Published online Mar 20, 2022. doi: 10.5493/wjem.v12.i2.26
Peer-review started: October 30, 2021
First decision: December 27, 2021
Revised: December 29, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: March 20, 2022
Processing time: 137 Days and 1.6 Hours
Even though coronavirus 2019 disease (COVID-19) clinical course in children is much milder than in adults, pneumonia can occur in the pediatric population as well. Here, we reported a single-center pediatric case series of COVID-19 from Kazakhstan during the first wave of pandemic.
To analyze the main clinical and laboratory aspects in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) positive and negative children diagnosed with pneumonia.
This is a retrospective analysis of 54 children, who were medically assessed as close contacts of COVID-19 adults in their family setting, between June and September 2020. These children were all hospitalized: We compared the clinical and laboratory characteristics of children affected with pneumonia in the presence (group 1) or absence (group 2) of SARS-CoV-2 infection.
Overall, the main clinical manifestations at the admission were fever, cough, loss of appetite, fatigue/weakness, nasal congestion and/or rhinorrhea, and dyspnea. Based on the SARS-CoV-2 polymerase chain reaction (PCR) test, 24 positive children with pneumonia (group 1) and 20 negative children with pneumonia (group 2) were identified; 10 positive children did not show any radiological findings of pneumonia. No significant differences were found between the two pneumonia study groups for any clinical and laboratory parameters, except for C-reactive protein (CRP). Of course, both pneumonia groups showed increased CRP values; however, the COVID-19 pneumonia group 1 showed a significantly higher increase of CRP compared to group 2.
In our case series of children assessed for SARS-CoV-2 infection based on contact tracing, the acute inflammatory response and, in detail, CRP increase resulted to be more pronounced in COVID-19 children with pneumonia than in children with SARS-CoV-2-unrelated pneumonia. However, because of multiple limitations of this study, larger, controlled and more complete clinical studies are needed to verify this finding.
Core Tip: This is a single-center pediatric case series of coronavirus 2019 disease (COVID-19) from Kazakhstan during the first wave of pandemic. We analyzed the main clinical aspects in those children diagnosed with pneumonia. In detail, we compared the clinical and laboratory characteristics of children affected with pneumonia in the presence (group 1) or absence (group 2) of severe acute respiratory syndrome coronavirus-2 infection. No significant differences were found between these study groups for any clinical and laboratory parameters, except for C-reactive protein (CRP). Of course, both pneumonia groups showed increased CRP values, overall; however, COVID-19 pneumonia group showed a significantly higher increase of CRP compared to pneumonia children without COVID-19.
- Citation: Zhamankulov A, Rozenson R, Morenko M, Akhmetova U, Tyo A, Poddighe D. Comparison between SARS-CoV-2 positive and negative pneumonia in children: A retrospective analysis at the beginning of the pandemic. World J Exp Med 2022; 12(2): 26-35
- URL: https://www.wjgnet.com/2220-315x/full/v12/i2/26.htm
- DOI: https://dx.doi.org/10.5493/wjem.v12.i2.26
In December 2019, a new type of coronavirus infection rapidly spread from Wuhan city (in Hubei province, China), which was implicated in many cases of pneumonia and severe respiratory distress. On February 11th, 2020, the Research Group of the International Committee on Taxonomy of Viruses defined this new coronavirus as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the World Health Organization (WHO) named the related infectious disease as coronavirus 2019 disease (COVID-19). On March 11th, 2020, the WHO announced a pandemic of COVID-19[1-3]. The Republic of Kazakhstan borders with China, and the first case of COVID-19 was registered on March 13th, 2020, in Almaty city. Accordingly, several restrictions were promptly implemented like in most parts of the world, which also affected the general medical practice and patients’ management all over the country[4]. Overall, COVID-19 in children is characterized by a milder clinical course, in terms of both clinical manifestations and risk of complications[5]. According to the report from the American Academy of Pediatrics, as of September 17th, 2020 (thus, related to the first wave of pandemic), the proportion of pediatric COVID-19 diagnoses in the United States was only 10.3% of all the COVID-19 registered cases; the mortality rate in children was < 0.2%[6]. A study from China, including 2,143 pediatric patients, confirmed a mild clinical course of COVID-19 in most children and, indeed, only 5.9% of cases were diagnosed as severe in the same period[7]. Therefore, most pediatric COVID-19 cases showed an asymptomatic or mild clinical course[8-9]. The most commonly reported symptoms in children were fever and cough and, in general, respiratory manifestations (such as rhinorrhea, nasal congestion, undifferentiated upper airways inflammatory syndrome, dyspnea); however, gastrointestinal symptoms (including nausea, vomiting, abdominal pain, and diarrhea) were described as well[9-11]. Here, we reported a pediatric case series of COVID-19 from Kazakhstan. In detail, we analyzed the development of pneumonia in children medically and microbiologically assessed for SARS-CoV-2 infection in the context of a household contact tracing strategy implemented at the beginning of the pandemic.
We retrospectively analyzed the medical records of 54 children aged 5 days to 17 years, who were medically assessed and hospitalized since they were close contacts of COVID-19 adult patients in their family setting. In detail, all these children were consecutively admitted and assessed at the Emergency Department of the multidisciplinary Children's Municipal Hospital No. 1 in Nur-Sultan (Kazakhstan), from June 8th to September 15th, 2020, because they were diagnosed with SARS-CoV-2 infection and/or affected with pneumonia. Indeed, this case series is a part of all those pediatric patients that received medical attention at the Emergency Department of Children's Municipal Hospital No. 1, because of previous close contact with a family member diagnosed with COVID-19, as already mentioned. All these children underwent SARS-CoV-2 polymerase chain reaction (PCR) test, but only those who resulted to be PCR positive and/or were diagnosed with pneumonia (even despite the negative PCR result), were admitted to the department of Pulmonology. Indeed, children who had contact with family members diagnosed with COVID-19 but resulted to be PCR negative and without pneumonia, were not admitted to the hospital and, thus, were discharged from the Emergency Department; unfortunately, these data could not be reliably retrieved. In order to assess the infection with SARS-CoV-2 in these children, the biospecimen was obtained by oropharyngeal swab, and the samples were placed in 3 mL of transport medium, in order to be delivered to the authorized laboratory according to the rules approved by the Ministry of Health of Republic of Kazakhstan (protocol No. 15990). The analysis of the viral RNA presence (by SARS-CoV-2 PCR test) was carried out by using the diagnostic kit KH-G-M-565-48-CE (manufactured by Shanghai Kehua Bio-engineering Co., Ltd; analyzer Xi'an Tian Long Science and Technology Co., Ltd., Shaanxi, China). Upon admission to the hospital, these children underwent a complete clinical examination (including an accurate collection of personal and family history) and first-level diagnostic work-up (including a complete blood cell count -CBC-, erythrocyte sedimentation rate -ESR-, urinalysis and general biochemistry). The biochemical analyses included plasmatic calcium, glucose, sodium, potassium, chloride, urea, creatinine, total protein, alanine aminotransferase, aspartate aminotransferase, bilirubin, creatine phosphokinase, in addition to serum C-reactive protein (CRP). All patients received a chest X-ray, in addition to the SARS-CoV-2 PCR test, as mentioned above. Additionally, according to the attending physician’s recommendation for individual patients, the coagulation panel (including D-dimer) and additional laboratory tests (such as procalcitonin, lactate dehydrogenase, vitamin D) were performed in some patients only. Moreover, based upon the actual clinical condition and previous results, some children variably received a chest computerized tomography, abdominal ultrasound, renal ultrasound, echocardiography, electrocardiogram, cranial sonography (in patients younger than 1 year). Whenever these children received this additional diagnostic work-up, it was performed within the first week after the hospital admission. The clinical monitoring was established based on individual patients’ condition. Temperature normalization, resolution of clinical symptoms, and 2 negative consecutive SARS-CoV-2 PCR tests were the adopted criteria to discharge these pediatric patients from the hospital. Data collection and descriptive analysis were carried out by Microsoft® Excel 2010 for Windows. Wherever appropriate and feasible, the statistical data analysis was performed: The differences in specific variables/parameters between two groups of patients were assessed for statistical significance by using the GraphPad Prism® software (version 4.0). In detail, laboratory parameters were expressed as mean ± SD error of the mean, because of the small and variable size of the study groups; accordingly, unpaired t-test (with Welch’s correction) was used to compare two groups: P value < 0.05 was considered statistically significant.
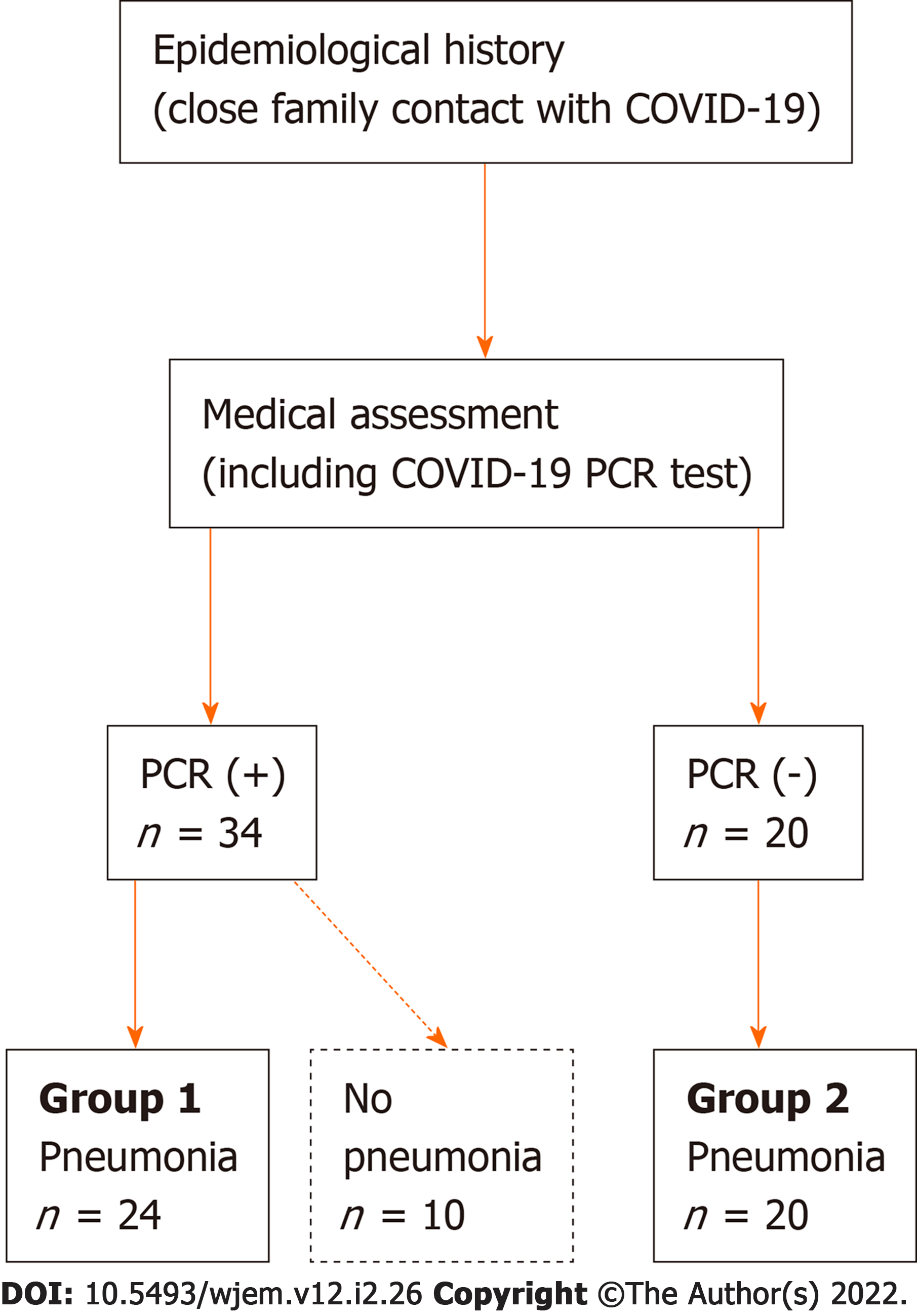
Fifty-four children (age range: 5 days to 17 years; mean age and SD: 56 ± 55 mo) were assessed because of a positive SARS-CoV-2 PCR test and/or clinical/radiological finding of pneumonia after a close contact with a family member diagnosed with COVID-19. As graphically summarized in Figure 1, based on the SARS-CoV-2 PCR test and the radiological findings, 24 COVID-19 children with pneumonia (group 1) and 20 COVID-19 negative children with pneumonia (group 2) were identified, in addition to 10 SARS-CoV-2 PCR positive children who did not show any radiological findings of pneumonia. The detailed clinical and demographic characteristics of these 44 pneumonia children enrolled in the study are shown in Table 1. Overall, among all those 34 SARS-CoV-2 PCR positive children, 4 patients were completely asymptomatic (11.8%), 6 children were affected with upper airway acute respiratory infection (17.6%), and 24 patients developed mild to moderate pneumonia (70.6%). Among these 24 patients diagnosed with pneumonia (who represent our study population), the lung disease was bilateral in 17 cases, segmental in 5 cases, and subsegmental in 2 patients. Among those 20 SARS-CoV-2 PCR negative children diagnosed with lung disease, 15 children developed bilateral pneumonia and 5 patients showed unilateral subsegmental (always right-sided) pneumonia. All these radiological aspects are also summarized in Table 1.
| Group 1 | Group 2 | |
| (PCR+ pneumonia) | (PCR- pneumonia) | |
| Patients | ||
| Number | 24 | 20 |
| Gender | ||
| Male | 16 (66. 7%) | 9 (45.0%) |
| Female | 8 (33. 3%) | 11(55.0%) |
| Age | ||
| 0-5 yr | 14 (58. 3%) | 11 (55.0%) |
| 5-10 yr | 4 (16. 7%) | 4 (20.0%) |
| > 10 years | 6 (25. 0%) | 5 (25.0%) |
| Clinical manifestations | ||
| Cough | 17 (70. 8%) | 15 (75.0%) |
| Fever | 17 (70. 8%) | 16 (80. 0%) |
| Dyspnea | 7 (29. 2%) | 7 (35. 0%) |
| Loss of appetite | 15 (62. 5%) | 13 (65.0%) |
| Fatigue | 15 (62. 5%) | 13 (65. 0%) |
| Weakness | 15 (62. 5%) | 13 (65. 0%) |
| Vomiting/nausea | 2 (8. 3%) | 3 (15. 0%) |
| Diarrhea | 1 (4. 2%) | 0 (0. 0%) |
| Flatulence | 1 (4. 2%) | 0 (0. 0%) |
| Rhinorrhea | 8 (33. 3%) | 9 (45. 0%) |
| Sweating | 0 (0. 0%) | 0 (0. 0%) |
| Chest pain | 0 (0. 0%) | 0 (0. 0%) |
| Dizziness | 1 (4. 2%) | 0 (0. 0%) |
| Joint pain | 1 (4. 2%) | 0 (0. 0%) |
| Seizures | 0 (0. 0%) | 0 (0. 0%) |
| Chest X ray findings | ||
| Bilateral pneumonia | 17 (70. 8%) | 15 (75. 0%) |
| Segmental pneumonia | 5 (20. 8%) | |
| Subsegmental pneumonia | 2 (8. 3%) | 5 (25.0%) |
| Comorbidity | ||
| CHD | 1 (4. 2%) | 0 (0. 0%) |
| PTI | 1 (4. 2%) | 0 (0. 0%) |
| AML | 1 (4. 2%) | 0 (0. 0%) |
| Partial epilepsy | 0 (0. 0%) | 0 (0. 0%) |
Overall, the main clinical manifestations at the admission were fever, cough (which was reported to be dry and not productive in most cases), loss of appetite, fatigue and weakness, nasal congestion and/or rhinorrhea, dyspnea, as summarized in Table 1. Gastrointestinal symptoms, such as vomiting/nausea, diarrhea, and flatulence, were unusual in our patients, and were mostly reported in children younger than 3 years. Only one 16-year patient complained of intense sweating, chest pain and dizziness, but he was affected with congenital heart disease (pulmonary artery stenosis). The differential descriptive analysis of all clinical manifestations according to the group designation is reported in Table 1. Therefore, the main chief complaints were fever and cough, overall. No statistically significant differences were noticed between these two groups in terms of frequency and type of clinical manifestations. Cough (overall, reported in around 72% of all pneumonia patients) was present in 70.8% and 75% patients of the COVID-19 positive and negative groups, respectively. Fever (that was detected in > 75% of the study participants, overall) was reported in 70.8% and 80% patients of COVID-19 positive and negative groups, respectively. As regards other concerning respiratory symptoms, dyspnea was detected in both groups without any statistical differences and, respectively, in 29.2% and 35% of COVID-19 positive and negative groups.
All the available laboratory results are summarized in Table 2. No statistically significant differences were found between the study groups for any laboratory parameters, except for CRP. In detail, there was a statistically significant difference between COVID-19 positive and negative patients, in terms of CRP values (group 1: 41.47 ± 11.23 mg/L, group 2: 15.10 ± 4.21 mg/L; P = 0.0361). However, no inter-group significant differences were detected as regards ESR. In terms of CBC, no significant differences were detected between these pneumonia groups in the main hematological parameters (hemoglobin, thrombocytes count and total white blood cells). However, in terms of differential cell blood count (as described in Table 2), both groups of children with pneumonia showed a relative lymphocyte reduction and, conversely, neutrophil increase. As already mentioned, no significant differences were found for all the other biochemical parameters; however, as explained, these data were not available for all study participants as regards many parameters, which may have affected the results of the statistical analysis, of course.
| Laboratory parameters | Group 1 | Group 2 |
| (PCR + pneumonia) | (PCR - pneumonia) | |
| n = 24 | n = 20 | |
| HGB (g/L) | 120 ± 3.97 | 119 ± 3.4 |
| MCV (fL) | 85.2 ± 2.6 | 83.9 ± 1.59 |
| PLT (109/L) | 280 ± 19.4 | 338 ± 18.6 |
| WBC (109/L) | 10.3 ± 0.85 | 9.5 ± 0.77 |
| Lymphocytes (%) | 28.3 ± 2.91 | 32.9 ± 3.4 |
| Lymphocytes (109/L) | 2.7 ± 0.31 | 3.1 ± 0.35 |
| Neutrophils (%) | 64.3 ± 3.35 | 60.8 ± 3.8 |
| Neutrophils (109/L) | 7.3 ± 0.75 | 6.3 ± 0.69 |
| Monocytes (%) | 5 ± 0.47 | 6.1 ± 0.64 |
| Monocytes (109/L) | 0.5 ± 0.06 | 0.5 ± 0.06 |
| ESR (mm/h) | 19.1 ± 2.36 | 18.4 ± 1.88 |
| CRP (mg/L) | 41.5 ± 11.2 | 15.1 ± 4.21 |
| Total bilirubin (µmol/L) | 7.2 ± 0.67 | 9.07 ± 0.94 |
| Total proteins (g/L) | 66.5 ± 1.85 | 62.3 ± 1.56 |
| Creatinine (µmol/L) | 43 ± 2.84 | 41.6 ± 4.32 |
| Urea ( mmol/L) | 3.24 ± 0.29 | 3.47 ± 0.41 |
| Ca (mmol/L) | 2.25 ± 0.04 | 2.24 ± 0.05 |
| K (mmol/L) | 4.53 ± 0.24 | 4.79 ± 0.21 |
| Na (mmol/L) | 137 ± 0.50 | 138 ± 0.71 |
| Cl1 (mmol/L) | 102 ± 1.22 | 104 ± 1.18 |
| Glucose1 (mmol/L) | 4.66 ± 0.18 | 5.54 ± 0.58 |
| ALT1 (U/L) | 24.6 ± 8.24 | 24.4 ± 4.78 |
| AST1 (U/L) | 29.6 ± 3.88 | 30.5 ± 5.42 |
| CK1 (U/L) | 70.2 ± 18.7 | 64 ± 14.3 |
| LDH1 (U/L) | 399 ± 120 | 323 ± 189 |
| PCT1 (ng/mL) | 0.5 ± 0.11 | 0.3 ± 0.09 |
| D dimer1 (μg/mL) | 1.4 ± 0.35 | 0.1 ± 0.02 |
| 25 OH vitD1 (ng/mL) | 27.3 ± 3.79 | 25.3 ± 2.67 |
Unfortunately, data on additional radiological investigations were available for a minority of patients, except for abdominal ultrasound, which was performed in 34 patients: It resulted abnormal with diffuse and reactive changes in the liver in only 4 COVID-19 patients (11.8%), who actually did not complain of any abdominal symptoms. No additional ultrasonographic alterations were reported. In detail, as regards the kidneys, no pathological changes were observed at all. Only 3 children (complaining of chest pain) underwent chest ultrasound: All showed signs of a small pleural effusion. In detail, among these patients, 2 were diagnosed with COVID-19 and one was SARS-CoV-2 negative.
Currently, a few articles on COVID-19 from Central Asia can be retrieved in the medical literature: As regards the first wave of pandemic, those are mainly epidemiological studies describing the outbreak situation until June 2020[12-15]. Our study is a single-center pediatric case series describing the clinical, laboratory and radiological characteristics of SARS-CoV-2 positive and negative Kazakhstani children with pneumonia, who were identified based on contact tracing in the household setting. The clinical manifestations of these COVID-19 children in our study were not qualitatively and quantitatively different from those emerging from previous and larger case series during the first phase of the pandemic[16-18]. Interestingly, > 60% of our patients were younger than 5 years; however, this distribution may be easily biased by the different parental awareness for infants and young children: Indeed, as explained, we assessed all consecutive pediatric contacts of COVID-19 adults, who were addressed for medical evaluation at the hospital. Respiratory symptoms were the most frequent clinical manifestations and were complicated with pneumonia in several patients. Among 54 pediatric contacts with family members affected with COVID-19, only 34 children resulted to be SARS-CoV-2 PCR positive, and 24 of them (70.6%) were concomitantly diagnosed with pneumonia. This diagnostic rate of pneumonia among COVID-19 children was quite high in our case series, compared to similar studies from different countries (see later), in which contact tracing strategy was the main method used for participants’ recruitment, like in the present study. For instance, Alsharrah et al[19] described a retrospective and monocentric case series including 134 pediatric COVID-19 patients who mostly (84%) acquired the infection from household contacts: 67.9% and 32.1% of these children were reported as asymptomatic or affected with mild symptoms or pneumonia, respectively. In detail, only 12 COVID-19 patients (around 9%) showed “abnormal chest X-ray findings”, which is clearly a much lower rate of COVID-19 pneu
In conclusion, in addition to a relatively high prevalence of pneumonia among Kazakhstani COVID-19 children diagnosed after contact tracing during the first wave of pandemic, we observed a significant difference in CRP values between SARS-CoV-2 positive and negative children affected with pneumonia, which may deserve further verification and investigations with larger clinical studies, due to the several limitations of this retrospective case series.
Even though coronavirus 2019 disease (COVID-19) clinical course in children is much milder than in adults, pneumonia can occur in the pediatric population as well.
To report a single-center pediatric case series of COVID-19 from Kazakhstan during the first wave of pandemic.
To analyze the main clinical and laboratory aspects in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive and negative children diagnosed with pneumonia.
Retrospective analysis of 54 children, who were medically assessed because they were close contacts of COVID-19 adults in their family setting, between June and September 2020. The clinical and laboratory characteristics of children affected with pneumonia in the presence (group 1) or absence (group 2) of SARS-CoV-2 infection, were compared.
No significant differences were found between the study groups for any clinical and laboratory parameters, except for C-reactive protein. Both pneumonia groups showed higher C-reactive protein values than COVID-19 children without pneumonia, overall; however, the COVID-19 pneumonia group 1 showed a significantly higher increase of C-reactive protein compared to group 2 (SARS-CoV-2 negative pneumonia).
In our case series of children assessed for SARS-CoV-2 infection based on contact tracing, the acute inflammatory response and, in detail, C-reactive protein increase resulted to be more pronounced in COVID-19 children with pneumonia than in children with SARS-CoV-2 negative pneumonia.
Larger, controlled and more complete clinical studies are needed to verify the different aspects of (acute) systemic inflammation in children with SARS-CoV-2 pneumonia.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Kazakhstan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, India; Bersot CD, Brazil S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17639] [Article Influence: 3527.8] [Reference Citation Analysis (0)] |
| 2. | She J, Liu L, Liu W. COVID-19 epidemic: Disease characteristics in children. J Med Virol. 2020;92:747-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 282] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 3. | Cucinotta D, Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2621] [Reference Citation Analysis (0)] |
| 4. | Mukusheva Z, Assylbekova M, Poddighe D. Management of pediatric rheumatic patients in Kazakhstan during the coronavirus disease 2019 (COVID-19) pandemic. Rheumatol Int. 2020;40:1351-1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Rabi FA, Al Zoubi MS, Al-Iede MM, Kasasbeh G, Badran EF. Coronaviruses in children: A review of potential mechanisms of childhood protection. Acta Paediatr. 2021;110:765-772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | American Academy of Pediatrics and Children's Hospital Association. Children and COVID-19: State Data Report. Available from: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%207.30.20%20FINAL.pdf. |
| 7. | Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2412] [Article Influence: 482.4] [Reference Citation Analysis (0)] |
| 8. | Tiruneh FT. Clinical Profile of Covid-19 in Children, Review of Existing Literatures. Pediatric Health Med Ther. 2020;11:385-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | de Souza TH, Nadal JA, Nogueira RJN, Pereira RM, Brandão MB. Clinical manifestations of children with COVID-19: A systematic review. Pediatr Pulmonol. 2020;55:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 10. | Zimmermann P, Curtis N. COVID-19 in Children, Pregnancy and Neonates: A Review of Epidemiologic and Clinical Features. Pediatr Infect Dis J. 2020;39:469-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 244] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 11. | Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020;55:2565-2575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 12. | Maukayeva S, Karimova S. Epidemiologic character of COVID-19 in Kazakhstan: A preliminary report. North Clin Istanb. 2020;7:210-213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Semenova Y, Glushkova N, Pivina L, Khismetova Z, Zhunussov Y, Sandybaev M, Ivankov A. Epidemiological Characteristics and Forecast of COVID-19 Outbreak in the Republic of Kazakhstan. J Korean Med Sci. 2020;35:e227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Kim K, Choi JW, Moon J, Akilov H, Tuychiev L, Rakhimov B, Min KS. Clinical Features of COVID-19 in Uzbekistan. J Korean Med Sci. 2020;35:e404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Bayesheva D, Boranbayeva R, Turdalina B, Fakhradiyev I, Saliev T, Tanabayeva S, Zhussupov B, Nurgozhin T. COVID-19 in the paediatric population of Kazakhstan. Paediatr Int Child Health. 2021;47:76-82. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1701] [Cited by in RCA: 1489] [Article Influence: 297.8] [Reference Citation Analysis (1)] |
| 17. | Hoang A, Chorath K, Moreira A, Evans M, Burmeister-Morton F, Burmeister F, Naqvi R, Petershack M. COVID-19 in 7780 pediatric patients: A systematic review. EClinicalMedicine. 2020;24:100433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 18. | Chang TH, Wu JL, Chang LY. Clinical characteristics and diagnostic challenges of pediatric COVID-19: A systematic review and meta-analysis. J Formos Med Assoc. 2020;119:982-989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 19. | Alsharrah D, Alhaddad F, Alyaseen M, Aljamaan S, Almutairi N, Ayed M, Papenburg J, Alghounaim M. Clinical characteristics of pediatric SARS-CoV-2 infection and coronavirus disease 2019 (COVID-19) in Kuwait. J Med Virol. 2021;93:3246-3250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Nunziata F, Bruzzese E, Poeta M, Pierri L, Catzola A, Ciccarelli GP, Vassallo E, Montella E, Lo Vecchio A, Guarino A. Health-care organization for the management and surveillance of SARS-CoV-2 infection in children during pandemic in Campania region, Italy. Ital J Pediatr. 2020;46:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Poddighe D. Common finding of mild hyponatremia in children evaluated at the Emergency Department and its correlation with plasma C-reactive protein values. Minerva Pediatr. 2016;68:173-176. [PubMed] |
| 22. | Zhao Y, Sun L, Bouchard HC, Zhang XX, Wan G, Hao YW, He SX, Jiang YY, Pang L. Coronavirus Disease 2019 versus Influenza A in Children: An Observational Control Study in China. Biomed Environ Sci. 2020;33:614-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Li Y, Wang H, Wang F, Du H, Liu X, Chen P, Wang Y, Lu X. Comparison of hospitalized patients with pneumonia caused by COVID-19 and influenza A in children under 5 years. Int J Infect Dis. 2020;98:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 24. | Poddighe D, Aljofan M. Clinical evidences on the antiviral properties of macrolide antibiotics in the COVID-19 era and beyond. Antivir Chem Chemother. 2020;28:2040206620961712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |