Copyright
©The Author(s) 2015.
World J Immunol. Nov 27, 2015; 5(3): 113-130
Published online Nov 27, 2015. doi: 10.5411/wji.v5.i3.113
Published online Nov 27, 2015. doi: 10.5411/wji.v5.i3.113
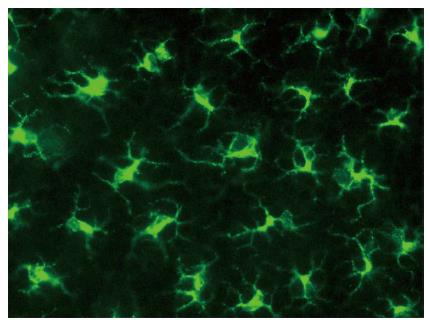
Figure 1 Murine Langerhans cells in ear skin reside in the epidermis, stained here for major histocompatibility complex class II (green).
Reprinted from Jakob T, Ring J, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol 2001; 108: 688-696 Copyright (2001), with permission from Elsevier.
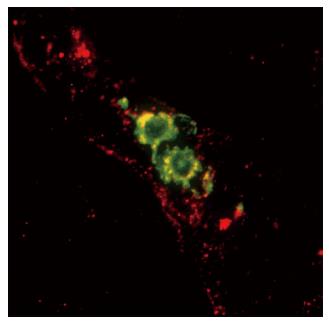
Figure 2 Mature skin dendritic cells egress via lymphatics that display secondary lymphoid tissue chemokine (SLC/CCL21) constitutively.
Shown here are two mature DC expressing MHC class II (green) located within a lymphatic vessel that presents SLC (red). Reprinted from Jakob T, Ring J, Udey MC. Multistep navigation of Langerhans/dendritic cells in and out of the skin. J Allergy Clin Immunol 2001; 108: 688-696 Copyright (2001), with permission from Elsevier. Courtesy of Saeki H and Hwang S, National Cancer Institute, Bethesda, MD.
Figure 3 Scanning electron microscope images demonstrate that dendritic cells cultured on fibronectin or laminin for 48 h exhibit widely differential morphologies.
Taken in part from García-Nieto S, Johal RK, Shakesheff KM, Emara M, Royer PJ, Chau DY, Shakib F, Ghaemmaghami AM. Laminin and fibronectin treatment leads to generation of dendritic cells with superior endocytic capacity. PLoS One 2010; 5: e10123. Copyright (2011), published under the Creative Commons Attribution (CC BY) license. FN: Fibronectin; LAM: Laminin.
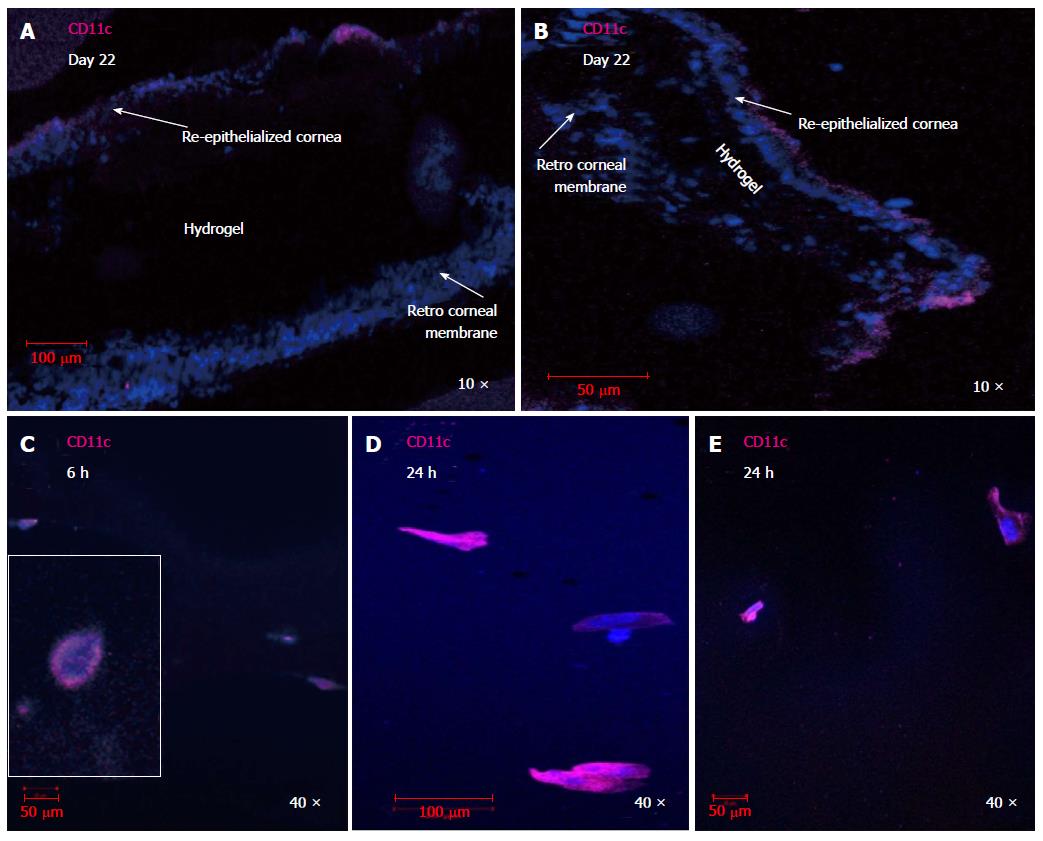
Figure 4 CD11c+ dendritic cell are involved in the host response to transplanted hydrogels.
CD11c+ DC were detected in re-epithelialized layers surrounding RHCIII hydrogels transplanted in murine corneas, 22 d post transplantation (A, B). CD11c+ dendritic cells infiltrated hydrogels transplanted into murine corneas as early as 6 h (C) or 24 h (D, E) after transplantation and showed a transformation in morphology from rounded immature DC to well-differentiated DC at the latter time point after interacting with the three dimensional crosslinked collagen matrix. Images were taken within the hydrogel in (C-E) as shown in the schematic. DAPI: Nuclear staining shown in blue; CD11c in magenta; DC: Dendritic cell.
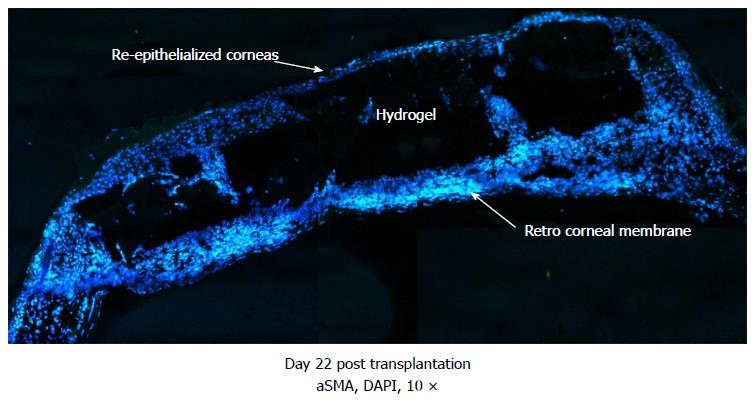
Figure 5 Merged images showing the formation of dense retro-corneal membrane separating corneal hydrogel from lens and posterior areas of eye, shown on day 22 after transplantation of murine corneas with RHCIII hydrogels.
Newly generated membrane exhibits presence of alpha smooth muscle actin, likely produced by myofibroblasts recruited during the wound healing process. Tissue ingrowths into the RHCIII hydrogel are evidence of active remodelling of the artificial matrix scaffold by immune/inflammatory cells and ECM components, towards long-term integration with natural tissue. DAPI: Nuclear staining shown in blue; Alpha smooth muscle actin in green; ECM: Extracellular matrix.
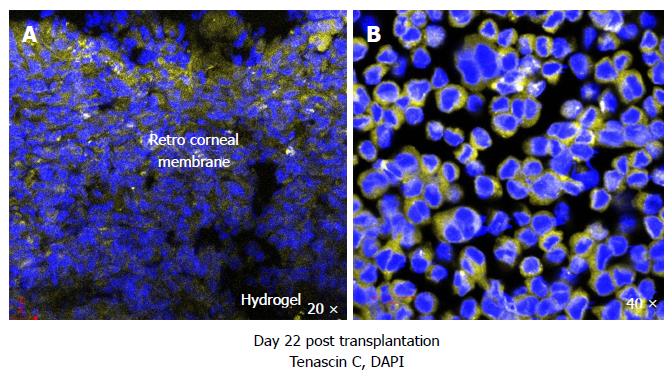
Figure 6 Murine corneas transplanted with RHCIII hydrogels stained positive for extracellular matrix constituent tenascin C in the retro-corneal membrane, a marker of epithelial to mesenchymal transition, indicative of active wound healing (A) and WEHI-164 murine fibrosarcoma cell line cultured with 5 ng/mL transforming growth factor-β1 for 48 h also produced tenascin C (positive control) (B).
DAPI: Nuclear staining shown in blue; Tenascin C in yellow.
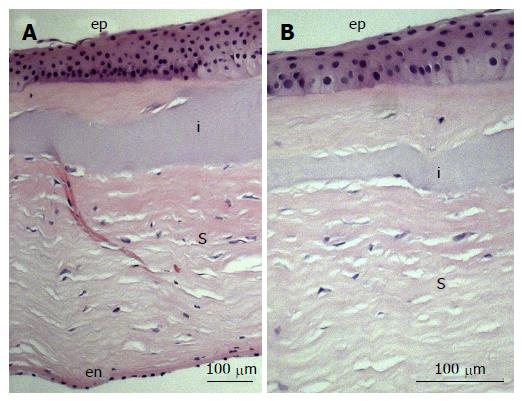
Figure 7 Immunohistochemistry image of biointeractive RHCIII hydrogel in a lamellar keratoplasty and removed after 4 years, upon regrafting of the patient.
Notably, regrowth of characteristically stratified corneal epithelium (ep), layered stroma (s) and endothelial monolayer (en) left intact during transplantation, can be observed (A). A portion of the RHCIII implant (i) is visible in a higher magnification image (B), displaying a uniform assimilation with the native stroma in a dynamic, ongoing remodelling process. Reprinted from Fagerholm P, Lagali NS, Ong JA, Merrett K, Jackson WB, Polarek JW, Suuronen EJ, Liu Y, Brunette I, Griffith M. Stable corneal regeneration four years after implantation of a cell-free recombinant human collagen scaffold. Biomaterials 2014; 35: 2420-2427. Copyright (2014), with permission from Elsevier.
- Citation: Shankar SP, Griffith M, Forrester JV, Kuffová L. Dendritic cells and the extracellular matrix: A challenge for maintaining tolerance/homeostasis. World J Immunol 2015; 5(3): 113-130
- URL: https://www.wjgnet.com/2219-2824/full/v5/i3/113.htm
- DOI: https://dx.doi.org/10.5411/wji.v5.i3.113