Published online Apr 27, 2020. doi: 10.5493/wjem.v10.i3.26
Peer-review started: December 9, 2019
First decision: December 26, 2019
Revised: February 4, 2020
Accepted: March 28, 2020
Article in press: March 28, 2020
Published online: April 27, 2020
Processing time: 140 Days and 22.9 Hours
With recent research advances, adipose-derived stromal/stem cells (ASCs) have been demonstrated to facilitate the survival of fat grafts and thus are increasingly used for reconstructive procedures following surgery for breast cancer. Unfortunately, in patients, following radiation and chemotherapy for breast cancer suggest that these cancer treatment therapies may limit stem cell cellular functions important for soft tissue wound healing. For clinical translation to patients that have undergone cancer treatment, it is necessary to understand the effects of these therapies on the ASC's ability to improve fat graft survival in clinical practice.
To investigate whether the impact on ASCs function capacity and recovery in cancer patients may be due to the chemotherapy.
ASCs were isolated from the cancerous side and noncancerous side of the breast from the same patients with receiving neoadjuvant chemotherapy (NAC) or not-receiving NAC. ASCs were in vitro treated with 5-fluorouracil (5-FU), doxorubicin (DXR), and cyclophosphamide (Cytoxan) at various concentrations. The stem cells yield, cell viability, and proliferation rates were measured by growth curves and MTT assays. Differentiation capacity for adipogenesis was determined by qPCR analysis of the specific gene markers and histological staining.
No significant differences were observed between the yield of ASCs in patients receiving NAC treatment and not-receiving NAC. ASCs yield from the cancerous side of the breast showed lower than the noncancerous side of the breast in both patients receiving NAC and not-receiving NAC. The proliferation rates of ASCs from patients didn’t differ much before and after NAC upon in vitro culture, and these cells appeared to retain the capacity to acquire adipocyte traits simile to the ASCs from patients not-receiving NAC. After cessation and washout of the drugs for another a week of culturing, ASCs showed a slow recovery of cell growth capacity in 5-FU-treated groups but was not observed in ASCs treated with DXR groups.
Neoadjuvant therapies do not affect the functioning capacity of ASCs. ASCs may hold great potential to serve as a cell source for fat grafting and reconstruction in patients undergoing chemotherapy.
Core tip: The functioning capacity and recovery potential of adipose-derived mesenchymal stromal/stem cells (ASCs) were demonstrated in terms of the stem cell yield, proliferation rates and adipogenic differentiation capabilities in breast cancer patients after exposure to neoadjuvant therapies (NAC) treatment. The yield of ASCs did not alter much after NAC treatment of patients. Moreover, the proliferation rates of ASCs derived from patients didn’t differ much before and after NAC upon in vitro culture, and these cells appeared to retain the differentiation capacity to acquire adipocyte traits simile to those ASC obtained from the patients not-receiving NAC.
- Citation: Hagaman AR, Zhang P, Koko KR, Nolan RS, Fromer MW, Gaughan J, Matthews M. Isolation and identification of adipose-derived stromal/stem cells from breast cancer patients after exposure neoadjuvant chemotherapy. World J Exp Med 2020; 10(3): 26-40
- URL: https://www.wjgnet.com/2220-315x/full/v10/i3/26.htm
- DOI: https://dx.doi.org/10.5493/wjem.v10.i3.26
Breast cancer is a leading cause of death in women with approximately 1.7 million new cases and over 522000 deaths per year[1]. However, the overall number of breast reconstructions has lately considerably increased, currently, there are about 93000 breast reconstructions performed in the United States per year[2,3]. Breast reconstruction including implant-and expander-based breast reconstruction, and nowadays, autologous fat grafting (non-vascularized autologous lipoaspirate fat) is being increasingly used for reconstructive procedures following surgery for breast cancer patients[4-6]. With recent research advances, cell-assisted lipotransfer studies concluded that it was more efficacious than autologous fat grafting alone, and adipose-derived mesenchymal stromal/stem cells (ASCs) have been demonstrated to facilitate the survival of fat grafts and thus increasingly used for reconstructive procedures following surgery for breast cancer[7,8]. ASCs, as an abundant source of adult mesenchymal stem cells, easily harvested, possess multiple characteristics that make them ideal for regenerative and complementary medicine applications[9,10]. Unfortunately, the problem of poor soft tissue wound healing in cancer patients post-exposure to radiation and chemotherapy suggests that these cancer treatment therapies may limit ASC cellular functions important for soft tissue wound healing[11-13]. Our previous in vitro human data demonstrates that direct exposure to chemotherapeutic agents (tamoxifen and paclitaxel) has a cytotoxic effect by decreasing ASCs proliferation rates and multi-potency differentiating abilities[13-15]. However, we have yet to examine the effects of chemotherapy on ASCs outcomes in patients. Importantly, for clinical translation to patients that have undergone radiation and/or chemotherapy, it is necessary to better understand the effects of these therapies on the ASC ability to improve fat graft survival in clinical practice. In addition, recent research has focused almost entirely on poor soft tissue wound healing in patients exposure to radiation/or chemotherapy, but little is known about the recovery of cell viability and function capability of stem cells in patients after chemotherapeutic-treatment[16]. Accordingly, this study aimed to determine the effects of chemotherapy on ASCs by examining human breast tissue specimens from cancer patients receiving neoadjuvant therapies (NAC) treatment and compared to the patients not-receiving NAC regarding their yield, proliferation capacity, and especially their potency to differentiate into a functional adipocyte-phenotype to clarify whether the impact on ASC function and recovery capacities may be due to the chemotherapy.
Autologous fat grafting (lipofilling) for breast reconstruction after surgery is performed frequently, investigations studying the functional capability and recovery of stem cells in tumor environment with chemotherapy treatment have been inadequate[17,18]. In the presents study, we involved a comparison study to analysis of the stem cells yield, proliferative and adipogenesis differentiation capacities of ASCs from the cancerous side of the breast and noncancerous side of the breast from the same patient who underwent scheduled bilateral mastectomies with reconstruction at the time of resection about 6-8 wk receiving neoadjuvant chemotherapy and compared with the patients who have not-receiving NAC.
Furthermore, nowadays, neoadjuvant therapy has become increasingly common for many patients, as this is associated with reduced recurrence rates and mortality[19]. According to the National Comprehensive Cancer Network, there are several combinations of neoadjuvant therapies including cytotoxic, endocrine, and biologic target agents for the treatment of breast cancer[20,21]. Herein, the second aim of this study was to investigate, in vitro, the cellular functions of ASCs after exposure to three commonly utilized clinical chemotherapeutic agents: 5-fluorouracil (5-FU), doxorubicin (DXR), and cyclophosphamide (Cytoxan) to determine whether the ASCs viability diminished in the presence of these agents commonly used neoadjuvant chemotherapies, or patterns of recovery after cessation of these chemotherapeutic drugs in treatment. The results of our study will provide important information and further understanding regarding the use of autologous ASCs in reconstructive procedures following chemotherapy such as in breast cancer patients.
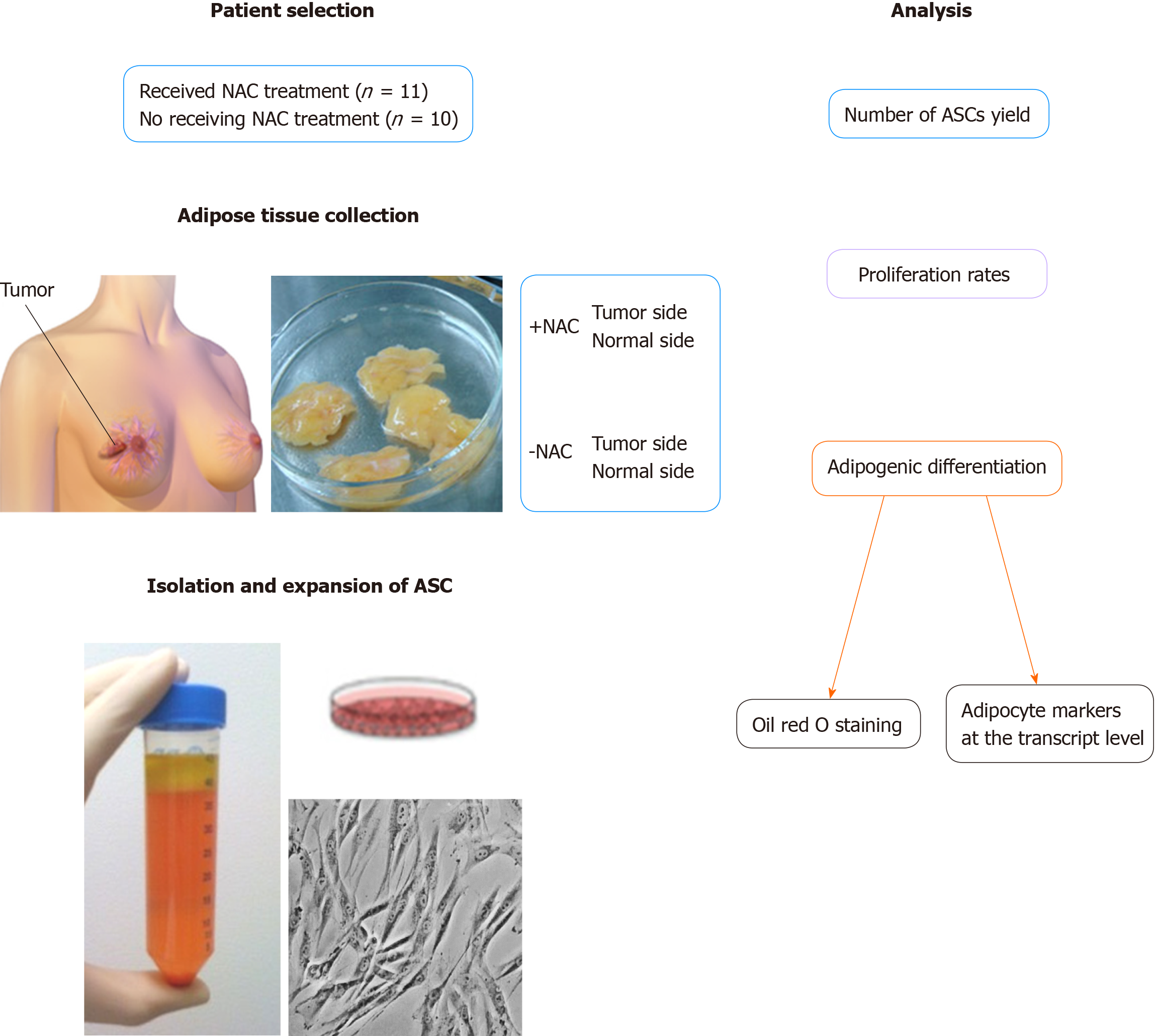
Breast cancer patients who underwent scheduled bilateral mastectomies with reconstruction included in this study (n = 21). All patients provided written informed consent following our institutional review board-approved protocol. Patients were included if they were 18 years and older, getting bilateral resection with reconstruction, and had a diagnosis of breast cancer on one side. Patients were excluded if they had bilateral premalignant and/or malignant lesions, had a unilateral prophylactic mastectomy, or if they were less than 18 years old. The demographic and clinical characteristics of all patients have summarized in Table 1. ASCs were isolated from the cancerous side and noncancerous side of the breast from the same patient who underwent scheduled bilateral mastectomies with reconstruction at the time of resection about 6-8 weeks receiving NAC treatment or not-receiving NAC regarding their yield, proliferation rates, and especially their potency to differentiate into a functional adipocyte-phenotype (Figure 1).
| Patient demographics | No neoadjuvant chemotherapy, n = 10 | Neoadjuvant chemotherapy, n = 11 | P value |
| Age, yr | 49 ± 6 | 49 ± 7 | 1.000 |
| Stage of cancer | |||
| 0 | 2 (9.5) | 0 (0) | 0.2143 |
| IA | 6 (28.5) | 2 (9.5) | 0.0805 |
| IIA | 1 (5) | 4 (19) | 0.3108 |
| IIB | 1 (5) | 2 (9.5) | 1.000 |
| IIIA | 0 (0) | 3 (14) | 0.2143 |
| Menopausal | 4 (19) | 5 (24) | 1.000 |
| Multiparous | 10 (48) | 9 (43) | 0.4762 |
| ER+ | 9 (43) | 8 (38) | 0.5865 |
| PR+ | 10 (48) | 5 (24) | 0.0124 |
| Her2+ | 3 (14) | 6 (28.5) | 0.3870 |
| Tobacco | 3 (14) | 1 (5) | 0.3108 |
Adipose tissue specimens were collected from the cancerous side of the breast and noncancerous side of the breast from the same patient receiving NAC treatment or not-receiving NAC. Adipose tissue was washed with phosphate-buffered saline (PBS) and the isolation of ASCs was performed as previously described[22]. Adipose tissue was incubated for 1 h at 37 °C with collagenase I solution (Worthington) with shaking. After centrifugation (1500 rpm/10 min), the stromal vascular fraction (SVF) was cultured in basal medium 199 (Mediatech; Manassas, VA) supplemented with 10% fetal bovine serum (Gemini Bio Products; West Sacramento, CA). After 24 h incubation, the media was aspirated to remove any unattached cells and washed with PBS; complete culture media was subsequently replaced twice weekly.
The primary isolated ASCs from patients receiving NAC or not-receiving NAC were submitted for proliferation and differentiation experimentation at passages 3–5. Proliferation rates were assessed by constructing growth curves during 11-d periods. ASCs were plated at 2.5 × 103 cells/cm2 and fed every other day. At various time points, cells were trypsin-released and counted with a Coulter counter (Beckman Coulter, Inc., Fullerton, CA, United States).
To evaluate the adipogenic differentiation capacity of ASCs, ASCs from patients receiving NAC or not-receiving NAC were cultured in AdipoDiff Medium (Gbico Life Technologies) for three weeks. Commitment toward an adipocyte lineage was measured by evaluating mRNA expression of the adipocyte markers of lipoprotein lipase (LPL) and peroxisome proliferator activated receptor gamma (PPAR-γ) by quantitative PCR, and histological Oil red O staining. The differentiated ASCs were incubated with cooled methanol for 5 min and stained with fresh Oil Red-O (Sigma-Aldrich) solution for 20 min then the photomicrographs were taken to identify lipid droplets. In addition, the adipogenic differentiation levels of ASCs were quantified by extracting the oil red O dye with 100% isopropanol and using optical density readings taken at a wavelength of 495 nm (100% isopropanol as a background).
Total RNA was extracted using TRIzol (Life technologies; Grand Island, NY, United States) reagent. To detect the specific genes expressed in adipogenic differentiated ASCs, RT-PCR was performed using the following primers: Peroxisome proliferator-activated receptor gamma (PPAR-γ) with 5’-primers (5’-GCTTTTGGCATACTCTG TGATCTC-3’) and 3’-primers (5’-TCAGGGCTGCCAGTTTCG-3’); and Lipoprotein lipase (LPL) with 5-primers (5’-GCTCGTGGGAGCACTTCACT-3’) and 3’-primers (5’-TCCGCGTGATTGCAGAGA-3’). mRNA levels were quantified using SYBR Green Real-Time PCR with the 7500 Fast Real-Time PCR system (Applied Biosystems). PCR reaction conditions were 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The housekeeping gene GAPDH (5’-primers: 5’-GGATCTCGCTCC TGGAAGATG-3’) and 3’-primers: 5’-GCACCGTCAAGGCTGAGAAC-3’) were amplified in separate tubes to normalize for variance in input RNA. For relative quantification, the efficiency of amplification for each primer pair was determined using the cDNA target and the 2-ΔΔct method.
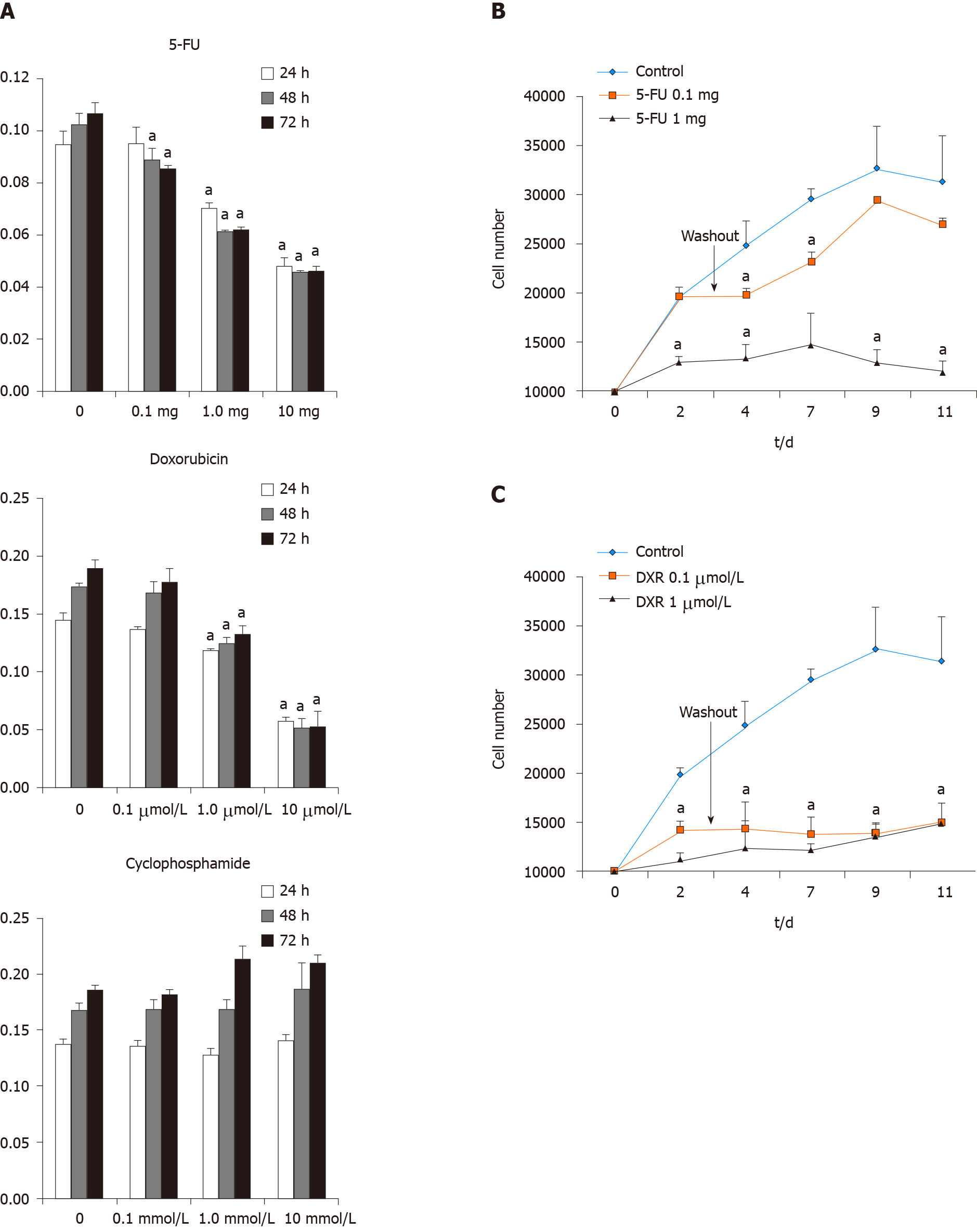
For evaluation of the cellular function and recovery potential of ASCs after exposure to chemotherapeutic agents, ASCs isolated from female patients during reconstructive procedures were treated with 5-FU, DXR, and Cytoxan to determine whether the ASC viability diminished in the presence of these three commonly utilized clinical chemotherapeutic agents. 5-FU, DXR, and Cytoxan (Sigma-Aldrich) was dissolved to make a stock concentration solution, aliquoted and kept in -20 °C refrigerator for freshly using each time. ASCs were plated at a density of 1 × 104 cells per well in a volume of 1ml in 24 well plates and then treated with 5-FU (0.1, 1, and 10 mg/mL), DXR (0.1, 1, and 10 µmol/L), and Cytoxan (0.1, 1, and 10 mmol/L) at the various concentrations. At 24, 48, and 72 h, MTT assay (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) was used to evaluate the effect of 5-FU, DXR, and Cytoxan on ASCs cell viabilities. At various time points, the culture medium was replaced with a medium containing MTT solution (5 mg/mL) and incubate at 37 °C with 5% CO2 for 3 h. The wells were then decanted and the purple formazan crystals formed were dissolved in 200 µL dimethyl sulfoxide solution (Sigma-Aldrich). The absorbance of the plate was read on a microplate reader at 570 nm. Fresh cells were used as controls. All assays were done in triplicate.
To evaluate the potential of cellular function recovery of ASCs after treated with the chemotherapeutic agents, we extended the cell culture period. ASCs were plated into 24 well plates at 5 × 103 cells /cm2 and exposure to 5-FU (0.1 or 1 mg/mL) or DXR (0.1 or 1 µmol) for 72 h then the drugs have removed. The cells were cultured in medium without the drug for an additional 8 d, and the proliferation rates were assessed by constructing growth curves for 11 d. At different time points, cells were trypsin released and counted with a coulter counter (Beckman Coulter, Inc, Fullerton, Calif).
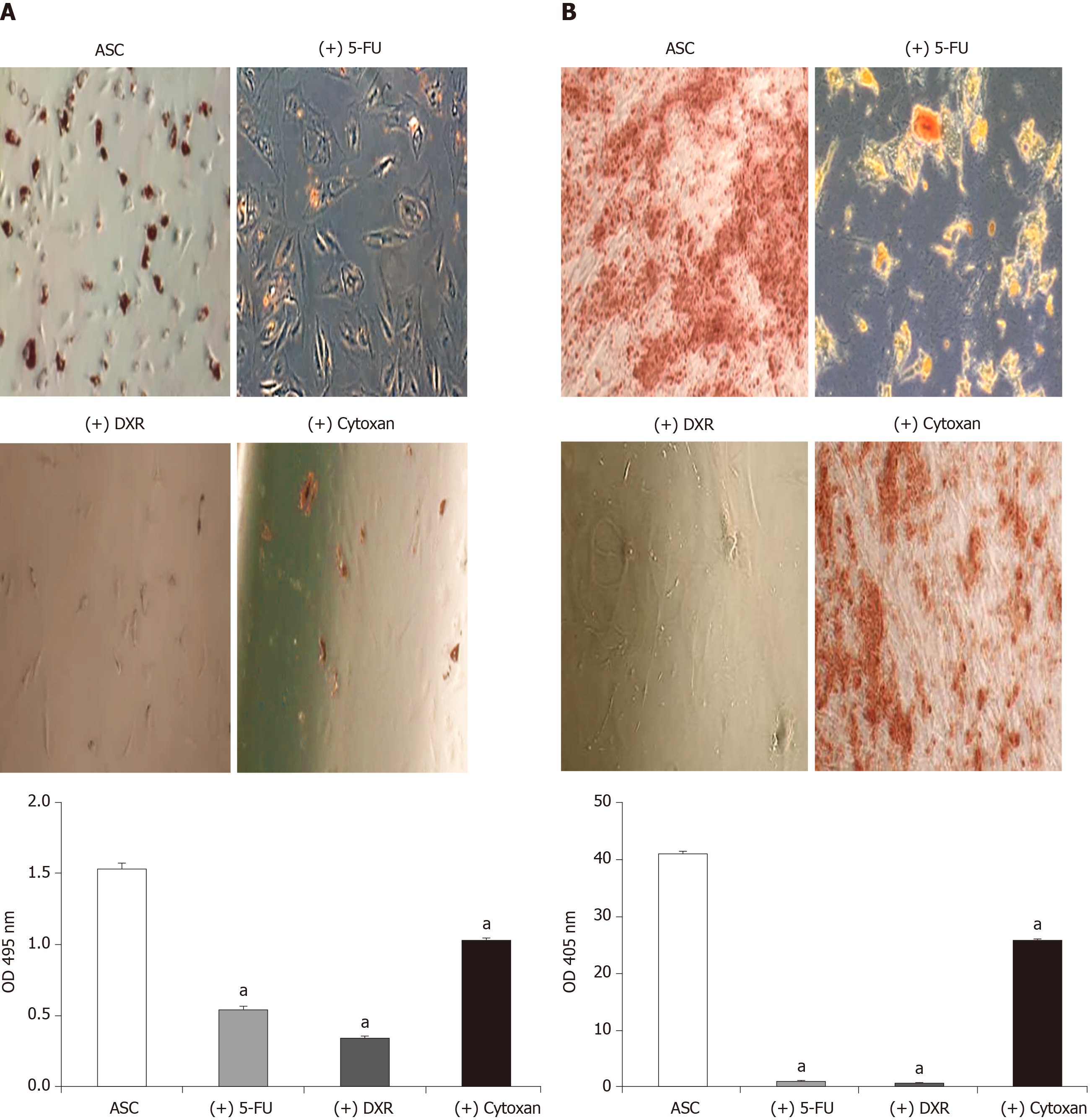
To evaluate the multipotency differentiation with these chemotherapeutic agents, ASCs were cultured in AdipoDiff Medium with or without 5-FU (1 mg), DXR (1 µmol/L) and Cytoxan (1 mmol) for three weeks. For osteogenic differentiation, ASC cells were cultured in OsteoDiff Medium (Gibco LifeTechnologies) with or without above chemo-drugs for 3 wk; the cells were then stained with Alizarin Red S solution for 20 min to identify calcium deposits.
Cell counts were analyzed as continuous variables and are presented as means with standard deviations. Categorical variables are presented as frequencies with percent. Group differences of cell counts were analyzed using analysis of variance for repeated measures with follow-up pair-wise comparisons. The count data were tested for normality using the Shapiro Wilk test. In order to use parametric methods on non-normal data, the counts were transformed to normalized ranks prior to analysis. A P value of 0.05 was regarded as being statistically significant. All data were analyzed using SAS v9.4 (SAS Institute, Cary, NC, United States).
Human breast adipose tissue specimens were collected from a breast cancer patient’s cancerous side of the breast and noncancerous side of the breast in same patients who underwent scheduled bilateral mastectomies with reconstruction at the time of resection about 6-8 wk receiving NAC treatment or not-receiving NAC (age: 31-59 years, n = 21). Table 1 describes the characteristics of the experimental population. The stage of cancer including zero, IA, IIA, IIB, and IIIA patients, and as known with 43% ER and 48% PR positive.
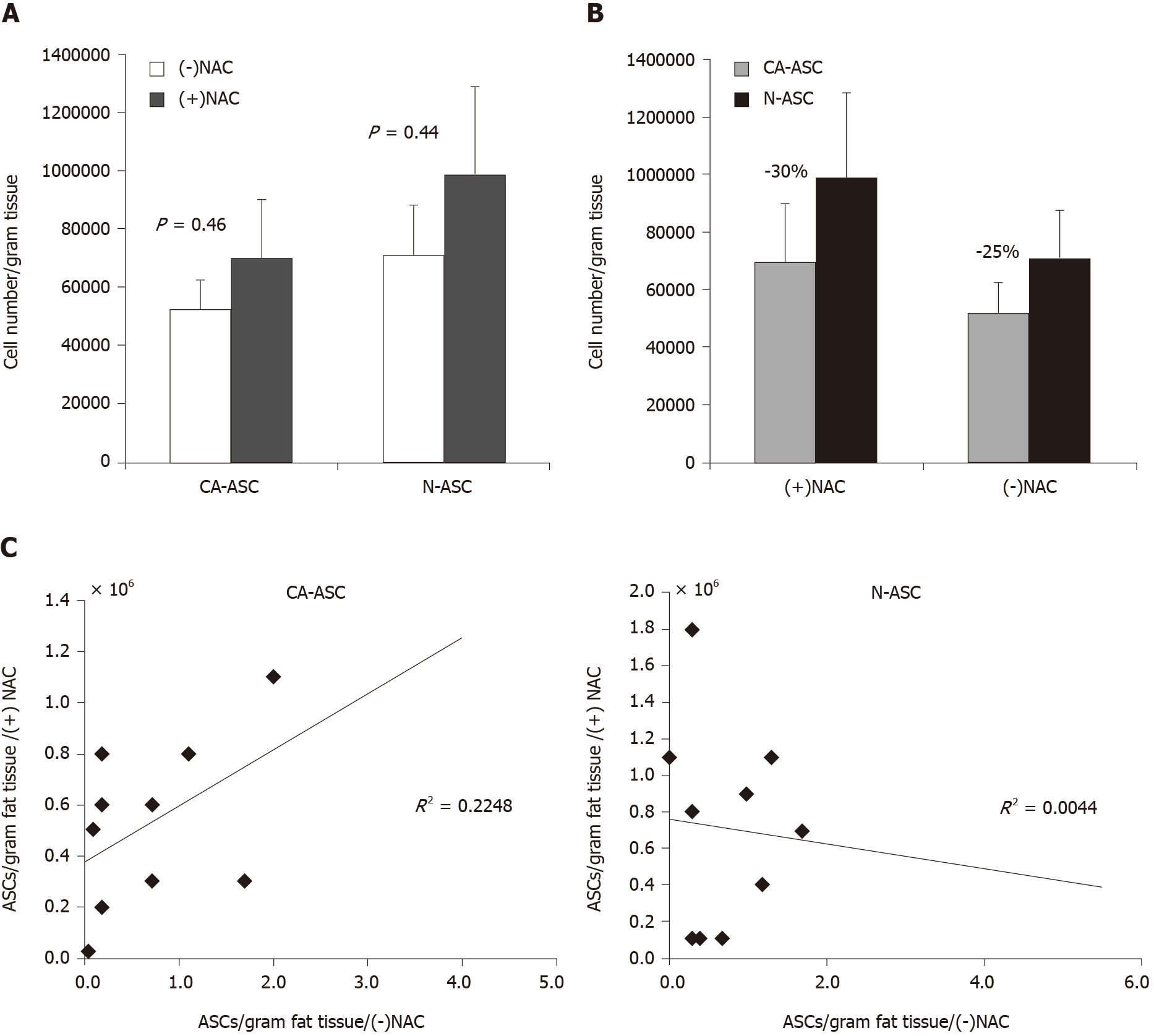
To determine the influence of NAC treatment on stem cell yield, ASCs were isolated from breast cancer patients with receiving NAC treatment or not-receiving NAC. In addition, we have compared the numbers of ASCs yield from the cancerous side of the breast and noncancerous side of the breast from the same patient. As shown in Figure 2A, we observed that the number of ASCs yield was not shown reduced in patients receiving NAC treatment when compared to patients not-receiving NAC from either the cancerous side of the breast (CA-ASC, 0.69 ± 0.20 million/g vs 0.52 ± 0.10 million/g, P = 0.46) or noncancerous side of the breast (N-ASC, 0.99 ± 0.29 m million/g vs 0.71 ± 0.17 million/g, P = 0.44). However, compared to the noncancerous side of the breast, we found that the numbers of ASCs yield from the cancerous side of breast were lower than the noncancerous side of breast in both patients receiving NAC treatment (30% decreased, P = 0.43) and not-receiving NAC (25% decreased, P = 0.36) (Figure 2B). Furthermore, univariate analysis of the ASCs harvest from the cancerous side of the breast or normal side of the breast adipose failed to reveal any significant correlation between patients receiving NAC and not-receiving NAC (Figure 2C).
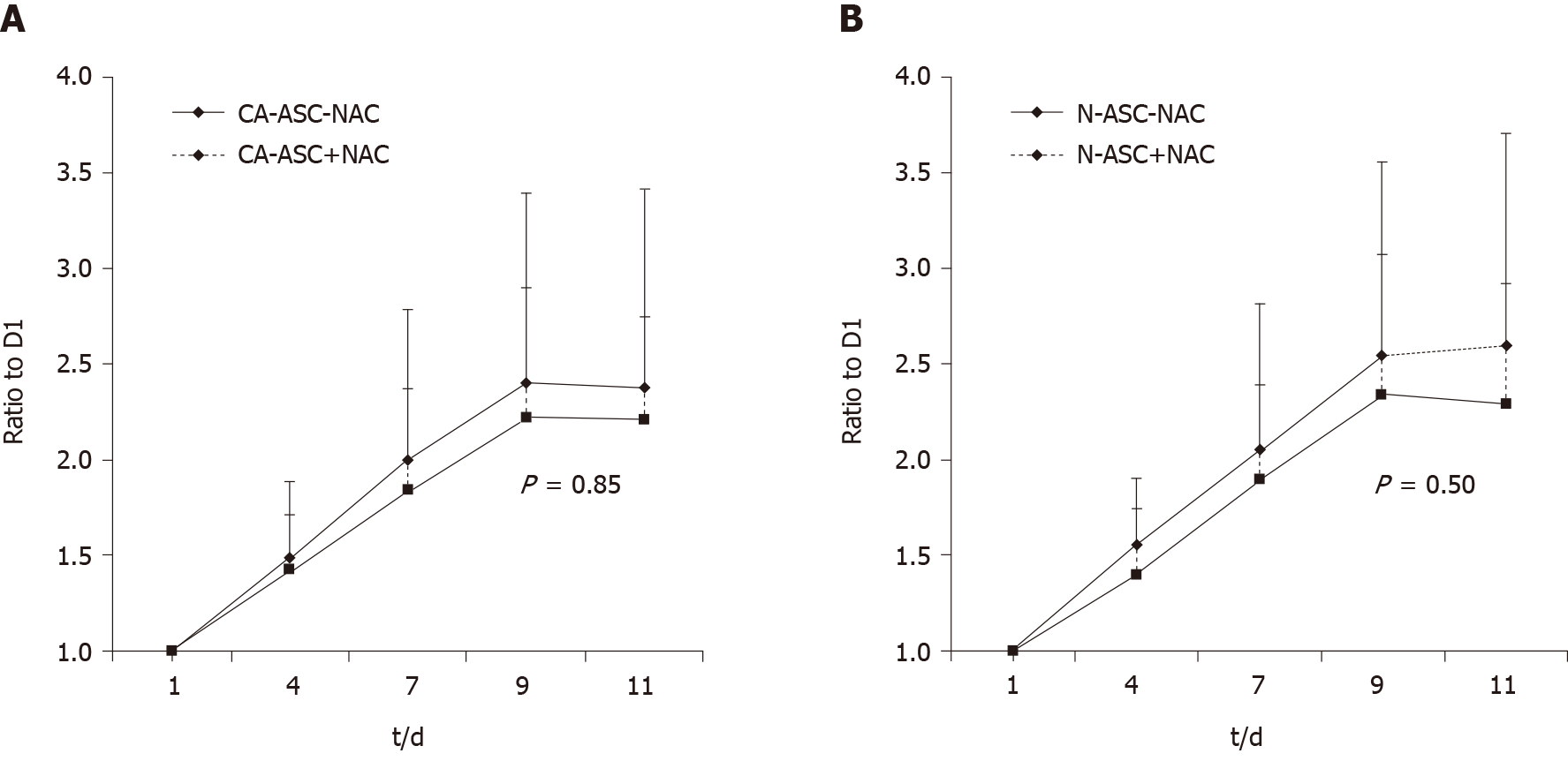
We then analysis of the proliferation rates of ASCs from the patients receiving NAC or not-receiving NAC. Compared to the ASCs from patients not-receiving NAC, a trend toward lower proliferation rates was observed in the ASCs that from patients receiving NAC treatment in both that from the cancerous side of the breast (Figure 3A) and noncancerous side of the breast (Figure 3B) as measured by growth curves during 11 d culturing. However, this difference was not shown statistically significantly.
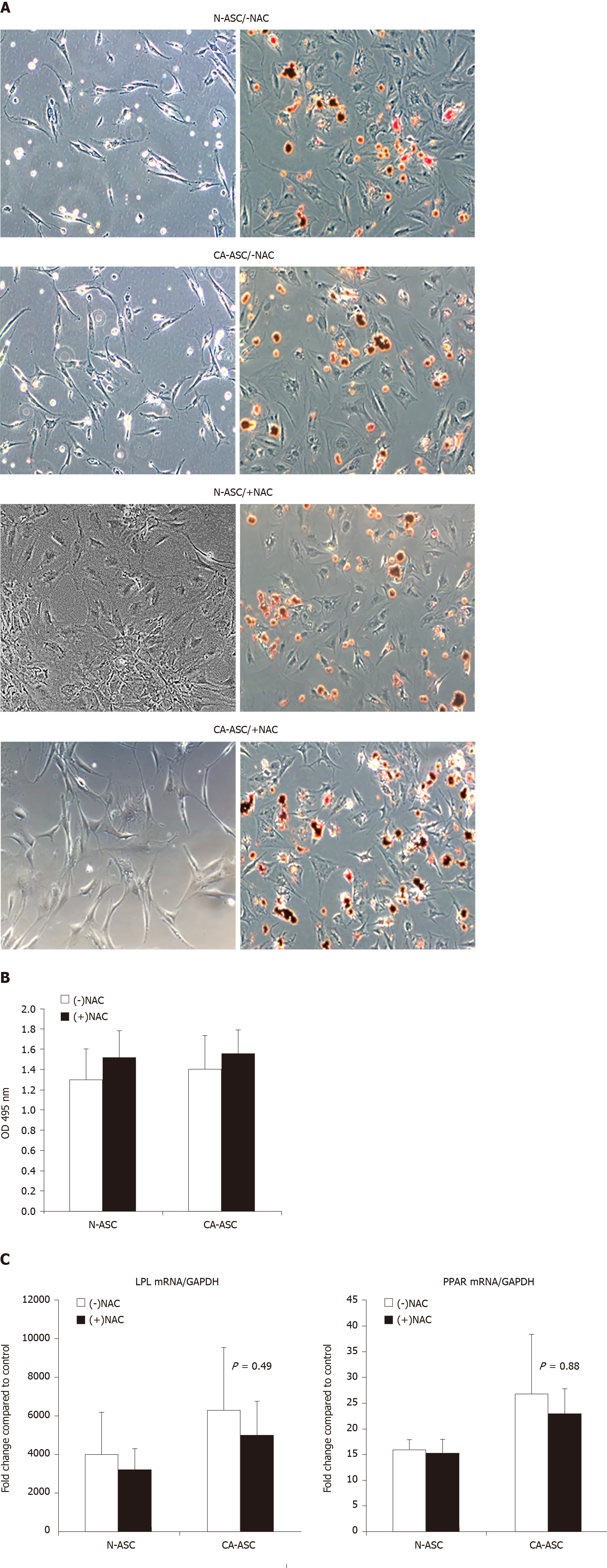
We further analyzed whether in patients receiving NAC treatment affects ASCs adipogenic differentiation capability. ASCs from patients receiving NAC or not-receiving NAC from the patient’s cancerous side of breast or noncancerous side of the breast were cultured in adipogenic differentiation medium for three weeks. After three weeks of culture, the ASC has differentiated into the adipocyte phenotype. Oil Red O stain demonstrated that the lipid droplets in the differentiated ASCs that from the patients receiving NAC treatment were similar to the ASCs from patients not-receiving NAC. After extract the Oil red O dye for quantifying the positive adipogenic cells, there was not displayed a significant difference between the ASCs from patients receiving NAC vs ASCs from patients not-receiving NAC (Figure 4B). In addition, we have determined the differentiated ASCs expressing the adipocyte markers at the transcript levels. After three weeks of differentiation, qPCR results reveal that ASCs newly expressed the messenger RNA for the adipocyte-specific genes of PPAR-γ and LPL. Compared to the ASCs that from patients not-receiving NAC, we found that there was a trend toward decreased mRNA expression of PPAR-γ and LPL in ASCs that from patients receiving NAC treatment (Figure 4C). However, this difference was also not shown statistically significantly.
To investigate the effects of chemotherapeutic agents on cell viability of ASCs, the cell growth arrest rates were determined at various doses of 5-FU (0.1, 1, and 10 mg/mL), DXR (0.1, 1, and 10 µmol/L), and Cytoxan (0.1, 1, and 10 mmol/L) using MTT assays. In the control group, the cell number increased over the 3-d time course, whereas for ASCs treated with 5-FU and DXR demonstrated decreased growth rates in a dose-dependent and time-dependent manner. However, in ASCs treated with cyclophosphamide groups, the cell viability and growth rates were not shown inhibited when compare to without drug treatment the control groups (Figure 5A). After the withdrawal of the drugs for another 8 d of culturing, in the lower doses of 5-FU treated groups, ASCs showed recovery of the cell's growth capacity which compared to the higher doses of 5-FU treated groups. However, full recovery was not achieved as the numbers of ASCs still significantly lower than the control group (Figure 5B). The recovery of cell viability was not observed in ASCs treated with DXR groups at either low- or high- dose followed by cessation of the drugs for an additional 8 d culturing (approximately 50% decreased vs non-treatment control group, P < 0.05; Figure 5C).
The adipogenic and osteogenic differentiation capacities of ASCs were decreased in the 5-FU, DXR and Cytoxan treated group when compared with the non-treated control group evidenced by oil red O (Figure 6A) and alizarin red staining (Figure 6B). After extracting the dye from oil red O for positive adipogenic cells and alizarin red for positive osteogenic cells, ASCs displayed the impact of adipogenic (2.1 fold decreased in 5-FU treated group, 2.3 fold in DXR treated group, and 1.3 fold in Cytoxan treated group, P < 0.05; Figure 6A) and osteogenic differentiation potential (20 fold decreased in 5-FU treated group, 20 fold in DXR treated group, and 1.5 fold decreased in Cytoxan treated group, P < 0.05; Figure 6B).
Mesenchymal stem/stromal cells are present in many human tissues and organs, in patients post-exposure to cancer treatment therapies may limit their cellular functions important to improve fat graft survival and soft tissue wound healing. In this study, we investigated whether the ASCs have the potential for recovery of the cellular function after exposure to the NAC treatment in breast cancer patients. The major findings of this study include that the yield of ASCs didn’t alter much after NAC treatment of patients. Moreover, the proliferation rates of ASCs derived from patients didn’t differ much before and after NAC upon in vitro culture, and these cells appeared to retain the differentiation capacity to acquire adipocyte traits similar to those ASCs obtained from patients not-receiving NAC. Further, we compared the ability of ASCs derived from the patient’s cancerous side of the breast and noncancerous side of the breast in patients receiving NAC or not-receiving NAC. Our study results suggest that ASCs may hold great potential and represents to serve as a cell source for clinical practice in fat grafting and reconstruction in breast cancer patients undergoing chemotherapy treatment.
Over the years, stem cell-based therapies have been tested and used in many clinical settings such as outcomes of breast cancer and breast reconstruction[7,23]. In the use of autologous stem cell-based target therapy, an additional challenge is making the cell source readily available for implantation. This study specifically evaluates the availability of ASCs from breast tissue in cancer patients with received NAC treatment. After isolation, we found that there no significant differences were observed between the numbers of ASCs yield in patients receiving NAC treatment with not-receiving NAC and in either from the cancerous side of the breast or noncancerous side of the breast. Of note, we acknowledge that we did not collect the control data that from before starting the treatment in the same patient, we cannot definitively comment upon isolation differences that may exist with this population; nonetheless, the current study demonstrates that ASCs can be isolated in great abundance from the breast adipose tissue in cancer patients after post chemotherapy. In addition, it is of great interest to know we recently described that the ASC can be isolated from the breast smaller fat specimens (approximately 5 g), and expansion to commitment toward an adipocyte lineage upon in vitro cultured in derivative-specific differentiation medium. This finding is important; the use of harvested autologous stem cells back to the treating surgeon is likely to be necessary for the maintenance of cell function before implantation. Another important point, the ASCs yield and proliferation rates of ASCs from the cancerous side of the breast was showed lower than the ASCs that from the noncancerous side of the breast in both patients receiving NAC and not-receiving NAC. However, the ability of ASCs to differentiate toward an adipocyte lineage from the cancerous side of the breast was showed similar to those ASCs that obtained from the noncancerous side of the breast suggested that the chemotherapy does not significantly alter the ability of adipocyte characteristics acquired by the stem cells in the tumor environment. To our knowledge, this is the first direct demonstration showing that ASCs from the cancerous side of the breast were capable of surviving at their differentiation potential in patients receiving the cancer treatment. Nevertheless, evaluation of the effects of chemotherapy in breast cellular interactions between the mesenchymal stem cells/or cancer stem cells and tumor cells will require in our future study[24,25].
In addition to confirming the availability of these stem cells in a cancer population, this study provides evidence that ASCs from cancer patients after exposure to chemo-treatment can retain their proliferation and differentiation capacity to acquire several important adipocyte characteristics. The results are consistent with other in vitro studies showing that human mesenchymal stromal cells were able to maintain their multi-differentiation potential after treatment with various chemotherapeutic agents[26-28]. This may also become important; several studies have shown that grafted fat has an increased survival rate when transplanted with the adipose-derived stem cells[10,29-31].
Previous work from our lab has shown that in vitro direct exposure chemo- therapeutic agents of paclitaxel and tamoxifen modulated human ASC cellular functions[13-15]. In the present study, we evaluated the recovery of ASC cellular functions after exposure to another three clinically useful chemotherapeutic agents of 5-FU, DXR and cyclophosphamide by using in vitro culture system. The predominant neoadjuvant chemotherapeutics for breast cancer are Taxol’s, doxorubicin, cyclophosphamide, and 5-fluoruracil, and there remains limited research describing how these drugs affects stem cells function[32]. In our study, we found that after cessation and washout of the drugs for a week, ASCs showed recovery of the cell growth capacity in lower doses of 5-FU treated groups which compared to the higher doses of 5-FU treated groups. However, the recovery of cell viability was not seen in ASCs treated with higher doses of 5-FU groups and doxorubicin treated groups, after the withdrawal of the drugs respectively. As this time-lapse is considerably longer than the 8 d withdrawal period examined in our in vitro setting, the ASCs likely recovered from the cytotoxic effects of their therapy with additional time. Further studies are needed to investigate the timeframe of ASC functional recovery after treated with chemotherapeutic drugs. Additionally, in our study, we have observed that cyclophosphamide did not inhibit ASCs growth. This correlates with previous studies and provides that not all chemotherapeutic agents affect the viability of stem cells[33]. Moreover, a study by Beane et al[33] demonstrated that the growth of ASCs was inhibited by clinically relevant concentrations of vincristine, cytarabine, and etoposide, but these cells contrarily appeared resistant to high-dose methotrexate treatment.
There are only a few in vitro studies showing human mesenchymal stem cell (bone marrow or ASC) characteristics in the presence of chemotherapeutic drugs and radiation[7,13-15,23]. Unfortunately, no human studies are evaluating the recovery of ASC differentiation capabilities in patients receiving neoadjuvant chemotherapy. Therefore, to the best of our knowledge, our results are the first time in which a human study directly demonstrates that ASCs have the potential to recover differentiation capacity in patients after receiving chemotherapy. We would note that by observing the in vitro culture system direct exposure chemotherapeutic agents to human ASCs and in patients with receiving the chemotherapy treatment, our results indicated that despite the in vitro evidence of the negative effects of chemotherapeutic agents on the ASCs, it might not be clinically relevant as ex vivo examination of patient’s ASC shows these cells can functionally recover after various neoadjuvant chemotherapy regimens. Our data may well provide new information to better understand the use of autologous ASCs for fat grafting and reconstruction in breast cancer patients undergoing treatment.
These are several limitations to this study, including the relatively small numbers of patients and the comparison of ASCs before posting the chemotherapy and after cessation of NAC treatment. Therefore, determine the temporal effects of chemotherapy before, during chemo-treatment and after cessation to evaluate the ASCs function recovery potential in patients will be required in our future studies. In addition, at this time, the limited tissue samples that could be obtained from the patients limited the number of studies we could perform. We were only able to look at the adipogenic differentiation of ASCs in the ex vivo study and unable to look at the osteogenic or endothelial differentiation and their stem cell characteristics. Furthermore, this was a pilot study consisting of only 21 patients; a larger randomized study of ex vivo samples is needed to confirm our findings that neoadjuvant chemotherapy does not affect the ASCs functions. We did not take into account the different variations of neoadjuvant chemotherapy regimens the patients received due to the small sample size. However, all of the patients who did receive neoadjuvant chemotherapy did receive Taxol and completed therapy about 4-6 wk prior to the isolation of ASCs.
In conclusion, this study provides new and novel insight information regarding the use of autologous mesenchymal stem cells derived from adipose tissue in cancer patients after exposure to chemotherapy, namely, (1) the availability of these cells for use does not appear to be adversely affected by post-chemotherapy (in fact, the yield of ASCs didn’t alter much after NAC treatment of patients); (2) the proliferation rates of ASCs derived from patients didn’t differ much before and after NAC upon in vitro culture; and (3) these cells can appear to retain the differentiation capacity to acquire adipocyte traits simile to those ASCs that obtained from the patients not-receiving NAC. Further, we evaluated the cellular function recovery of ASCs after exposure to three clinically useful chemotherapeutic agents. The study of our results indicated that despite the in vitro evidence of the negative effects of chemotherapeutic agents on the ASCs, it might not be clinically relevant as ex vivo examination shows these cells from patients can functionally recover after various neoadjuvant chemotherapy regimens. Overall, the present study is the first report that the breast adipose tissue appears to will be a viable source of autologous stem cells and ASCs may hold great potential and represents to serve as a cell source for use in fat grafting and reconstruction in cancer patients undergoing chemotherapy.
In cancer patients, post-exposure to radiation and chemotherapy suggests that these cancer treatment therapies may limit stem cells cellular functions important for soft tissue wound healing. For clinical translation to patients that have undergone cancer treatment, it is necessary to understand the effects of these therapies on the adipose-derived stromal/stem cell (ASC)'s ability to improve fat graft survival in clinical practice. Herein, we examined the effects of chemotherapy on ASCs outcomes in patients with receiving neoadjuvant therapies and compared to the patients not-receiving neoadjuvant chemotherapy (NAC) treatment.
Recent research has focused almost entirely on poor soft tissue wound healing in patients exposure to radiation/or chemotherapy, but little is known about the recovery of cell viability and function capability of stem cells in patients receiving chemotherapeutic-treatment. This research focused on characteristics of stem cells isolated from human adipose tissue: (1) The cellular function of ASCs diminished in direct exposure to chemotherapeutic agents, and potency to recovery after cessation of these drugs in treatment; and (2) Availability in receiving NAC treatment of population most likely the ASCs may hold great potential to serve as a cell source for fat grafting and reconstruction in cancer patients undergoing chemo-treatment. This research will provide new and novel insight information regarding the use of autologous mesenchymal stem cells derived from adipose tissue in cancer patients after exposure to chemotherapy.
The main objective of this study was to investigate whether the impact on ASCs function capacity and recovery in cancer patients may be due to the chemotherapy. In addition, we evaluated in vitro, whether ASCs have the potential for recovery of cellular function after exposure to three commonly utilized clinical chemotherapeutic agents.
We analyzed the stem cells yield, proliferation rates, and adipogenesis differentiation capacity of ASCs from breast cancer patients with receiving NAC treatment or not-receiving NAC. We also measured the recovery of the cellular functions of ASCs after treated with three chemotherapeutic agents by in vitro culture system.
We reveal that the yield of ASCs didn’t alter much after NAC treatment of patients. The proliferation rates of ASCs derived from patients didn’t differ much before and after NAC upon in vitro culture, and these cells appeared to retain the capacity to acquire adipocyte traits simile to the ASCs that from patients not-receiving NAC. By observing the in vitro culture system, the study indicates that the full recovery of cell proliferation rates was not observed in ASCs after the withdrawal of drug treatment in a short time interval.
We conclude that (1) the availability of ASCs for use does not appear to be adversely affected by post-chemotherapy; (2) despite the in vitro evidence of the negative effects of chemotherapeutic agents on the ASCs, it might not be clinically relevant as ex vivo examination of patient’s ASC shows these cells can functionally recover after various neoadjuvant chemotherapy regimens; and (3) the breast adipose tissue appears to will be a viable source of autologous stem cells and ASCs may hold great potential and represents to serve as a cell source for use in fat grafting and reconstruction in patients undergoing chemotherapy such as in breast cancer patients.
The study of our results provides novel insight into the use of autologous stem cell-based target therapy in reconstructive procedures in cancer patients that have received chemotherapy. Further study is needed to determine the temporal effects of chemotherapy before, during chemo-treatment and after cessation to evaluate ASC function and recovery potential in patients to clarify whether the impact on ASC function and recovery components may be due to the chemotherapy.
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kode JA, Leung PC S-Editor: Gong ZM L-Editor: A E-Editor: Li X
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20504] [Article Influence: 2050.4] [Reference Citation Analysis (20)] |
| 2. | Malhaire C, Hequet D, Falcou MC, Feron JG, Tardivon A, Leduey A, Guillot E, Mosseri V, Rouzier R, Couturaud B, Reyal F. Outcome of oncoplastic breast-conserving surgery following bracketing wire localization for large breast cancer. Breast. 2015;24:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Surgeons ASoP. Report of the 2010 plastic surgery statistics: ASPS national clearinghouse of plastic surgery procedural statistics. Available from: www.plasticsurgery.org. |
| 4. | Schmauss D, Machens HG, Harder Y. Breast Reconstruction after Mastectomy. Front Surg. 2015;2:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118:108S-120S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 832] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 6. | Pearl RA, Leedham SJ, Pacifico MD. The safety of autologous fat transfer in breast cancer: lessons from stem cell biology. J Plast Reconstr Aesthet Surg. 2012;65:283-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Toyserkani NM, Quaade ML, Sørensen JA. Cell-Assisted Lipotransfer: A Systematic Review of Its Efficacy. Aesthetic Plast Surg. 2016;40:309-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 8. | Fu S, Luan J, Xin M, Wang Q, Xiao R, Gao Y. Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg. 2013;132:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 502] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 10. | Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 11. | Mitchem J, Herrmann D, Margenthaler JA, Aft RL. Impact of neoadjuvant chemotherapy on rate of tissue expander/implant loss and progression to successful breast reconstruction following mastectomy. Am J Surg. 2008;196:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Zweegman S, Kessler FL, Kerkhoven RM, Heimerikx M, Celie JW, Janssen JJ, Huijgens PC, Drager AM, van den Born J. Reduced supportive capacity of bone marrow stroma upon chemotherapy is mediated via changes in glycosaminoglycan profile. Matrix Biol. 2007;26:561-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Pike S, Zhang P, Wei Z, Wu N, Klinger A, Chang S, Jones R, Carpenter J, Brown SA, DiMuzio P, Tulenko T, Liu Y. In vitro effects of tamoxifen on adipose-derived stem cells. Wound Repair Regen. 2015;23:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 14. | Choron RL, Chang S, Khan S, Villalobos MA, Zhang P, Carpenter JP, Tulenko TN, Liu Y. Paclitaxel impairs adipose stem cell proliferation and differentiation. J Surg Res. 2015;196:404-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Harris WM, Zhang P, Plastini M, Ortiz T, Kappy N, Benites J, Alexeev E, Chang S, Brockunier R, Carpenter JP, Brown SA. Evaluation of function and recovery of adipose-derived stem cells after exposure to paclitaxel. Cytotherapy. 2017;19:211-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, Paolucci P, Conte P, Horwitz EM. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Kronowitz SJ, Mandujano CC, Liu J, Kuerer HM, Smith B, Garvey P, Jagsi R, Hsu L, Hanson S, Valero V. Lipofilling of the Breast Does Not Increase the Risk of Recurrence of Breast Cancer: A Matched Controlled Study. Plast Reconstr Surg. 2016;137:385-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Charvet HJ, Orbay H, Wong MS, Sahar DE. The Oncologic Safety of Breast Fat Grafting and Contradictions Between Basic Science and Clinical Studies: A Systematic Review of the Recent Literature. Ann Plast Surg. 2015;75:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Oh E, Chim H, Soltanian HT. The effects of neoadjuvant and adjuvant chemotherapy on the surgical outcomes of breast reconstruction. J Plast Reconstr Aesthet Surg. 2012;65:e267-e280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Holmes D, Colfry A, Czerniecki B, Dickson-Witmer D, Francisco Espinel C, Feldman E, Gallagher K, Greenup R, Herrmann V, Kuerer H, Malik M, Manahan E, O'Neill J, Patel M, Sebastian M, Wheeler A, Kass R. Performance and Practice Guideline for the Use of Neoadjuvant Systemic Therapy in the Management of Breast Cancer. Ann Surg Oncol. 2015;22:3184-3190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Breast Cancer Version 2. Plymouth Meeting, PA: National Comprehensive Cancer Network, 2016. Available from: www.NCCN.org. |
| 22. | Zhang P, Moudgill N, Hager E, Tarola N, Dimatteo C, McIlhenny S, Tulenko T, DiMuzio PJ. Endothelial differentiation of adipose-derived stem cells from elderly patients with cardiovascular disease. Stem Cells Dev. 2011;20:977-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Gentile P, Casella D, Palma E, Calabrese C. Engineered Fat Graft Enhanced with Adipose-Derived Stromal Vascular Fraction Cells for Regenerative Medicine: Clinical, Histological and Instrumental Evaluation in Breast Reconstruction. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 24. | Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front Pharmacol. 2018;9:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 25. | Kusuma GD, Carthew J, Lim R, Frith JE. Effect of the Microenvironment on Mesenchymal Stem Cell Paracrine Signaling: Opportunities to Engineer the Therapeutic Effect. Stem Cells Dev. 2017;26:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 299] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Li J, Law HK, Lau YL, Chan GC. Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. Br J Haematol. 2004;127:326-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Liang W, Xia H, Li J, Zhao RC. Human adipose tissue derived mesenchymal stem cells are resistant to several chemotherapeutic agents. Cytotechnology. 2011;63:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Mueller LP, Luetzkendorf J, Mueller T, Reichelt K, Simon H, Schmoll HJ. Presence of mesenchymal stem cells in human bone marrow after exposure to chemotherapy: evidence of resistance to apoptosis induction. Stem Cells. 2006;24:2753-2765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Lopatina T, Kalinina N, Karagyaur M, Stambolsky D, Rubina K, Revischin A, Pavlova G, Parfyonova Y, Tkachuk V. Adipose-derived stem cells stimulate regeneration of peripheral nerves: BDNF secreted by these cells promotes nerve healing and axon growth de novo. PLoS One. 2011;6:e17899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 30. | Piccinno MS, Veronesi E, Loschi P, Pignatti M, Murgia A, Grisendi G, Castelli I, Bernabei D, Candini O, Conte P, Paolucci P, Horwitz EM, De Santis G, Iughetti L, Dominici M. Adipose stromal/stem cells assist fat transplantation reducing necrosis and increasing graft performance. Apoptosis. 2013;18:1274-1289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 31. | Harris WM, Plastini M, Kappy N, Ortiz T, Chang S, Brown S, Carpenter JP, Zhang P. Endothelial Differentiated Adipose-Derived Stem Cells Improvement of Survival and Neovascularization in Fat Transplantation. Aesthet Surg J. 2019;39:220-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Beane OS, Fonseca VC, Darling EM. Adipose-derived stem cells retain their regenerative potential after methotrexate treatment. Exp Cell Res. 2014;327:222-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Taylor CW, Horgan K, Dodwell D. Oncological aspects of breast reconstruction. Breast. 2005;14:118-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |