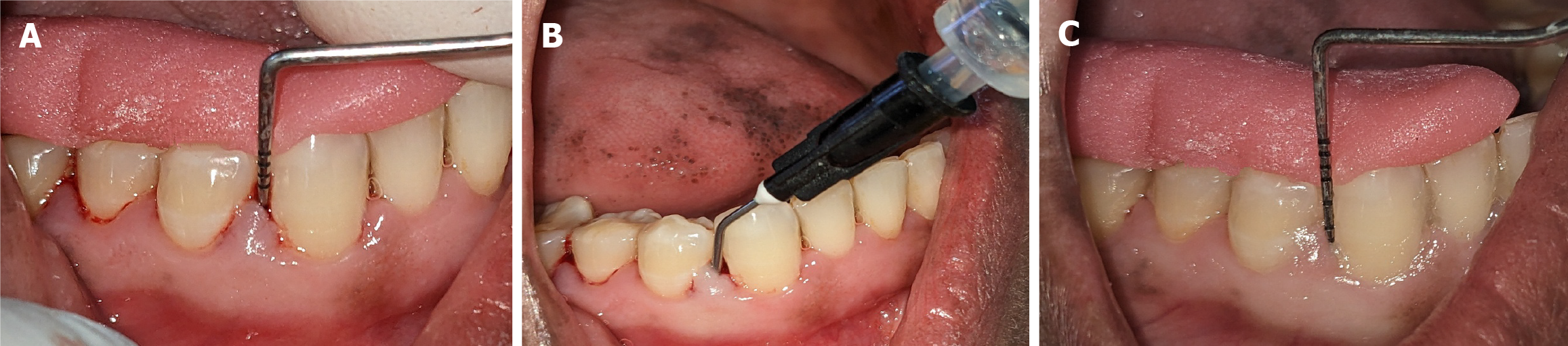
A single, calibrated clinician identified teeth that satisfied the study’s inclusion criteria. All participants received phase I nonsurgical periodontal therapy, which included ultrasonic SRP. Their baseline OHI-S, PI, CAL, and PPD measurements were recorded. Two symmetrical quadrants exhibiting PPDs of 4-7 mm were randomly assigned to two treatment groups using a coin toss method. For each of the 20 participants (10 per group), three sites were selected within each quadrant, all with PPDs of 4-7 mm. Consequently, 60 periodontal pockets were included, 30 receiving green tea extract gel (GT1, GT2, and GT3) and 30 receiving ornidazole gel (OR1, OR2, and OR3). Group I: Green tea extract gel (Figure 2). Group II: Ornidazole (Ornigreat) gel (Figure 3).
The LDD was performed using a 2 mL disposable syringe with a blunt needle to apply the respective gels into three periodontal pockets per quadrant. Care was taken to avoid tissue trauma. The participants were instructed to refrain from brushing the treated areas for 12 hours, avoid flossing or using interproximal cleaning devices for 10 days, and abstain from using mouthwash during the study period. The participants’ clinical parameters were reassessed after one month.
The normality of the data was confirmed using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Therefore, parametric tests were applied: (1) Descriptive statistics: The variables are presented as the mean ± SD; and (2) Inferential statistics: Within-group comparisons used paired t-tests, and between-group comparisons used unpaired t-tests.
The statistical analyses were conducted using SPSS Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, United States). A P < 0.05 was considered statistically significant.
The mean OHI-S was 2.89 ± 0.29 mm at baseline, decreasing significantly to 1.26 ± 0.17 mm after one month. The OHI-S decreased significantly by 1.62 ± 0.34 mm from baseline to one month (P < 0.001; Table 1).
| Intra group comparison | t | df | P value | ||||||
| Mean difference | SD | SEM | 95%CI of the difference | ||||||
| Lower | Upper | ||||||||
| OHI simplified | Baseline vs 1 month | 1.62500 | 0.34774 | 0.07776 | 1.46225 | 1.78775 | 20.899 | 19 | < 0.001a |
| Plaque index | Green tea | 0.71250 | 0.25346 | 0.05667 | 0.59388 | 0.83112 | 12.572 | 19 | < 0.001a |
| Ornidazole gel | 0.65750 | 0.23881 | 0.05340 | 0.54573 | 0.76927 | 12.313 | 19 | < 0.001a | |
| Probing pocket depth | Green tea | 2.80000 | 0.70830 | 0.09144 | 2.61703 | 2.98297 | 30.621 | 59 | < 0.001a |
| Ornidazole gel | 2.48333 | 0.77002 | 0.09941 | 2.28441 | 2.68225 | 24.981 | 59 | < 0.001a | |
| Clinical attachment level | Green tea | 2.86667 | 0.81233 | 0.10487 | 2.65682 | 3.07651 | 27.335 | 59 | < 0.001a |
| Ornidazole gel | 2.51667 | 0.77002 | 0.09941 | 2.31775 | 2.71559 | 25.316 | 59 | < 0.001a | |
At baseline, the mean PI was 1.51 ± 0.16 mm in Group I and 1.66 ± 0.17 mm in Group II. After one month, the mean PI decreased to 0.80 ± 0.18 mm in Group I and 1.00 ± 0.19 mm in Group II.
Intragroup comparison: From baseline to one month, the PI decreased significantly by 0.71 ± 0.25 mm in Group I and 0.65 ± 0.23 mm in Group II (P < 0.001; Table 1).
Intergroup comparison: At baseline, the mean PI differed significantly between groups (0.14 ± 0.25 mm, P < 0.020; Table 2).
| Baseline and 1 month | Inter-group comparison | t | df | P value | ||||
| Mean difference | SD | SEM | 95%CI of the difference | |||||
| Lower | Upper | |||||||
| Plaque index | -0.14550 | 0.25591 | 0.05722 | -0.26527 | -0.02573 | -2.543 | 19 | 0.020a |
| Probing pocket depth | 0.03333 | 0.78041 | 0.10075 | -0.16827 | 0.23493 | 0.331 | 59 | 0.742 |
| Clinical attachment level | 0.01667 | 0.83345 | 0.10760 | -0.19864 | 0.23197 | 0.155 | 59 | 0.877 |
After one month, the mean PI was significantly lower in Group I than in Group II (mean difference = 0.28 ± 0.06 mm, P = 0.005; Table 3).
| Baseline and 1 month | Inter-group comparison | t | df | P value | ||||
| Mean difference | SD | SEM | 95%CI of the difference | |||||
| Lower | Upper | |||||||
| Plaque index | -0.20050 | 0.28376 | 0.06345 | -0.33330 | -0.06770 | -3.160 | 19 | 0.005a |
| Probing pocket depth | -0.28333 | 0.78312 | 0.10110 | -0.48563 | -0.08103 | -2.802 | 59 | 0.007a |
| Clinical attachment level | -0.33333 | 0.79547 | 0.10269 | -0.53882 | -0.12784 | -3.246 | 59 | 0.002a |
At baseline, the mean PPD was 5.20 ± 0.81 mm in Group I and 5.20 ± 0.70 mm in Group II. After one month, the mean PPD decreased to 2.40 ± 0.67 mm in Group I and 2.70 ± 0.45 mm in Group 2.
Intragroup comparison: The PPD decreased significantly by 2.80 ± 0.70 mm in Group I and 2.48 ± 0.77 mm in Group II from baseline to one month (P < 0.001; Table 1).
Intergroup comparison: At baseline, the mean PPD did not differ significantly between groups (0.03 ± 0.78, P = 0.742; Table 2).
After one month, the mean difference in PPD between groups was 0.28 ± 0.78 mm, improving significantly in Group I (P = 0.007; Table 3).
At baseline, the mean CAL was 5.35 ± 0.935 mm in Group I and 5.33 ± 0.77 mm in Group II. After one month, the mean CAL decreased to 2.40 ± 0.72 mm in Group I and 2.81 ± 0.53 mm in Group 2.
Intragroup comparison: The CAL decreased significantly by 2.80 ± 0.70 mm in Group I and 2.51 ± 0.77 mm in Group II from baseline to one month (P < 0.001; Table 1).
Intergroup comparison: At baseline, the mean CAL did not differ significantly between groups (0.03 ± 0.78 mm, P = 0.742; Table 2).
After one month, the mean difference in CAL between groups was 0.33 ± 0.79 mm, improving significantly in Group I
The treatment of periodontitis typically involves mechanical debridement to alleviate inflammation and promote clinical attachment gain. However, this approach alone may not eliminate pathogenic bacteria, which often persist in gingival crevices or other inaccessible areas[19]. In order to address this, locally delivered antimicrobial agents have become an integral component of periodontal therapy, especially given growing concerns about antibiotic resistance[20]. Among recent innovations, green tea extract gel has emerged as a promising adjunctive agent due to its diverse therapeutic properties, including antioxidant, anti-inflammatory, antibacterial, and antiviral effects[20]. When applied to periodontal pockets, green tea extract gel inhibits periodontopathogenic bacteria and protects against tissue damage by solidifying in situ for sustained action[21-23]. Similarly, ornidazole (Ornigreat) gel has shown efficacy as an LDD agent by targeting Gram-negative anaerobic bacteria, which are pivotal in periodontal tissue destruction[13,14]. While individual studies have examined these agents, our study is the first to directly compare green tea extract gel and ornidazole gel in treating periodontitis after SRP.
Participants’ OHI-S significantly improved from baseline to one month, with a mean change of 1.62 ± 0.34 mm (P < 0.001). This finding is consistent with Lertpimonchai et al[24], who demonstrated that inadequate oral hygiene increases the risk of periodontitis by two- to five-fold. Maintaining proper oral hygiene is pivotal in achieving favorable treatment outcomes, as reflected in our study.
Participants’ PI also significantly decreased from baseline to one month (P < 0.001), with intergroup comparisons favoring the green tea extract gel (P = 0.05). These findings align with Rattanasuwan et al[25], who observed substantial plaque reduction after applying green tea extract gel as an LDD agent. These results underscore the dual role of green tea extract gel in mitigating plaque accumulation and promoting gingival health.
The reduction in PPD in our study directly indicated therapeutic success. Both groups demonstrated significant improvements in PPD from baseline to one month, with green tea extract gel significantly outperforming ornidazole gel
The improvements in CAL further substantiated the efficacy of both agents, with green tea extract gel demonstrating slightly superior outcomes to ornidazole gel (P = 0.002). This finding is consistent with earlier studies that highlighted the role of green tea catechins in reducing CAL loss, PI, and PPD[27,28]. The antioxidant properties of catechins likely contributed to these improvements by mitigating ROS-induced damage and suppressing the growth of periodontopathogens such as P. gingivalis[12].
The overall findings of our study confirm that subgingivally applying green tea extract gel or ornidazole gel after SRP significantly reduced clinical periodontal parameters at one month, resulting in a return to normal sulcus depth in most treated sites[29-31]. Despite significant improvements in OHI-S and comparable efficacy between the two examined agents, PI, PPD, and CAL reductions were greater with green tea extract gel, likely due to its unique antioxidant and antibacterial properties.
A follow-up study assessed the efficacy of using green tea gel (5 mg/mL EGCG) in treating periodontitis, using it as a substitute for distilled water in an ultrasonic scaler[32]. It measured clinical parameters like PPD, CAL, bleeding index, gingival index (GI), and PI at baseline and 6 and 12 weeks post-treatment. While it observed improvements, PPD, CAL, GI, and PI did not differ significantly between groups. The bleeding index had decreased significantly in the EGCG group at 12 weeks. Additionally, the potential for unblinding due to visible interventions may have introduced bias. Future research should involve larger randomized controlled trials, longer follow-up periods, comprehensive microbiological and biochemical analyses, cost-effectiveness assessments, and examining the synergistic effects of combining green tea extract gel with ornidazole gel.
Our study aimed to evaluate the clinical efficacy of green tea extract gel compared to ornidazole gel in treating periodontitis. Its results showed that green tea extract gel significantly outperformed ornidazole gel in reducing PPD, CAL, and PI. However, several limitations may influence the interpretation of our results. One limitation is the small sample size of 20 participants, which might limit the generalizability of our findings. A small sample may increase the risk of type II errors and may not accurately reflect the larger population of patients with periodontitis. In addition, the short follow-up period of just one month may not be adequate to fully evaluate the sustainability of the treatment effects. Future research should incorporate longer follow-up periods to assess the long-term effectiveness of these therapies.
Another limitation of our study is the lack of microbiological analysis, which limits understanding of the specific mechanisms through which green tea extract gel exerts its therapeutic effects. However, based on existing literature, it is plausible that the catechins in green tea, particularly EGC gallate, play a key role in modulating the periodontal microbiome. Future studies should consider including a microbiological assessment to determine the impact of these treatments on the periodontal microbiome, particularly the “red complex” pathogens associated with periodontitis. In addition, the potential for unblinding due to the visible nature of the treatment gels could also have introduced bias in our clinical evaluation. Future studies should use a double-blind design to help mitigate this bias. Moreover, the formulation of green tea extract gel used in our study was prepared in-house using a maceration method and specific ratios of ethanol, Carbopol gel base, and other additives. Therefore, a thorough comparison between the research formulation and commercial alternatives is warranted. Future research should evaluate the clinical outcomes of in-house-prepared and commercially available green tea products, ensuring that formulations are standardized and comparable to establish clearer therapeutic guidelines for clinical use. Our findings suggest that while green tea extract gel could be a promising adjunct therapy for managing periodontal disease, a more robust research framework is needed to validate our findings and apply them effectively in clinical practice.
Our study used maceration to extract active compounds like EGCG from green tea extract gel. This gel was designed for periodontal therapy, enhancing mucosal absorption and minimizing systemic effects. A previous three-month follow-up study showed sustained improvement in periodontal health after applying green tea extracts[32]. Clinical validation, testing, and comparative studies are needed to evaluate the efficacy of both in-house and commercial products.
The key findings of this study revealed statistically significant enhancements in clinical periodontal parameters, such as a reduction in PI and PPD, as well as an improvement in CAL, after applying both green tea extract gel and ornidazole gel. Despite the study's inherent limitations, the observed clinical trends suggest that green tea extract gel may offer a comparable or potentially superior LDD approach compared to ornidazole gel. This could be attributed to its robust antioxidant and antibacterial properties. These results imply that green tea extract gel may be an effective adjunctive therapy to SRP in managing periodontitis, especially for patients seeking alternatives to conventional antimicrobial interventions. However, additional research with larger groups, extended follow-up periods, and in-depth microbiological and biochemical assessments is needed to confirm these initial findings.
| 1. | Cafiero C, Spagnuolo G, Marenzi G, Martuscelli R, Colamaio M, Leuci S. Predictive Periodontitis: The Most Promising Salivary Biomarkers for Early Diagnosis of Periodontitis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Cheng YL, Jordan L, Chen HS, Kang D, Oxford L, Plemons J, Parks H, Rees T. Chronic periodontitis can affect the levels of potential oral cancer salivary mRNA biomarkers. J Periodontal Res. 2017;52:428-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Lima de Sousa T, Dourado D, Rodrigues JS, de Souza Rebouças J, Montes MAJR, Formiga FR. Treatment of periodontal disease: does drug delivery matter? Front Bioeng Biotechnol. 2024;12:1427758. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Kochman J, Jakubczyk K, Antoniewicz J, Mruk H, Janda K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules. 2020;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 5. | Rasaei N, Asbaghi O, Samadi M, Setayesh L, Bagheri R, Gholami F, Soveid N, Casazza K, Wong A, Suzuki K, Mirzaei K. Effect of Green Tea Supplementation on Antioxidant Status in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Zhao L, La VD, Grenier D. Antibacterial, antiadherence, antiprotease, and anti-inflammatory activities of various tea extracts: potential benefits for periodontal diseases. J Med Food. 2013;16:428-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Zhao T, Li C, Wang S, Song X. Green Tea (Camellia sinensis): A Review of Its Phytochemistry, Pharmacology, and Toxicology. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 106] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 8. | Singh N, Puzhankara L, Kedlaya MN, Ramanarayanan V. Effectiveness of tea tree oil versus chlorhexidine in the treatment of periodontal diseases: a systematic review. Evid Based Dent. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 9. | Basudan AM. Nanoparticle based periodontal drug delivery - A review on current trends and future perspectives. Saudi Dent J. 2022;34:669-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 10. | Chen H, Yang H, Weir MD, Schneider A, Ren K, Homayounfar N, Oates TW, Zhang K, Liu J, Hu T, Xu HHK. An antibacterial and injectable calcium phosphate scaffold delivering human periodontal ligament stem cells for bone tissue engineering. RSC Adv. 2020;10:40157-40170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Andhare MG, Shetty S, Vivekanandan G, Shetty RM, Rahman B, Shetty SR, Siddeshappa ST, Desai V. Clinical efficacy of green tea, aloe vera and chlorhexidine mouthwashes in the treatment of dental biofilm induced gingivitis: A multi-arm, double-blinded, randomized controlled clinical trial. Int J Dent Hyg. 2024;22:504-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Jin B, Chen H, Liu P, Wang Y, Guo Y, Wang C, Jia Y, Zou R, Niu L. Assessing the association between tea intake and risk of dental caries and periodontitis: a two-sample Mendelian randomization study. Sci Rep. 2024;14:4728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Wang MJ, DA Y, Zheng LC. [Study on the efficacy and safety of metronidazole combined with periodontal tissue regeneration in the treatment of periodontitis]. Shanghai Kou Qiang Yi Xue. 2020;29:293-297. [PubMed] |
| 14. | Alauzet C, Berger S, Jean-Pierre H, Dubreuil L, Jumas-Bilak E, Lozniewski A, Marchandin H. nimH, a novel nitroimidazole resistance gene contributing to metronidazole resistance in Bacteroides fragilis. J Antimicrob Chemother. 2017;72:2673-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Liu B, Hu J, Zhao L, Zhao H. The Effect of Local Application of Tea Tree Oil Adjunctive to Daily Oral Maintenance and Nonsurgical Periodontal Treatment: A Systematic Review and Meta-Analysis of Randomised Controlled Studies. Oral Health Prev Dent. 2024;22:211-222. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Roth JVS, Guarenghi GG, Ferro RM, Valenga HM, Haas AN, Prates RC, Steffens JP. Gingival bleeding as a predictor of handgrip strength-an observational study and a pilot randomized clinical trial. Clin Oral Investig. 2024;28:109. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Heitz-Mayfield LJA. Conventional diagnostic criteria for periodontal diseases (plaque-induced gingivitis and periodontitis). Periodontol 2000. 2024;95:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 18. | Silness J, Loe H. Periodontal Disease In Pregnancy. II. Correlation Between Oral Hygiene And Periodontal Condtion. Acta Odontol Scand. 1964;22:121-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4958] [Cited by in RCA: 5453] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 19. | Zhang QW, Lin LG, Ye WC. Techniques for extraction and isolation of natural products: a comprehensive review. Chin Med. 2018;13:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1041] [Cited by in RCA: 698] [Article Influence: 99.7] [Reference Citation Analysis (0)] |
| 20. | Petersilka GJ, Ehmke B, Flemmig TF. Antimicrobial effects of mechanical debridement. Periodontol 2000. 2002;28:56-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Slots J, Jorgensen MG. Effective, safe, practical and affordable periodontal antimicrobial therapy: where are we going, and are we there yet? Periodontol 2000. 2002;28:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Vargas-Sanchez PK, Pitol DL, de Sousa LG, Beloti MM, Rosa AL, Rossi AC, Siéssere S, Bombonato-Prado KF. Green tea extract rich in epigallocatechin gallate impairs alveolar bone loss in ovariectomized rats with experimental periodontal disease. Int J Exp Pathol. 2020;101:277-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Li Y, Cheng L, Li M. Effects of Green Tea Extract Epigallocatechin-3-Gallate on Oral Diseases: A Narrative Review. Pathogens. 2024;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 24. | Lertpimonchai A, Rattanasiri S, Arj-Ong Vallibhakara S, Attia J, Thakkinstian A. The association between oral hygiene and periodontitis: a systematic review and meta-analysis. Int Dent J. 2017;67:332-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 25. | Rattanasuwan K, Rassameemasmaung S, Sangalungkarn V, Komoltri C. Clinical effect of locally delivered gel containing green tea extract as an adjunct to non-surgical periodontal treatment. Odontology. 2016;104:89-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Kudva P, Tabasum ST, Shekhawat NK. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planing in chronic periodontitis patients: A clinicomicrobiological study. J Indian Soc Periodontol. 2011;15:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Fournier-Larente J, Morin MP, Grenier D. Green tea catechins potentiate the effect of antibiotics and modulate adherence and gene expression in Porphyromonas gingivalis. Arch Oral Biol. 2016;65:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Zeng J, Yuan Q, Luan Q. Efficacy of (-)-epigallocatechin gallate delivered by a new-type scaler tip during scaling and root planing on chronic periodontitis: a split-mouth, randomized clinical trial. BMC Oral Health. 2021;21:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Cai Y, Chen Z, Liu H, Xuan Y, Wang X, Luan Q. Green tea epigallocatechin-3-gallate alleviates Porphyromonas gingivalis-induced periodontitis in mice. Int Immunopharmacol. 2015;29:839-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Penmetsa GS, Subbareddy B, Mopidevi A, Arunbhupathi P, Baipalli V, Pitta S. Comparing the Effect of Combination of 1% Ornidazole and 0.25% Chlorhexidine Gluconate (Ornigreat™) Gel and Aloe vera Gel in the Treatment of Chronic Periodontitis: A Randomized, Single-Blind, Split-Mouth Study. Contemp Clin Dent. 2019;10:226-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 31. | Wang B, Wang J, Shao J, Kouwer PHJ, Bronkhorst EM, Jansen JA, Walboomers XF, Yang F. A tunable and injectable local drug delivery system for personalized periodontal application. J Control Release. 2020;324:134-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | Zeng J, Wang Y, Yuan Q, Luan Q. The effect of (-)-epigallocatechin gallate as an adjunct to non-surgical periodontal treatment: a randomized clinical trial. Trials. 2022;23:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |