Published online Mar 20, 2024. doi: 10.5493/wjem.v14.i1.84284
Peer-review started: July 28, 2023
First decision: October 9, 2023
Revised: November 24, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: March 20, 2024
Processing time: 234 Days and 18.6 Hours
Photodynamic therapy (PDT) can take place in the presence of three elements: Light with an appropriate wavelength; a photosensitizer; and the presence of oxygen. This type of treatment is very effective overall against bacterial, viral and mycotic cells. In the last 10 years many papers have been published on PDT with different types of photosensitizers (e.g., methylene blue, toluidine blue, indo
Core Tip: In recent years there are more and more multidrug resistant infections at the oral level, and this has led researchers to find alternative solutions to conventional pharmacology that have no impact on systemic health. Among these, there is photody
- Citation: Casu C, Orrù G. Potential of photodynamic therapy in the management of infectious oral diseases. World J Exp Med 2024; 14(1): 84284
- URL: https://www.wjgnet.com/2220-315x/full/v14/i1/84284.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i1.84284
There are over 780 results in PubMed when searching the keyword “Photodynamic Therapy for Oral Infection,” with an exponential growth of the works published in the last 10 years. All this denotes the strong scientific interest of this instrument in the field of infectious pathologies of the oral cavity.
Photodynamic therapy (PDT) can take place in the presence of three elements: Light with an appropriate wavelength; a photosensitizer; and the presence of oxygen. When a photosensitizer is activated by light, it will either lose energy or form an oxygen triplet[1]. This second type of PDT is linked to the amount of oxygen, while the first type of PDT can work in anaerobic conditions[2]. The interaction that the photosensitizer has with oxygen can determine the formation of hydrogen peroxide, hydroxyl radicals, superoxide radical oxygen, and singlet oxygen. These products determine the killing of microorganisms due to damage to the cell membrane and their metabolic activity[1].
The maximum absorption of light by the photosensitizer occurs at a wavelength between 600 nm and 800 nm. In fact, a higher wavelength would not have enough energy to stimulate oxygen to produce radicals[1]. The penetration of the wavelength comprised between 700-1100 nm is wider than that comprised between 400-700 nm[2].
Among the infections that afflict the oral cavity, endodontic infections are caused by microorganisms that colonize the inside of the tooth and can give rise to infections in the periapical bone tissue. One of the most important microorganisms in this infectious process is Enterococcus faecalis (E. faecalis), which is also the most studied in vitro and ex vivo model. The difficulty of eradicating this microorganism responsible for endodontic re-infections and refractory infections has prompted researchers to make use of PDT in eradicating this microorganism. Researchers have found that the photosensitizer curcumin activated by LED (450 nm, 67 mW/cm2, and 20.1 J/cm2) has shown very interesting results in reducing the colony forming units of E. faecalis[1]. Other authors have tested Ce6 methyl ester (Zn(II)e6Me), a chlorophyll-derived photosensitizer activated by red light (627 nm, 75 mW, 3150 J/cm2) for 90 s, with success against E. faecalis. Furthermore, an in vitro study found that a PDT using 500 g/mL of Chlorella plus 660 nm diode laser at an energy density of 23.43 J/cm2 was effective against E. faecalis biofilms[1].
The most frequent infectious disease at the gingival level is periodontitis. It is an infectious process mediated by various microorganisms, including Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans that leads to inflammation and destruction of bone and gingival tissue with loss of stability of the dental elements. Curcuma longa activated by blue LED lights (450-470 nm, output power density 1.2 W/cm2) and Ce6 have shown the most effectiveness among the natural photosensitizers[1]. PDT carried out with Curcuma longa and diode light at 460 nm has also shown efficacy in drug-induced gingival hypertrophy[3].
Rose bengal with a light source for illumination (375 nm, 3 mW/cm2) could be very useful for reducing the bacterial load of Streptococcus mutans, the most important etiological agent of dental cavities. Ozonated water used as a photosensitizer and activated by 460 nm LED lights has also demonstrated efficacy against Streptococcus mutans[4].
The most frequent oral viral infectious diseases are associated with herpes simplex type 1. Clinically, this is a condition that involves the formation of vesicles filled with infectious liquid that when they explode give rise to erosion and the formation of crusts if they affect the vermilion border of the lips. In this context, the gold standard treatment is represented by topical or systemic antivirals, particularly in extensive systemic cases. However, the reduction of effectiveness of traditional pharmacological treatments has prompted several researchers to find different solutions to mitigate these infections, including PDT[5]. For example, the most common photosensitizer for the treatment of Herpes simplex infection is methylene blue at concentrations between 0.1% and 1%, activated by lights with a wavelength of 660 nm. In another in vitro study, indocyanine green also showed good results when activated by wavelengths of 810 nm[6,7].
Human papillomavirus (HPV) plays a prominent role in causing viral infections in the mouth. According to the World Health Organization reports, it is estimated that in developed countries a growing prevalence of HPV-related cancer is reported each year, particularly for males. There are over 200 subtypes of HPV, but some of these can trigger malignant tissue transformations, including HPV 16. Surgical excision and histological evaluation represent the treatment of choice. Sometimes these lesions can reach considerable dimensions, are located in sites that are difficult to reach and can be recurring. These reasons have led clinicians to try PDT on the oral cavity in a patient with a large squamous papilloma in the soft palate. In this case, a formulation containing 5-aminolevulenic acid is injected at the perilesional level and activated with wavelengths of 630 nm at a power of 100 J/cm2 and at 300 mW/cm2 of power density for 6 min. After two sessions, the lesion healed[8].
PDT has also been proposed in the treatment of oral lesions from coronavirus disease 2019. In fact, in vitro studies have shown that methylene blue was able to inhibit the severe acute respiratory syndrome coronavirus 2 spike protein and its angiotensin converting enzyme-2 receptor. It was also used to treat the typical crusted lip lesions associated with coronavirus disease 19 at very low concentrations[9].
Oral candidiasis, as previously mentioned, is an infection that increasingly shows resistance to the main commercial antifungals, such as azoles. PDT was proposed 15 years ago as an alternative, especially in relapsing cases of oral candidiasis. Among the most tested photosensitizers, methylene blue, which is associated with laser and non-red laser lights, has demonstrated excellent antifungal properties at different concentrations[10-13]. Toluidine blue has also been widely proposed in different concentrations for the treatment of Candida infections in the oral cavity. A recent review on the subject has highlighted that most of these are in vitro studies, where the parameters used are very different between the various studies, even if almost all report the efficacy of PDT. In addition to the more common Candida albicans, it has also been tested against different species of Candida in some studies, such as Candida glabrata, Candida krusei, Candida parapsilosis and Candida tropicalis. Toluidine blue has been shown to be effective against all types of Candida spp. mentioned[14]. Most of the in vitro studies evaluated both the activity of PDT in its planktonic form and in the biofilm of the fungus. The prevailing method was that of microplates[14].
A recent compound, indocyanine green, activated by a light with a wavelength of 808 nm has also been proposed as a photosensitizer. In fact, by comparing this approach with a traditional treatment based on nystatin, a significant infection reduction was observed, which was similar to the healthy group control patient[15]. The same result was reported in another study on refractory subprosthetic candidiasis in which PDT showed better results than the group treated only pharmacologically[16]. Furthermore, in another study, PDT with indocyanine green was shown to be more effective in vitro than PDT performed with methylene blue[17].
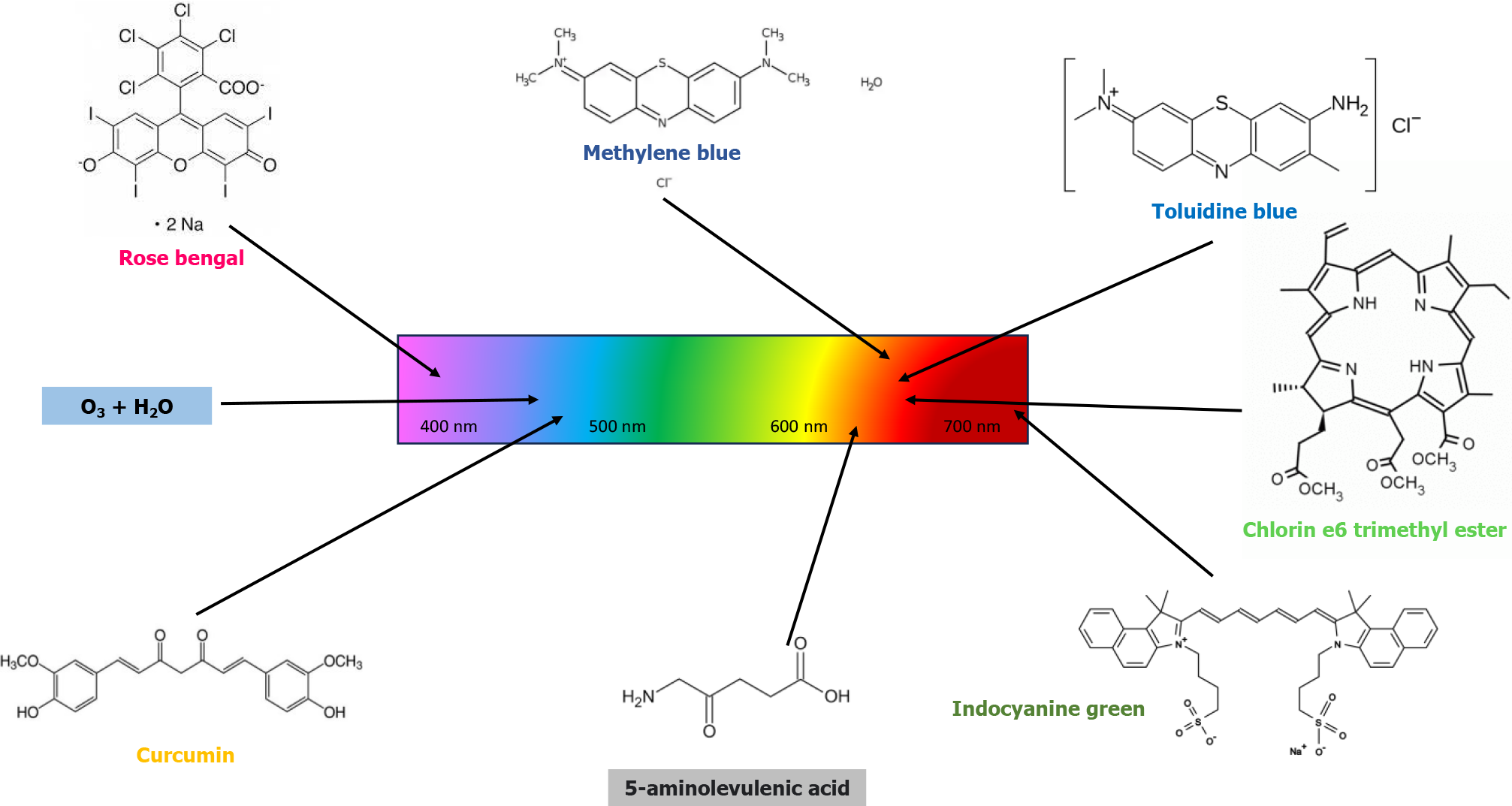
Other photosensitizers tested in vitro against Candida were erythrosine and rose bengal, activated by green diode lights around 532 nm[18,19]. Erythrosine was also effective against Candida dubliniensis, demonstrating good efficacy in the planktonic form but less activity on the biofilm[20]. Among the natural photosensitizers reported are 5-aminolevulenic acid[21] and derivatives from Curcuma longa, which was first carried out in 2013[22]. In a study using LED light at 460 nm, 5-aminolevulenic acid showed in vitro antifungal activity higher than that of methylene blue, which was activated by red light at 660 nm[23]. A photosensitizer based on Curcuma longa/3% hydrogen peroxide, activated by polarized light (wavelength from 380 to 3400 nm), showed greater antifungal activity than activation with LED light at 460 nm (the wavelength most widely used in PDT to activate curcumin)[24]. Furthermore, PDT has also shown efficacy in other oral pathologies, perhaps by virtue of its antimicrobial action against microorganisms that colonize the damaged mucosal surfaces[25] or which may be at the basis of the pathogenic process[26]. The main photosensitizers used in PDT vs oral infections, with relative wavelength of activation, are summarized in Figure 1.
The increasing resistance to antibiotics, antifungals and antivirals, the side effects of the drugs themselves on some categories of patients and the possibility of resolving some infections in a single session have led researchers to propose the use of PDT as a treatment tool for refractory infections of the oral cavity. The wide variability of the parameters used, including the wavelength, the type of photosensitizer, the pre-illumination and irradiation times, makes it difficult to have standard protocols for the treatment of each oral pathology. Further studies will be needed to find the most suitable protocols for each type of oral infection.
Cinzia Casu performed this research activity in the framework of the International PhD in Innovation Sciences and Technologies at the University of Cagliari, Italy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoun G, Lebanon; Liu HQ, China; Wu J, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Afrasiabi S, Partoazar A, Chiniforush N, Goudarzi R. The Potential Application of Natural Photosensitizers Used in Antimicrobial Photodynamic Therapy against Oral Infections. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Gao J, Chen Z, Li X, Yang M, Lv J, Li H, Yuan Z. Chemiluminescence in Combination with Organic Photosensitizers: Beyond the Light Penetration Depth Limit of Photodynamic Therapy. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Casu C, Murgia MS, Orrù G, Scano A. Photodynamic therapy for the successful management of cyclosporine-related gum hypertrophy: A novel therapeutic option. J Public Health Res. 2022;11:22799036221116177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 4. | Casu C, Orrù G, Fais S, Mazur M, Grassi R, Grassi RF, Nardi GM. Efficacy of ozonated water as a PS in photodynamic therapy: A tool for dental caries management? An in vitro study. J Public Health Res. 2023;12:22799036231182267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 5. | La Selva A, Negreiros RM, Bezerra DT, Rosa EP, Pavesi VCS, Navarro RS, Bello-Silva MS, Ramalho KM, Aranha ACC, Braz-Silva PH, Fernandes KPS, Bussadori SK, Horliana ACRT. Treatment of herpes labialis by photodynamic therapy: Study protocol clinical trial (SPIRIT compliant). Medicine (Baltimore). 2020;99:e19500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Khalil M, Hamadah O. Association of Photodynamic Therapy and Photobiomodulation As a Promising Treatment of Herpes Labialis: A Systematic Review. Photobiomodul Photomed Laser Surg. 2022;40:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Namvar MA, Vahedi M, Abdolsamadi HR, Mirzaei A, Mohammadi Y, Azizi Jalilian F. Effect of photodynamic therapy by 810 and 940 nm diode laser on Herpes Simplex Virus 1: An in vitro study. Photodiagnosis Photodyn Ther. 2019;25:87-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Jin J, Zhang Y, Zhiyue L. Successful treatment of oral human papilloma by local injection 5-aminolevulinic acid-mediated photodynamic therapy: A case report. Photodiagnosis Photodyn Ther. 2019;26:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Betsy J, Prasanth CS. Can photodynamic therapy be repurposed to treat oral lesions of COVID-19? Photodiagnosis Photodyn Ther. 2021;33:102175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | Freire F, Ferraresi C, Jorge AO, Hamblin MR. Photodynamic therapy of oral Candida infection in a mouse model. J Photochem Photobiol B. 2016;159:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Javed F, Samaranayake LP, Romanos GE. Treatment of oral fungal infections using antimicrobial photodynamic therapy: a systematic review of currently available evidence. Photochem Photobiol Sci. 2014;13:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Boltes Cecatto R, Siqueira de Magalhães L, Fernanda Setúbal Destro Rodrigues M, Pavani C, Lino-Dos-Santos-Franco A, Teixeira Gomes M, Fátima Teixeira Silva D. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: The state of the art. Photodiagnosis Photodyn Ther. 2020;31:101828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 13. | Martins Jda S, Junqueira JC, Faria RL, Santiago NF, Rossoni RD, Colombo CE, Jorge AO. Antimicrobial photodynamic therapy in rat experimental candidiasis: evaluation of pathogenicity factors of Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Wiench R, Skaba D, Matys J, Grzech-Leśniak K. Efficacy of Toluidine Blue-Mediated Antimicrobial Photodynamic Therapy on Candida spp. A Systematic Review. Antibiotics (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Tavangar A, Khozeimeh F, Razzaghi-Abyaneh M, Sherkat S. Sensitivity of Four Various Candida Species to Photodynamic Therapy Mediated by Indocyanine Green, an in vitro Study. J Dent (Shiraz). 2021;22:118-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Afroozi B, Zomorodian K, Lavaee F, Zare Shahrabadi Z, Mardani M. Comparison of the efficacy of indocyanine green-mediated photodynamic therapy and nystatin therapy in treatment of denture stomatitis. Photodiagnosis Photodyn Ther. 2019;27:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Azizi A, Amirzadeh Z, Rezai M, Lawaf S, Rahimi A. Effect of photodynamic therapy with two photosensitizers on Candida albicans. J Photochem Photobiol B. 2016;158:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Costa AC, Rasteiro VM, Pereira CA, Rossoni RD, Junqueira JC, Jorge AO. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses. 2012;55:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Freire F, Costa AC, Pereira CA, Beltrame Junior M, Junqueira JC, Jorge AO. Comparison of the effect of rose bengal- and eosin Y-mediated photodynamic inactivation on planktonic cells and biofilms of Candida albicans. Lasers Med Sci. 2014;29:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Costa AC, de Campos Rasteiro VM, Pereira CA, da Silva Hashimoto ES, Beltrame M Jr, Junqueira JC, Jorge AO. Susceptibility of Candida albicans and Candida dubliniensis to erythrosine- and LED-mediated photodynamic therapy. Arch Oral Biol. 2011;56:1299-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | AlGhamdi AS, Qamar Z, AlSheikh R, Al Hinai MTA, Abdul NS, Aljoghaiman EA, Ali S. Clinical efficacy of 5-aminolevulinic acid-mediated photodynamic therapy vs topical antifungal agent and surgical excision for the treatment of hyperplastic candidiasis. Photodiagnosis Photodyn Ther. 2023;41:103258. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Dovigo LN, Carmello JC, de Souza Costa CA, Vergani CE, Brunetti IL, Bagnato VS, Pavarina AC. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med Mycol. 2013;51:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 23. | Daliri F, Azizi A, Goudarzi M, Lawaf S, Rahimi A. In vitro comparison of the effect of photodynamic therapy with curcumin and methylene blue on Candida albicans colonies. Photodiagnosis Photodyn Ther. 2019;26:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Casu C, Orrù G, Scano A. Curcumin/H2O2 photodynamically activated: an antimicrobial time-response assessment against an MDR strain of Candida albicans. Eur Rev Med Pharmacol Sci. 2022;26:8841-8851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Casu C, Mannu C. Atypical Afta Major Healing after Photodynamic Therapy. Case Rep Dent. 2017;2017:8517470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Scano A, Casu C, Orrù G, Coni P. Editorial - Epigenetic mechanisms in oral cancer: new diagnostic and therapeutic strategies. Eur Rev Med Pharmacol Sci. 2022;26:7318-7320. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |