Copyright
©The Author(s) 2021.
World J Crit Care Med. Jul 9, 2021; 10(4): 66-80
Published online Jul 9, 2021. doi: 10.5492/wjccm.v10.i4.66
Published online Jul 9, 2021. doi: 10.5492/wjccm.v10.i4.66
Figure 1 Krebs cycle derived reducing equivalents (NADH, FADH2) donate electrons that are processed by the electron transport chain during oxidative phosphorylation.
Up to 5% of electrons (e-) will normally escape the electron transport chain (ETC) into the mitochondrial matrix (electron leakage)[14-16]. These electrons combine with molecular oxygen (O2) to form superoxide anion radical (O2-), which is metabolized by superoxide dismutase (SOD) to hydrogen peroxide (H2O2) that in turn is converted to glutathione disulfide (GS-SG) and water via glutathione peroxidase (GPX) and its reducing co-factor glutathione (GSH). Critical illness hypermetabolic states increase ETC activity leading to enhanced electron leakage and far greater H2O2 formation, which can deplete cellular GSH resulting in a build-up of H2O2 in cells and blood causing bioenergetic dysfunction and organ failure.
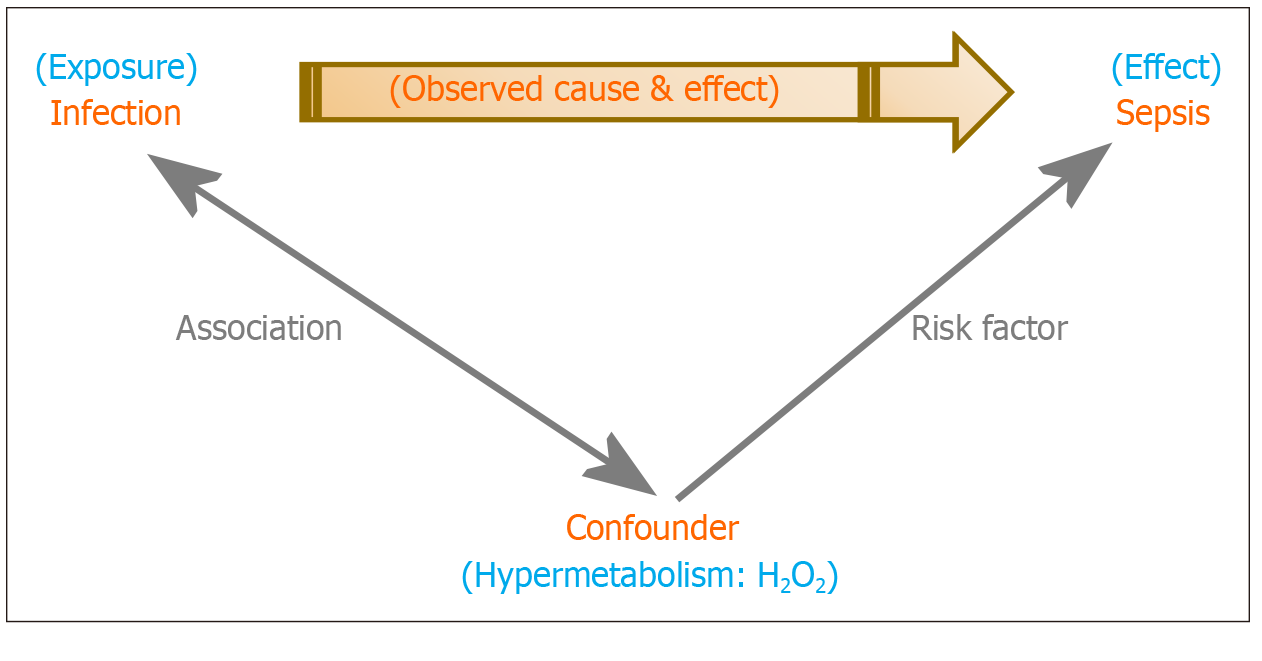
Figure 2 Confounding in Sepsis: The hypermetabolic state that accompanies a critical illness is a con
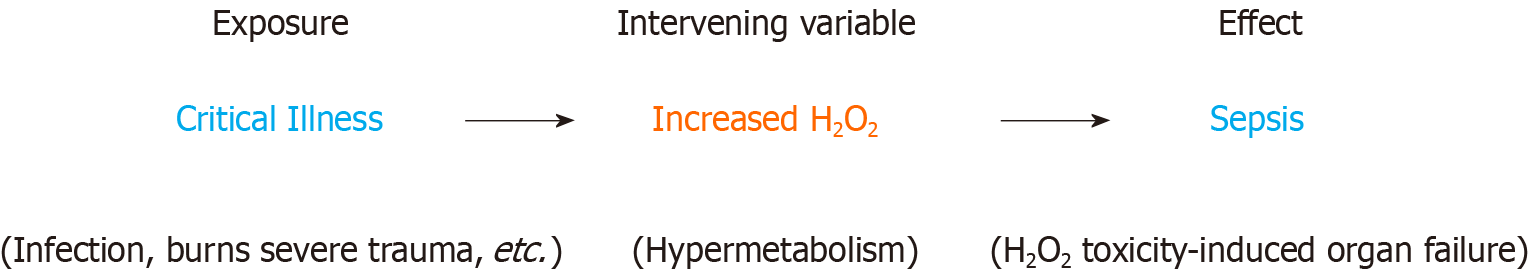
Figure 3 Sepsis and intervening variables: Hydrogen peroxide is an intervening variable between a critical illness (exposure), which triggers a systemic hypermetabolic response, and sepsis (effect).
Hypermetabolism, characterized by the systemic inflammatory response syndrome, is the clinical manifestation of supraphysiological cellular H2O2 production. This will eventually lead to reductive depletion and sepsis (H2O2 toxicity, bioenergetic organ failure) if allowed to persist. Prolonged critical illness (hypermetabolism) and dietary restriction severely limit the body’s ability to re-establish and maintain redox homeostasis. Under these circumstances, direct acting reducing equivalents must be supplied to the patient to aid in neutralizing excess H2O2. A hypermetabolic response to critical illness or injury may continue for years after hospital discharge and contribute to increased inpatient and post-discharge morbidity and mortality (chronic critical illness and post sepsis syndrome respectively)[52-55].
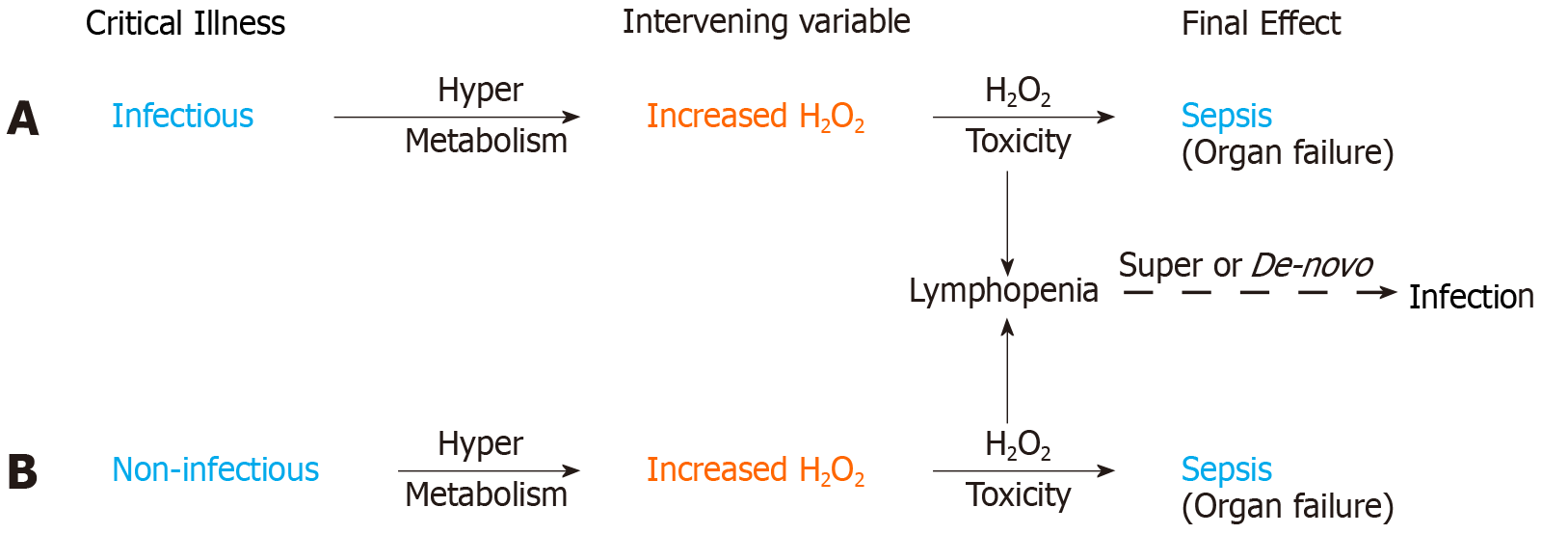
Figure 4 H2O2 induced immune system failure.
Sequences 4A and 4B illustrate the common hypermetabolic response in infectious and non-infectious critical illness leading to H2O2 toxicity induced organ failure and sepsis. Lymphocytes are highly sensitive to H2O2 induced apoptosis. Lymphopenia is thus a manifestation of H2O2 induced immune system failure secondary to a hypermetabolic response in both infectious and non-infectious critical illness. H2O2 induced lymphopenia will predispose to de-novo infection in otherwise sterile critical illness and may cause a super-infection in patients on appropriate antibiotics. H2O2 toxicity and/or super-infection may contribute to sepsis mortality despite appropriate antibiotics.
- Citation: Pravda J. Sepsis: Evidence-based pathogenesis and treatment. World J Crit Care Med 2021; 10(4): 66-80
- URL: https://www.wjgnet.com/2220-3141/full/v10/i4/66.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i4.66