Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.87916
Peer-review started: September 2, 2023
First decision: November 1, 2023
Revised: December 18, 2023
Accepted: February 3, 2024
Article in press: February 3, 2024
Published online: March 20, 2024
Processing time: 199 Days and 10.4 Hours
Diabetes, a globally escalating health concern, necessitates innovative solutions for efficient detection and management. Blood glucose control is an essential aspect of managing diabetes and finding the most effective ways to control it. The latest findings suggest that a basal insulin administration rate and a single, high-concentration injection before a meal may not be sufficient to maintain healthy blood glucose levels. While the basal insulin rate treatment can stabilize blood glucose levels over the long term, it may not be enough to bring the levels below the post-meal limit after 60 min. The short-term impacts of meals can be greatly reduced by high-concentration injections, which can help stabilize blood glucose levels. Unfortunately, they cannot provide long-term stability to satisfy the post-meal or pre-meal restrictions. However, proportional-integral-derivative (PID) control with basal dose maintains the blood glucose levels within the range for a longer period.
To develop a closed-loop electronic system to pump required insulin into the patient's body automatically in synchronization with glucose sensor readings.
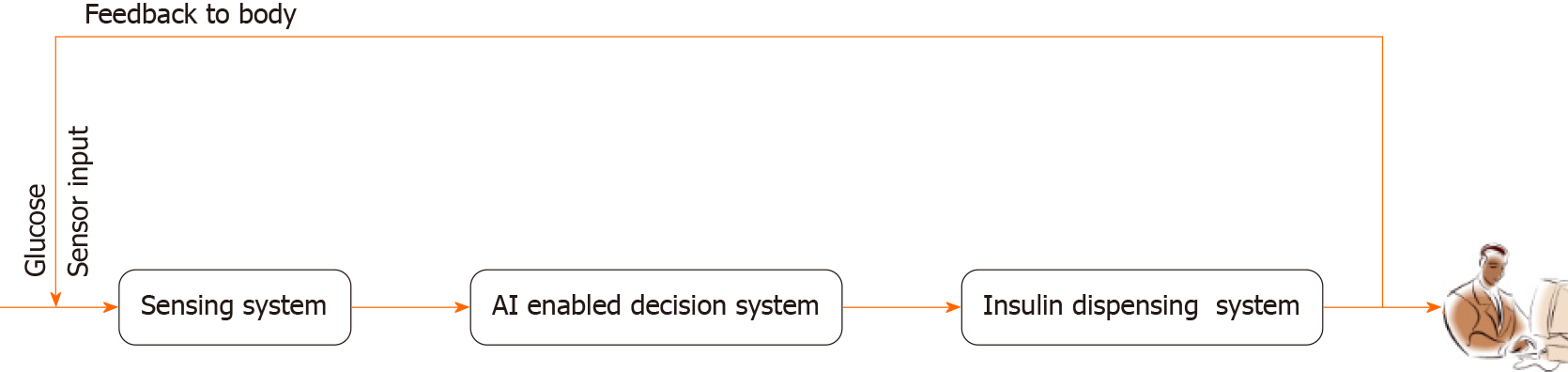
The proposed system integrates a glucose sensor, decision unit, and pumping module to specifically address the pumping of insulin and enhance system effectiveness. Serving as the intelligence hub, the decision unit analyzes data from the glucose sensor to determine the optimal insulin dosage, guided by a pre-existing glucose and insulin level table. The artificial intelligence detection block processes this information, providing decision instructions to the pumping module. Equipped with communication antennas, the glucose sensor and micropump operate in a feedback loop, creating a closed-loop system that eliminates the need for manual intervention.
The incorporation of a PID controller to assess and regulate blood glucose and insulin levels in individuals with diabetes introduces a sophisticated and dynamic element to diabetes management. The simulation not only allows visualization of how the body responds to different inputs but also offers a valuable tool for predicting and testing the effects of various interventions over time. The PID controller's role in adjusting insulin dosage based on the discrepancy between desired setpoints and actual measurements showcases a proactive strategy for maintaining blood glucose levels within a healthy range. This dynamic feedback loop not only delays the onset of steady-state conditions but also effectively counteracts post-meal spikes in blood glucose.
The WiFi-controlled voltage controller and the PID controller simulation collectively underscore the ongoing efforts to enhance efficiency, safety, and personalized care within the realm of diabetes management. These technological advancements not only contribute to the optimization of insulin delivery systems but also have the potential to reshape our understanding of glucose and insulin dynamics, fostering a new era of precision medicine in the treatment of diabetes.
Core Tip: This study describes an innovative diabetes management system that integrates real-time glucose monitoring with advanced artificial intelligence (AI) algorithms and closed-loop insulin delivery. This approach offers precise, personalized control of blood glucose levels, reducing the risk of complications. The simulations demonstrate the system's effectiveness in maintaining stable glucose levels, even in response to meals and exercise, thanks to the International Diabetes Federation controller and basal insulin dosage. Additionally, the study explores the use of ESP32 for monitoring input voltage levels to protect the system. Overall, this study provides a groundbreaking approach that combines AI, closed-loop control, and real-time monitoring to revolutionize diabetes care and improve patient outcomes.
- Citation: Medanki S, Dommati N, Bodapati HH, Katru VNSK, Moses G, Komaraju A, Donepudi NS, Yalamanchili D, Sateesh J, Turimerla P. Artificial intelligence powered glucose monitoring and controlling system: Pumping module. World J Exp Med 2024; 14(1): 87916
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/87916.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.87916
A significant worldwide health issue, diabetes mellitus is a complex metabolic condition. Diabetes, which is characterized by high blood glucose levels, is extremely dangerous for both people's health and the global healthcare system. According to the International Diabetes Federation, there were 463 million adult diabetics worldwide in 2019, and that number is expected to more than double to 700 million by 2045[1,2]. Continuous glucose monitoring and exact glucose control are essential for efficient diabetes treatment in order to reduce the risk of consequences such as cardiovascular disease, neuropathy, nephropathy, and retinopathy[2].
A type of medical technology or procedure known as a "open-loop glucose controlling system" is intended to control a person's blood glucose levels without requiring ongoing feedback or alterations based on real-time data[3]. It has certain advantages, but it also has a number of drawbacks. The drawbacks can be avoided by using a feedback system. Recent advancements in artificial intelligence (AI) and machine learning offer a promising avenue for redefining diabetes management paradigms[4]. The fusion of AI with glucose monitoring systems presents an opportunity to enhance the precision and personalization of diabetes care. Through the integration of AI algorithms, not only can glucose levels be continuously monitored, but patterns can also be analyzed, future trends predicted, treatment plans dynamically adjusted, and interventions even automated[5]. At the heart of this research paper lies the development and comprehensive evaluation of an AI-powered glucose monitoring and controlling system poised to revolutionize the landscape of diabetes care.
This pioneering system integrates real-time glucose monitoring through continuous glucose monitoring technology with sophisticated AI algorithms, capable of learning from individual responses to a spectrum of interventions. By harmonizing data-driven insights with automated decision-making, the system aspires to maintain glucose levels within a targeted range while simultaneously minimizing the risks of hypoglycemia and hyperglycemia.
The technological details of the AI-powered glucose monitoring and controlling system will be covered in detail in the following sections of this article. This entails a thorough investigation of data collection techniques, the careful selection and application of AI algorithms, the design of the system's architecture, and the utilized validation processes. Additionally, a thorough evaluation of the system's effectiveness will be carried out through precisely planned clinical trials, which will compare its performance to current glucose management techniques[6]. Such an innovative strategy has significant potential for transformation, promising to improve the quality of life for people with diabetes while simultaneously reducing the burden on healthcare systems around the world. By providing real-time insights, predictive abilities, and autonomous treatments, the integration of AI into glucose monitoring and management is poised to completely change the way that diabetes is treated. To treat illnesses, pharmaceutics is a major component of medical care. Injections under the skin are a simple and popular method of delivery. Manual injections, however, do not allow for continuous dosing and might be painful. They also demand frequent attention[7]. These drawbacks can be avoided via automated delivery. The treatment of diabetics is one common pharmacological dosing application. The patient's health depends on the tight monitoring of blood glucose levels. It is well recognized that using a micropump to give insulin continuously improves care compared to many daily injections.
One of the most exciting advancements is the development of insulin pumps that help deliver medications with ease and comfort. With the availability of two types of pumps, patch pumps, and durable pumps, patients have more options than ever before. The durable pumps used in conjunction with infusion sets are especially noteworthy as they provide a continuous supply of insulin, which reduces the need for routine disconnections. This is a notable change for patients who want to maintain their active lifestyles without having to worry about taking breaks for medication[8]. Another advantage of durable pumps is that they are more comfortable to use, thanks to the removal of tubing. Patients can now wear them without feeling self-conscious or weighed down and this helps increase patient compliance. With these innovative devices, patients can go about their daily activities without feeling like they must compromise their comfort or lifestyle.
Type 1 diabetes, characterized by an insufficient production of insulin, demands a vigilant approach to prevent dangerous fluctuations in blood sugar concentrations. Genetic predisposition, autoimmune factors, or underlying diseases can trigger the onset of this chronic condition. Managing type 1 diabetes involves a multi-faceted approach, including insulin injections, dietary adjustments, and regular exercise. However, the inherent challenge lies in the precise administration of insulin, as overdosing can lead to hypoglycemia with symptoms ranging from chills to uncon
Recognizing the complexities of insulin management in type 1 diabetes, there is a growing interest in automated solutions. An automated insulin pump, equipped with the capability to measure blood glucose levels and administer insulin in real-time, holds the promise of simplifying this process. Such a technological advancement could not only enhance the precision of insulin delivery but also alleviate the burden on individuals, contributing to an improved quality of life.
In pursuit of improved outcomes for those with insulin dependence, modeling blood glucose levels becomes a pivotal aspect. By developing sophisticated models that predict blood glucose fluctuations based on various factors, and refining insulin administration methods tailored to individual needs, we can aspire to maintain healthier blood glucose ranges. This not only addresses the immediate challenges of diabetes management but also contributes to the long-term well-being and overall health of individuals grappling with this chronic condition.
The proposed system being developed comprises an AI detection block. This block acts as the brain of the device, receiving input from a glucose sensor that measures blood sugar levels. Using advanced algorithms and machine learning, the AI then analyzes this data and makes decisions on how much insulin to release into the patient's bl
The system's cutting-edge AI detection block serves as its central component. It orchestrates a seamless interface with a cutting-edge glucose sensor, which is in charge of continuously measuring blood sugar levels. This complex component serves as the device's intellectual hub. The AI detection block takes on the difficult duty of analyzing the incoming glucose data through the integration of complicated algorithms and cutting-edge machine learning techniques, which ultimately results in educated recommendations for the administration of insulin. A ground-breaking closed-loop system that combines real-time monitoring with AI-driven decision-making streamlines the treatment of diabetes while also vastly improving its accuracy and dependability.
At the heart of this closed-loop system is the glucose sensor, a state-of-the-art technology designed to monitor blood sugar levels in near-real-time. This sensor acquires data through non-invasive or minimally invasive means, negating the need for traditional and often uncomfortable fingerstick tests. The data acquired are then seamlessly transmitted to the AI detection block, which serves as the digital nerve center of the entire system. The transformative power of this AI de
Through the utilization of advanced algorithms, the AI is capable of discerning patterns, trends, and potential ano
The Bergman Minimal Model, a widely accepted framework for describing blood glucose control in individuals with type 1 diabetes.
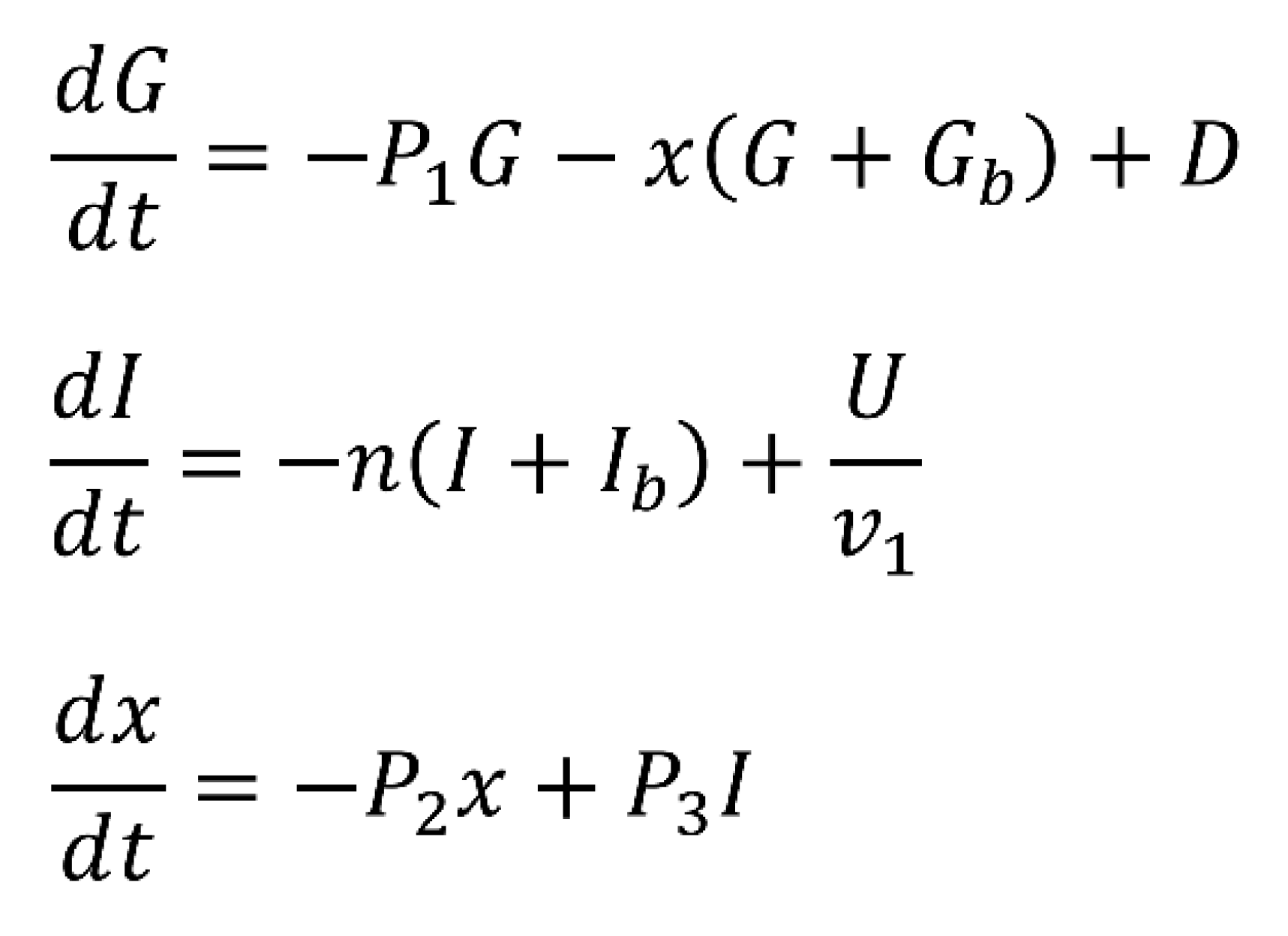
comprises a set of ordinary differential equations that model the dynamics of key variables: G (deviation of blood glucose concentration from basal levels), I (deviation of blood insulin concentration from basal levels), and X (proportionality variable describing insulin concentration in a remote compartment). These variables are crucial in understanding the intricate interplay of glucose and insulin in the body. The model is governed by parameters representing rates and conversions related to glucose and insulin processes. Basal blood glucose and basal blood insulin levels, along with parameters such as P1 (glucose removal rate), P2 (insulin removal rate from the remote compartment), and P3 (insulin appearance rate in the remote compartment), collectively contribute to the model’s accuracy. Disturbance variable D represents the intake of glucose from external sources, while U accounts for insulin input from an external source. To better mimic real-world scenarios, the disturbance variable D is time-dependent and represents glucose intake from external sources, primarily food. Its formulation includes factors like glucose distribution volume, determined by an individual’s weight and size, and the rate of glucose infusion, influenced by meal content, weight, and size. On the other hand, U represents external insulin input into the bloodstream.
The study introduces a proportional-integral-derivative (PID) controller to maintain blood glucose (G) at a desired setpoint. The PID controller involves terms such as Uff (feedforward controller output), Kp (proportional controller gain), Kd (derivative controller gain), and Ke (integral controller gain). This setup allows for a comprehensive and adaptive control mechanism, responding to deviations from the setpoint by adjusting insulin administration.
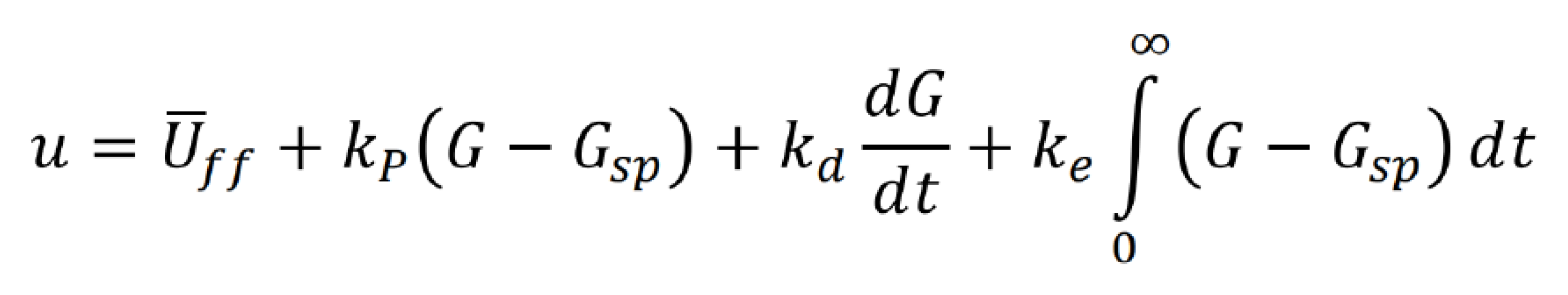
Additionally, the study explores an alternative input mechanism, a high-concentration insulin injection modeled similarly to the glucose disturbance function. This injection mechanism, represented by Uinject, involves an exponential decay with a decay rate of 10. The sharpness of the injection peak, denoted by Uinject, is estimated to scale with glucose intake from corresponding meals, providing a nuanced representation of real-world insulin injections the Bergman Model, coupled with PID control and alternative insulin injection mechanisms, provides a robust foundation for simulating and understanding the complexities of blood glucose control in type 1 diabetes. These models and control strategies aim to enhance our ability to predict, manage, and ultimately improve the lives of individuals with diabetes.
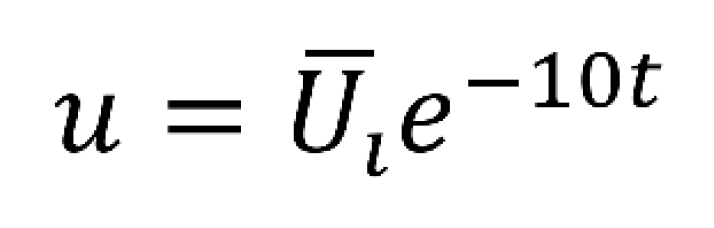
The Bergman Model, coupled with PID control and alternative insulin injection mechanisms, provides a robust foundation for simulating and understanding the complexities of blood glucose control. These models and control strategies aim to enhance our ability to predict, manage, and ultimately improve the lives of individuals with diabetes. Furthermore, the integration of the Bergman Model with PID control and innovative insulin injection methods represents a significant step toward personalized and precise diabetes management. By capturing the intricate dynamics of glucose-insulin interactions, these models offer a more nuanced understanding of the disease's complexities. The application of PID control introduces a dynamic and adaptive approach to insulin administration, allowing for real-time adjustments to deviations from target glucose levels. Exploring alternative insulin injection mechanisms adds versatility to treatment options, potentially catering to individual variations in insulin needs. This comprehensive approach not only advances our theoretical understanding of diabetes physiology but also holds promise for the development of more effective and patient-tailored therapeutic interventions. Ultimately, these advancements contribute to the overarching goal of improving the quality of life for individuals living with diabetes through enhanced predictive capabilities and refined management strategies.
The comprehensive methodology for implementing an insulin pump system with the ESP32 goes beyond mere integration and data analysis. It encompasses a multifaceted approach to address the various aspects crucial for the system's accuracy, reliability, and safety. Sensor integration forms the foundation, with the ESP32 connecting seamlessly to glucose sensors to capture real-time data. The microcontroller's processing capabilities are then harnessed to not only analyze glucose levels but also to implement sophisticated algorithms that intelligently calculate the precise insulin dosage required. This iterative process of data interpretation ensures that the insulin pump responds dynamically to the user's changing physiological needs. Communication protocols play a pivotal role in ensuring the effectiveness of the system. The ESP32 employs wireless technologies like Bluetooth or Wi-Fi to establish robust connections between the microcontroller and user interfaces, which can include liquid crystal displays or mobile applications. This facilitates real-time monitoring of glucose levels, user input for dosage adjustments, and the reception of alerts or notifications, enhancing the overall user experience. Safety is paramount in insulin pump systems, and the ESP32 is programmed to incorporate multiple layers of protection. Fail-safes and redundant checks are meticulously designed to prevent over-dosage or any potential risks, ensuring the user's safety and peace of mind. The system undergoes rigorous testing and calibration, including simulated scenarios with varying glucose levels and hardware stress tests, to validate its accuracy and reliability across diverse conditions. User interface design extends beyond functionality, focusing on creating an intuitive and user-friendly experience. The ESP32 facilitates the development of interfaces that not only provide essential information but also allow users to customize settings, track historical data, and receive timely notifications, fostering a sense of control over their health management. Efficient power management is a key consideration, especially in portable insulin pump systems. The ESP32's capabilities are leveraged to optimize power consumption, extending the lifespan of the battery and ensuring uninterrupted operation. This is essential for users who rely on the portability of the insulin pump for their daily activities. In conclusion, the methodology for implementing an insulin pump system with the ESP32 is a holistic process that integrates cutting-edge technology, prioritizes user experience and safety, and undergoes meticulous testing and validation. The result is a sophisticated and effective insulin delivery system that empowers users to manage their health with confidence and convenience.
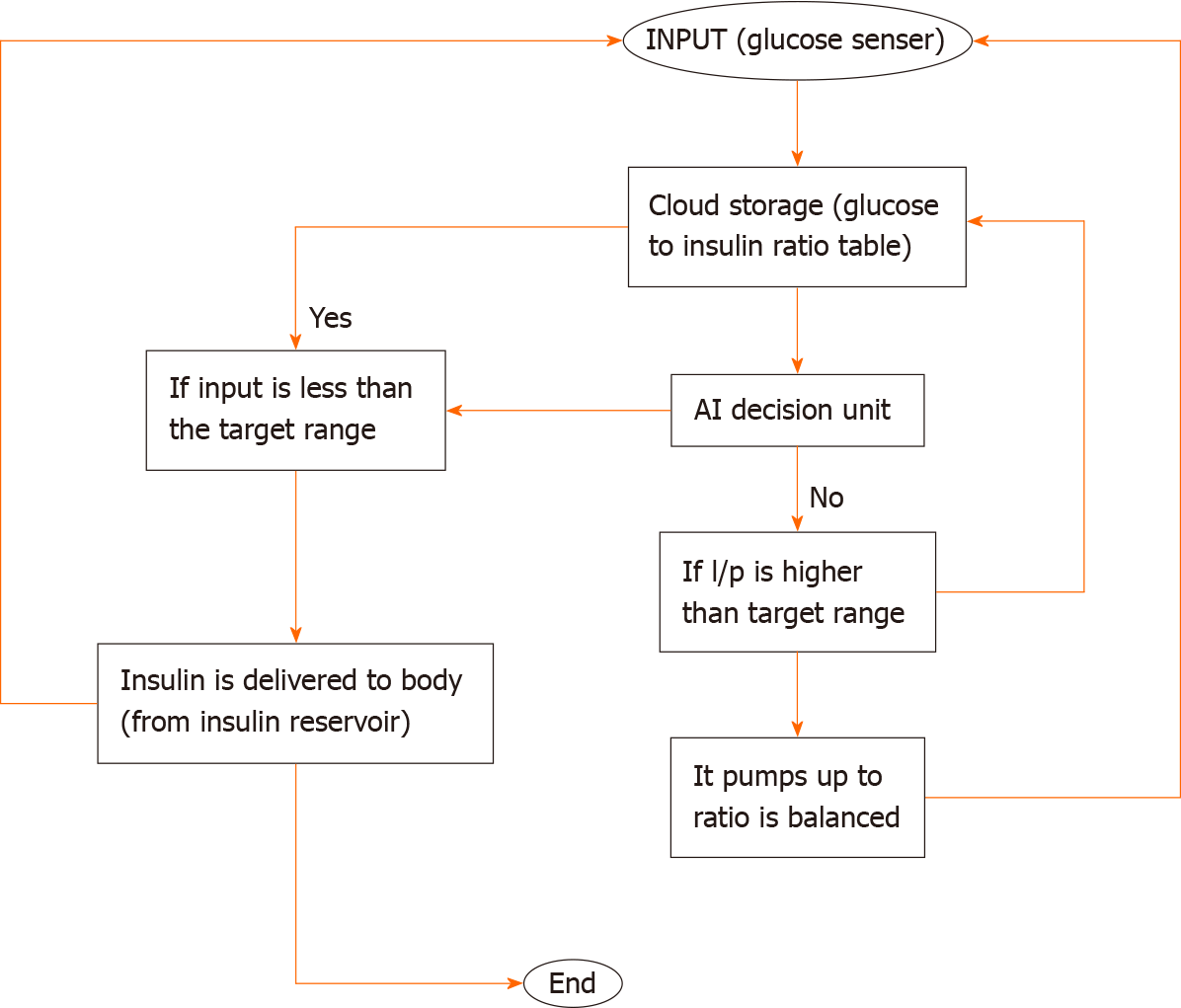
The necessary amount of insulin is pumped using a micropump combined with a microneedle, and the insulin is supplied to the body based on a choice using the pre-existing glucose and insulin levels. The control algorithm, which directs insulin delivery in accordance with glucose levels, is a crucial part. A gadget with a control algorithm receives data from a sensor concerning interstitial glucose levels. Subcutaneously, rapid-acting insulin is administered by an insulin pump. The control algorithm modifies the insulin supply. Wireless technology is used for system component communication (Figure 2).
The WiFi-controlled voltage controller plays a vital role in pumping insulin. The ESP32 is a highly versatile, low-cost SoC chip that has a built-in microprocessor, a full TCP/IP protocol stack, and can connect straight to your Wi-Fi network.
This chip has a built-in Microcontroller Unit, so you can either program it with your application code or just utilize the module as a Wi-Fi transceiver, which is what we do in this project. Using the same module as a transmitter and controller will be more effective. Here, we have utilized the ESP32 development board to create a monitoring system for input voltage levels. The primary objective of this project is to ensure that the input voltage level does not exceed the safe operating voltage of the ESP32, which is typically 3.3V. This is achieved by connecting a potentiometer as a voltage divider, allowing us to measure and monitor the input voltage in real time. If the voltage exceeds 3.3V, the system takes corrective action to prevent potential damage to the ESP32.
The integration of a WiFi-controlled voltage controller, specifically leveraging the ESP32 SoC chip, plays a crucial role in enhancing the efficiency and safety of the insulin pumping system (Figure 3). The ESP32, known for its versatility and cost-effectiveness, serves as a powerful tool to connect the insulin pumping system to a Wi-Fi network, enabling seamless communication and control. In the context of insulin delivery systems, maintaining the stability of the input voltage is paramount to the reliable operation of the ESP32. The ESP32, being a sensitive electronic component, typically operates at a safe voltage level of 3.3V. The implemented solution involves using the ESP32 development board to establish a real-time monitoring system for input voltage levels. To achieve this, a potentiometer is employed as a voltage divider, all
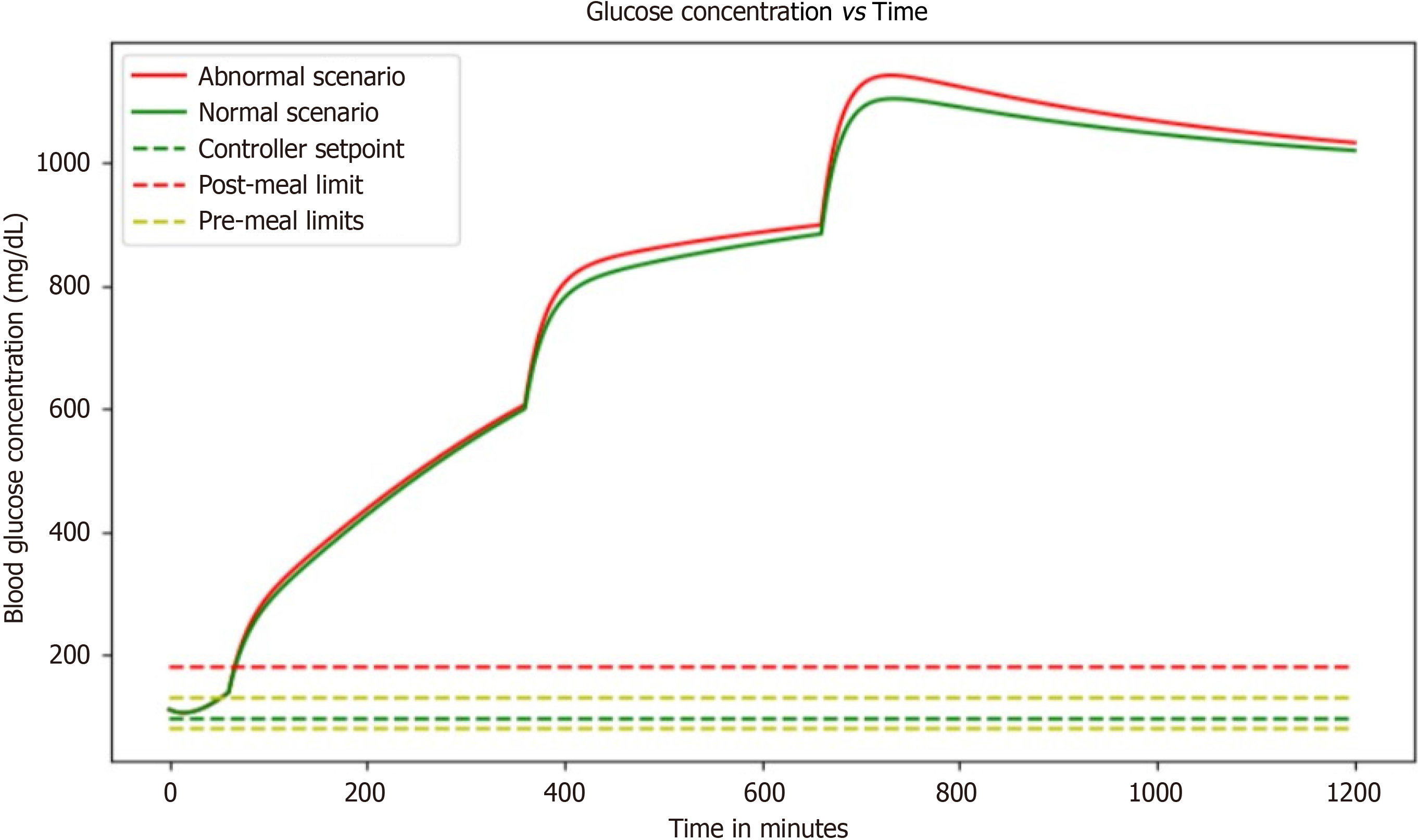
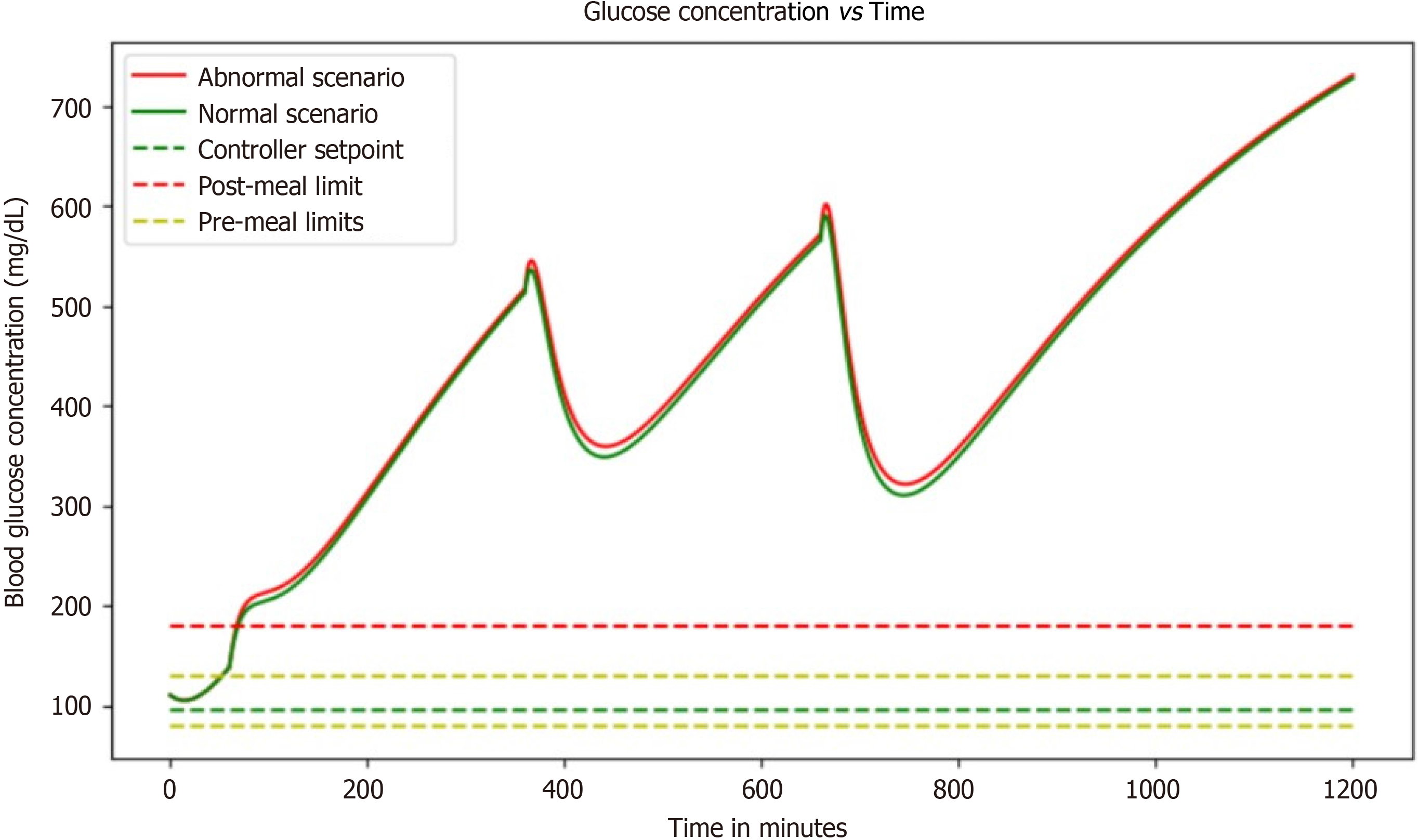
With no controller: Without a controller, this simulation replicates, the rapid blood glucose rise with respect to time and there is no control over it, which has a dangerous effect on people. In the absence of a controller within a blood glucose regulation simulation, a concerning scenario unfolds where blood glucose levels surge uncontrollably over time. This unchecked escalation poses significant risks to individuals, particularly those with diabetes. Without the mechanisms to modulate these fluctuations, blood glucose levels can spike rapidly, leading to a state known as hyperglycemia. Hyperglycemia, characterized by elevated blood sugar levels, can manifest in symptoms like excessive thirst, frequent urination, fatigue, and impaired vision.
This situation becomes even more critical for individuals with diabetes, potentially culminating in life-threatening diabetic ketoacidosis (DKA). DKA arises when the body resorts to breaking down fats for energy due to insufficient glucose utilization, resulting in the production of acidic ketones and severe symptoms such as vomiting and confusion. Prolonged exposure to uncontrolled hyperglycemia can also give rise to debilitating long-term complications, including cardiovascular issues, kidney problems, neuropathy, and retinopathy. Managing high blood sugar levels becomes an arduous task, affecting an individual's overall well-being, daily routines, and incurring substantial healthcare expenses. In essence, the absence of a controller in this simulation mimics the dangerous consequences of unregulated blood glucose, underscoring the critical importance of effective blood glucose management strategies for those affected by diabetes (Figure 4).
With dosage of basal insulin: The graph depicting the correlation between glucose concentration and basal insulin dosage offers valuable insights into the efficacy of the basal insulin regimen in the context of blood glucose level maintenance (Figure 5). This visual representation provides a clear understanding of how changes in the basal insulin dosage relate to fluctuations in glucose concentration. Basal insulin, which serves as the foundation of diabetes management, plays a pivotal role in stabilizing blood sugar levels between meals and during periods of fasting. As we examine the graph, patterns and trends become discernible. A rise in basal insulin dosage typically corresponds to a decline in glucose concentration, suggesting that an increase in basal insulin can effectively lower blood sugar levels. Conversely, a reduction in basal insulin dosage tends to result in an uptick in glucose concentration. These correlations underscore the delicate balance required in diabetes management, where the fine-tuning of basal insulin dosages is crucial for achieving and maintaining stable glucose levels. Furthermore, this graph can aid healthcare professionals and individuals with diabetes in optimizing their treatment plans. It provides a quantitative basis for adjusting basal insulin dosages to achieve target glucose levels, thus enhancing the precision of diabetes care. The data gleaned from this graphical representation can inform personalized insulin regimens tailored to individual needs, promoting better glycemic control and ultimately improving the quality of life for those managing diabetes. In summary, the glucose concentration vs basal insulin dosage graph serves as a powerful tool in diabetes management, offering insights that can lead to more effective treatment strategies and enhanced glucose level stability.
The simulation's ability to keep blood glucose levels steady and in an acceptable range is a significant breakthrough in diabetes management. Maintaining steady-state over extended periods of time is a critical aspect of diabetes mana
The simulation's remarkable capacity to maintain blood glucose levels within a steady and acceptable range represents a monumental leap forward in the field of diabetes management. This achievement holds profound implications for the well-being of individuals grappling with this chronic condition. The ability to sustain a steady-state of glucose levels over extended periods is a cornerstone of effective diabetes care. This stability significantly diminishes the risk of debilitating complications, including but not limited to kidney failure, vision impairment, and nerve damage, all of which can have profoundly negative impacts on an individual's quality of life.
Central to the success of this simulation is the seamless integration of basal insulin dosage with fast-acting insulin injections. This synergistic combination forms a holistic solution finely tuned to an individual's specific requirements. The basal dosage serves as the foundational support, providing a steady release of insulin that acts as a stabilizing force between meals and during periods of fasting. Complementing this, the fast-acting insulin injection steps in to address post-meal spikes in blood sugar, ensuring a comprehensive coverage of insulin needs throughout the day. What sets this simulation apart is its ability to offer a highly personalized approach to diabetes management. It takes into account the unique physiological and lifestyle characteristics of each patient, allowing for a level of customization that was previously unprecedented. By tailoring treatment strategies to the individual, this simulation maximizes the effectiveness of diabetes management, ultimately leading to improved outcomes and a higher quality of life. Moreover, the potential ripple effects of this breakthrough are immense. It not only empowers individuals with diabetes to take more control over their health but also represents a leap forward in the broader landscape of healthcare. This personalized approach sets a precedent for the development of tailored treatments in other areas of medicine, potentially revolutionizing how we approach a range of chronic conditions. The simulation's ability to maintain steady blood glucose levels is a game-changer in diabetes management. It not only mitigates the risk of serious complications but also ushers in a new era of personalized care, underscoring its profound significance in the field of healthcare and the lives of those affected by diabetes.
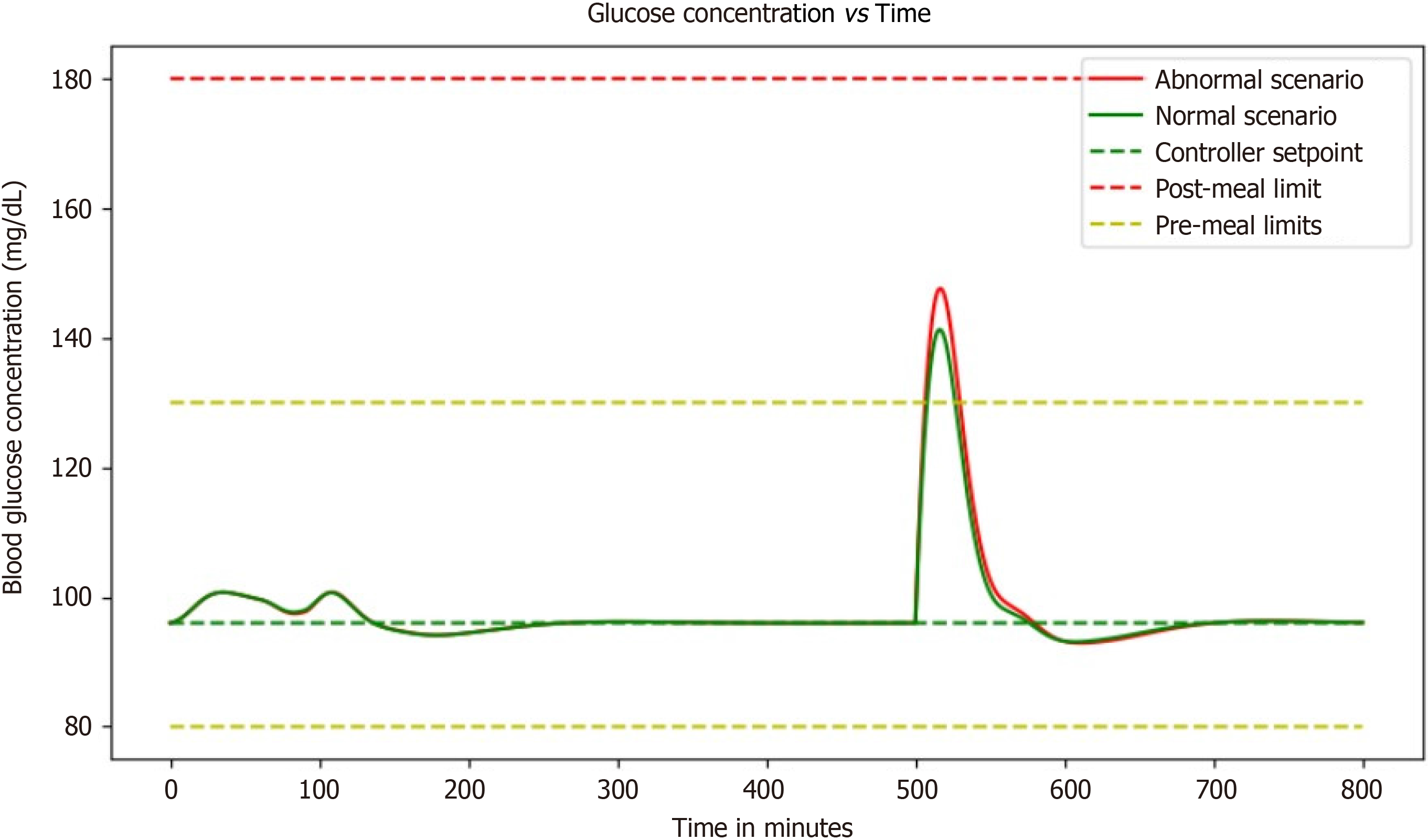
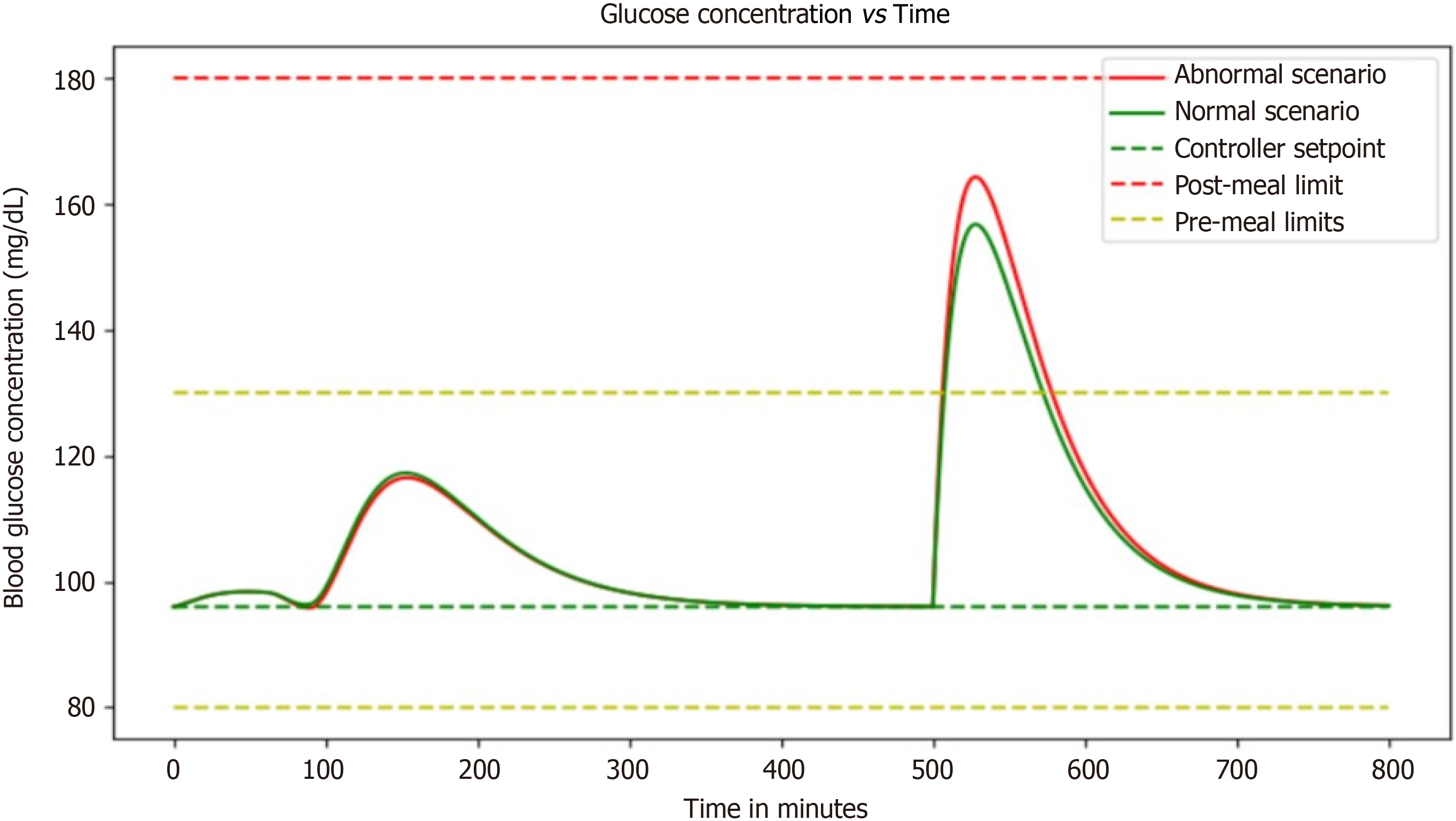
With the ability to see how blood glucose and insulin levels fluctuate in response to different inputs, this simulation provides an invaluable tool for healthcare professionals and patients alike. In particular, the scenario of a diabetic patient vigorously exercising before eating a small meal is fascinating. The simulation illustrates just how complex diabetes management can be. Even with only a basal dosage as a controller input, the patient's blood glucose and insulin concentrations are constantly changing. It is incredible to see how the addition of a small meal or some exercise can impact these levels so dramatically. As someone who's never had to manage diabetes, it is eye-opening to see just how much work goes into keeping blood glucose levels within a healthy range (Figure 7).
The ability of this simulation to visually represent the intricate dance between blood glucose and insulin levels in response to various inputs is an invaluable asset for both healthcare professionals and individuals navigating diabetes. It offers a dynamic and illuminating window into the complexities of managing this chronic condition. One particularly captivating scenario within this simulation involves a diabetic patient engaging in vigorous exercise before consuming a small meal.
This scenario vividly underscores the multifaceted nature of diabetes management. What stands out is the dynamic nature of blood glucose and insulin concentrations, even with the sole input of a basal dosage. It highlights the constant ebb and flow of these critical biomarkers, emphasizing that diabetes management is far from a static process. The intricate interplay between insulin, glucose, and external factors such as exercise and meal timing is on full display. Witnessing how even minor changes, like a small meal or a burst of physical activity, can exert profound effects on these levels is nothing short of astonishing. For individuals fortunate enough not to grapple with diabetes, this simulation serves as an eye-opening revelation. It sheds light on the immense effort and vigilance required to maintain blood glucose within a healthy range.
It underscores that diabetes management is not a one-size-fits-all endeavor but a highly individualized and nuanced process. Each person's response to various inputs can be unique, demanding a personalized approach that takes into account factors like lifestyle, dietary choices, and exercise routines. Moreover, the simulation's utility extends beyond raising awareness. It offers a practical tool for healthcare professionals to fine-tune treatment plans for their patients. By visually assessing how different scenarios impact blood glucose and insulin levels, healthcare providers can make more informed decisions about medication adjustments, dietary recommendations, and lifestyle modifications. This empowers individuals with diabetes to actively participate in their own care and enables healthcare teams to offer more tailored and effective guidance.
This simulation unveils the intricate web of factors influencing blood glucose and insulin dynamics, making it an essential resource for healthcare professionals and a revealing educational tool for those unfamiliar with the complexities of diabetes management. It underscores the relentless effort required to maintain blood glucose within a healthy range and the critical role of personalized care in this ongoing endeavor.
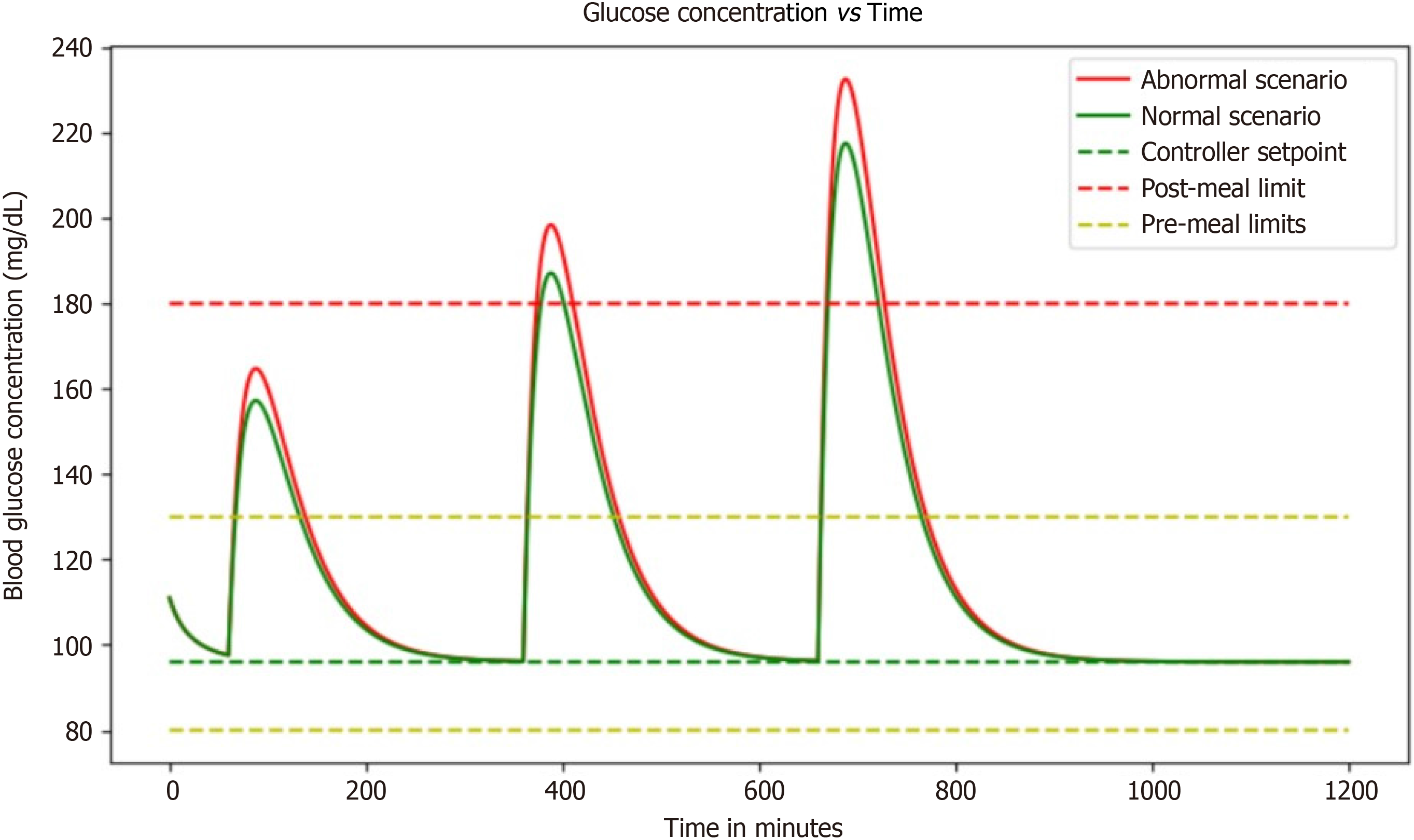
In this simulation, a PID controller was used to model the changing blood glucose and insulin levels in individuals with diabetes. Imagine being able to see how your body responds to different foods and activities without actually having to undergo those experiences in real life (Figure 8).
The goal of this simulation is to develop a way to effectively manage blood glucose levels in people with diabetes. The PID controller used in this simulation is a control mechanism that adjusts the input based on the error between the setpoint and the actual output.
In this case, the setpoint is the desired blood glucose level, and the actual output is the measured blood glucose level. By adjusting the insulin dosage based on these measurements, researchers hope to delay reaching a steady-state and counteract any spikes in blood glucose that occur after meals. It is fascinating to think about how this simulation can help better understand how the body processes glucose and insulin.
This simulation emerges as a captivating tool, offering an unprecedented opportunity to gain insights into the intricate processes of glucose and insulin regulation within the body. The PID-controlled insulin dosage adjustments create a dynamic feedback loop, mimicking the complexities of real-life physiological responses. It is not just a simulation; it is a window into the nuanced world of diabetes management, enabling researchers to explore, in a controlled virtual envir
By modeling these complex systems, predictions can be made about how different interventions will affect blood glucose levels. They can also test different scenarios and see how the body responds over time. This can lead to improved treatment options for people with diabetes, as well as a better understanding of the disease itself.
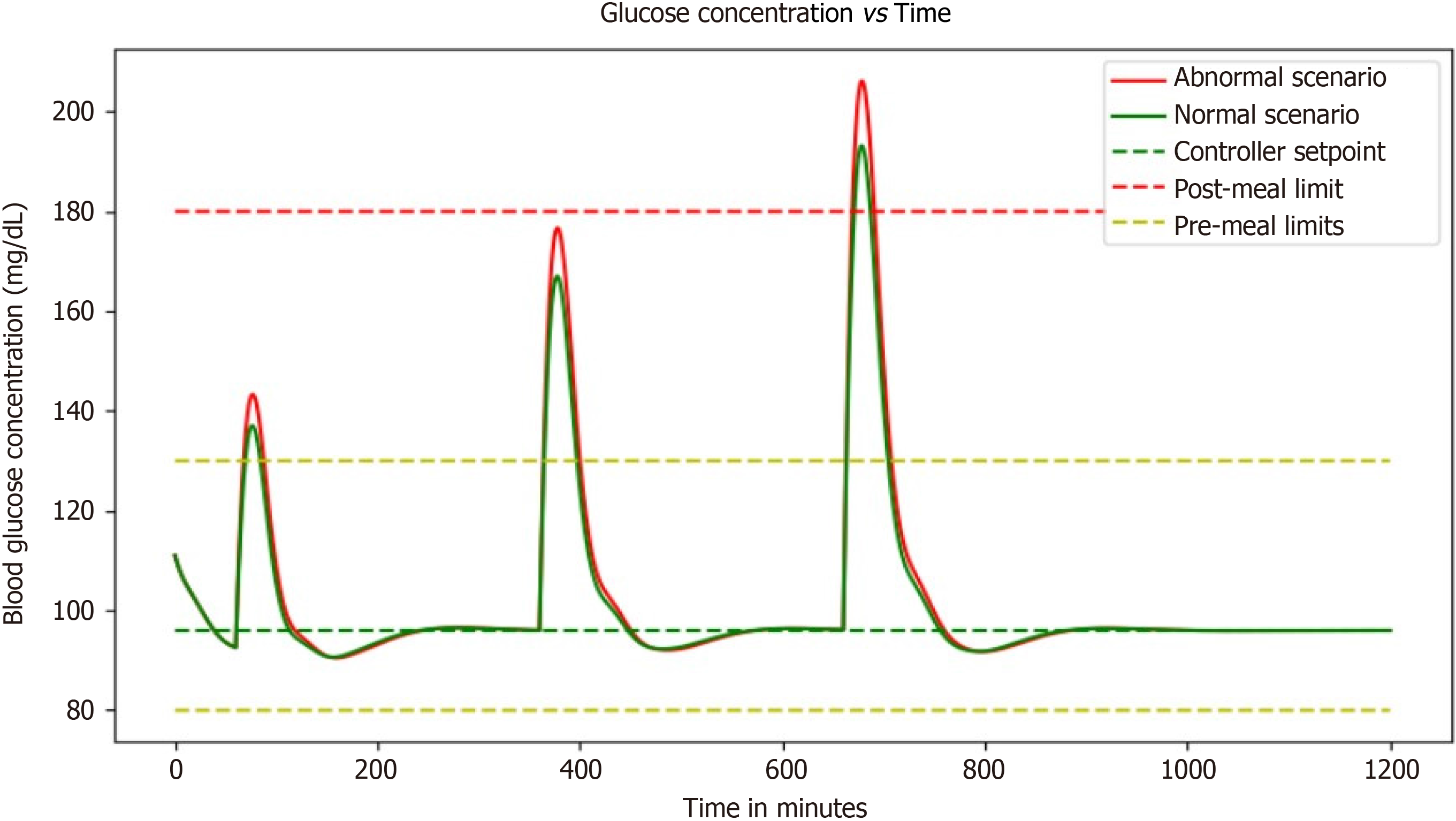
The simulation of the evolution of insulin and blood glucose levels in diabetic patients is an incredible technological advancement. With the help of a PID controller coupled with the basal input for steady-state, it has become possible to control the controller's input and mitigate the effects of blood sugar increase brought on by meals (Figure 9).
The simulation aims to delay the controller's arrival at a steady-state, which ultimately leads to little to no variation from the setpoint value. This means that diabetic patients can now have better control over their blood glucose levels, which is a huge relief for them. The use of a PID controller coupled with the basal input for steady-state is an excellent approach to modeling the evolution of insulin and blood glucose levels. The simulation enables diabetic patients to have a better understanding of their blood glucose levels and how they can manage them effectively. With the help of this simulation, patients can now avoid sudden spikes in their blood sugar levels that can be harmful to their health. This simulation brings hope to millions of people suffering from diabetes worldwide. By taking into account the basal dose in the expression, it is expected that a steady state would be attained with little to no variation from the setpoint value. This means that diabetic patients can now live healthier and happier lives without having to worry about fluctuating blood sugar levels. The simulation has made it possible for people with diabetes to have better control over their lives and health. It has given them a new lease on life, where they can enjoy their favorite foods without worrying about the consequences.
Blood glucose control is an essential aspect of managing diabetes and finding the most effective ways to control it. The latest findings suggest that a basal insulin administration rate and a single, high-concentration injection before a meal may not be sufficient to maintain healthy blood glucose levels. While the basal insulin rate treatment can stabilize blood glucose levels over the long term, it may not be enough to bring the levels below the post-meal limit after 60 min. The short-term impacts of meals can be greatly reduced by high-concentration injections, which can help stabilize blood glucose levels. Unfortunately, they cannot provide long-term stability to satisfy the post-meal or pre-meal restrictions. However, PID control with basal dose maintains the blood glucose levels within the range for a longer period of time.
In conclusion, the WiFi-controlled voltage controller used for insulin pumping systems, leveraging the ESP32 SoC chip, represents a remarkable advancement in the field of healthcare technology. The integration of this controller addresses a critical aspect of insulin delivery systems by ensuring the stability of the input voltage, a factor pivotal to the safe and reliable operation of the ESP32 microcontroller. By employing the ESP32's capabilities for real-time monitoring and corrective actions, the system demonstrates an innovative approach to safeguarding sensitive electronic components, preventing potential damage, and ultimately enhancing the overall performance and safety of insulin delivery.
Furthermore, the incorporation of a PID controller to assess and regulate blood glucose and insulin levels in in
In essence, the WiFi-controlled voltage controller and the PID controller simulation collectively underscore the ongoing efforts to enhance efficiency, safety, and personalized care within the realm of diabetes management. These technological advancements not only contribute to the optimization of insulin delivery systems but also hold the potential to reshape our understanding of glucose and insulin dynamics, fostering a new era of precision medicine in the treatment of diabetes.
The system in the future will be made more robust by integrating with the Internet of Things (IoT), and will be made customizable to the patient's needs.
This study proposes a novel approach to reduce the circuitry to be carried by the patient while using an automated glucose-controlling system by adopting cloud storage. The system uses a micropump and microneedle to pump the insulin into the body making it comfortable and affordable.
The system was successful in delivering the required amount of insulin in accordance with the glucose sensor readings.
Techniques like the IoT and artificial intelligence were used to design and work with the proposed system.
A closed-loop diabetes control system was designed, which was validated with several glucose sensor readings.
This system will provide a better and affordable solution for diabetic patients to control glucose levels in the body which can extend the life expectancy.
There exist very few but expensive closed-look automated diabetes detection control systems presenting a research gap for technical research.
The authors would like to thank Velagapudi Ramakrishna Siddhartha Engineering College, Sponsored by Siddhartha Academy of General Technical Education (SAGTE) m Vijayawada, for the financial support extended to carry out the project.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Engineering, biomedical
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Joda BA, Iraq; Long P, China; Zhao CF, China S-Editor: Liu JH L-Editor: Webster JR P-Editor: Zhao YQ
| 1. | International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104:1-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 346] [Article Influence: 31.5] [Reference Citation Analysis (1)] |
| 2. | Sakudo A. Near-infrared spectroscopy for medical applications: Current status and future perspectives. Clin Chim Acta. 2016;455:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 3. | Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J. Epidemiology of Type 2 Diabetes - Global Burden of Disease and Forecasted Trends. J Epidemiol Glob Health. 2020;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 1638] [Article Influence: 409.5] [Reference Citation Analysis (2)] |
| 4. | Villena Gonzales W, Mobashsher AT, Abbosh A. The Progress of Glucose Monitoring-A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors (Basel). 2019;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 5. | Bazaev NA, Masloboev IuP, Selishchev SV. [Optical methods for noninvasive blood glucose monitoring]. Med Tekh. 2011;29-33. [PubMed] |
| 6. | Bruen D, Delaney C, Florea L, Diamond D. Glucose Sensing for Diabetes Monitoring: Recent Developments. Sensors (Basel). 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 7. | Nathan DM; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 866] [Cited by in RCA: 1016] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 8. | Shokrekhodaei M, Quinones S. Review of Non-invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors (Basel). 2020;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |