Published online Nov 8, 2016. doi: 10.5409/wjcp.v5.i4.374
Peer-review started: July 6, 2016
First decision: September 5, 2016
Revised: September 25, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: November 8, 2016
Processing time: 127 Days and 8.1 Hours
To study the impact of vaccination critical illness due to H1N1pdm09, we compared the incidence and severity of H1N1pdm09 infection in Canada and France.
We studied two national cohorts that included children with documented H1N1pdm09 infection, admitted to a pediatric intensive care unit (PICU) in Canada and in France between October 1, 2009 and January 31, 2010.
Vaccination coverage prior to admission to PICUs was higher in Canada than in France (21% vs 2% of children respectively, P < 0.001), and in both countries, vaccination coverage prior to admission of these critically ill patients was substantially lower than in the general pediatric population (P < 0.001). In Canada, 160 children (incidence = 2.6/100000 children) were hospitalized in PICU compared to 125 children (incidence = 1.1/100000) in France (P < 0.001). Mortality rates were similar in Canada and France (4.4% vs 6.5%, P = 0.45, respectively), median invasive mechanical ventilation duration and mean PICU length of stay were shorter in Canada (4 d vs 6 d, P = 0.02 and 5.7 d vs 8.2 d, P = 0.03, respectively). H1N1pdm09 vaccination prior to PICU admission was associated with a decreased risk of requiring invasive mechanical ventilation (OR = 0.30, 95%CI: 0.11-0.83, P = 0.02).
The critical illness due to H1N1pdm09 had a higher incidence in Canada than in France. Critically ill children were less likely to have received vaccination prior to hospitalization in comparison to general population and children vaccinated had lower risk of ventilation.
Core tip: This article is on a two national cohorts study from Canada and France of critically ill children during influenza pandemic and reports that: (1) critically ill French children were much less likely to have received vaccine prior to hospitalization against influenza A(H1N1)pdm09 in comparison to children in the Canadian populations; and (2) in Canada, where vaccination rate was higher, the risk of severe respiratory failure was less among those critically ill children receiving vaccine.
- Citation: Fléchelles O, Brissaud O, Fowler R, Ducruet T, Jouvet P, the Pediatric Canadian Critical Care Trials Group H1N1 Collaborative and Groupe Francophone de Réanimation et Urgences Pédiatriques. Pandemic influenza 2009: Impact of vaccination coverage on critical illness in children, a Canada and France observational study. World J Clin Pediatr 2016; 5(4): 374-382
- URL: https://www.wjgnet.com/2219-2808/full/v5/i4/374.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i4.374
By March 2009, pandemic influenza A(H1N1)pdm09 had begun to spread from Mexico across the globe. The epidemiology of the first pandemic wave in Canada revealed that A(H1N1)pdm09 affected both young healthy patients and patients with underlying conditions. The severity of illness among children was high, predominantly due to severe hypoxic respiratory failure, resulting in prolonged pediatric intensive care unit (PICU) length of stay and mechanical ventilation, in comparison with seasonal influenza[1]. Countries from the Southern Hemisphere also reported early patterns of severity of illness including higher mechanical ventilation rate and higher mortality than previously observed with seasonal influenza[2,3].
To limit the impact of the pandemic influenza A(H1N1)pdm09 especially on children[4-6], a vaccination campaign was conducted just before the second wave. However, different vaccine coverage across countries was observed, especially between Canada and France[7-10]. In order to study the impact of pandemic influenza H1N1 vaccination prior to hospitalization on critical illness, we conducted a bi-national observational study in 42 centers across Canada and France on pandemic influenza A(H1N1)-associated critically illness in children, the most sensitive population affected by the pandemic. We originally hypothesized that the higher rate of vaccination coverage in children in Canada and previous exposure to influenza A(H1N1)pdm09 would have protected Canadian children from critical illness in the Fall of 2009.
The participating institutions’ research ethics boards approved study procedures in the two countries (Sainte-Justine IRB and Bordeaux IRB). The need for informed consent was waived given the non-interventional study design.
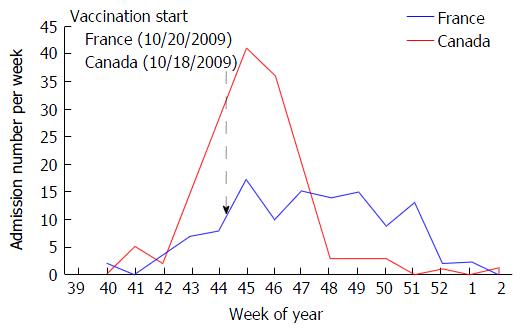
We studied pandemic influenza A(H1N1)pdm09 incidence and severity in children in Canada and France using two multicenter national databases designed for pandemic surveillance. A key difference between the two countries was that 54% of children in Canada and 18% in France had been vaccinated[7-10]. On the other hand, Canada and France are two similar industrialized countries with a gross domestic product par capital ranking, 15th and 23rd rank in the world, respectively - with similar per capita health expenditures[11,12]. Their climates during autumn are similar (average temperatures (low/high) are 0 °C to 15 °C in Canada and 5 °C to 20 °C in France). France and Canada have similar health care systems in that they are based on social health insurance to provide near universal coverage to the adult and pediatric populations. Family practitioners provide primary health care in each country and most vaccine delivery does not require out-of-pocket payment. The number of PICU is also similar (2.9 bed/100000 children under 15 years in Canada and 2.5 beds per 100000 children in France)[9,13]. During the pandemic, treatment recommendations were the same, those of the World Health Organization. Although oseltamivir was not prescribed initially to children under two years of age in Canada, and under one year of age in France, as of October 27, 2009 in Canada and December 10, 2009 in France, these restrictions were abolished[14,15]. Vaccination campaigns were organized in the two countries with the same priority groups and guidelines[16-18]. The campaigns started on October 18, 2009 in Canada and October 20, 2009 in France[19].
Data collection was prospective in all Canadian PICUs (n = 17). In France, data collection was both prospective and retrospective in 25 of 29 French PICUs. Four French PICUs did not participate to the study. All children admitted to a participating PICU in Canada and France, with documented A(H1N1)pdm09 infection between October 1 2009 and January 31 2010, were included. During this second wave of pandemic influenza A(H1N1)pdm09, all children admitted to PICU with clinical symptoms of H1N1 infection or strong epidemiologic link to patients with known H1N1 infection were tested for H1N1, in both countries. Proven A(H1N1)pdm09 corresponded to World Health Organization criteria in both countries: Any specimen yielding influenza A(H1N1)pdm09 by polymerase chain reaction and/or viral culture[20]. Variables in common between both databases were identified.
The data collected in both cohorts included demographic characteristics, vaccination history, comorbid conditions, admission severity of illness according to the Pediatric Logistic Organ Dysfunction (PELOD)[21] and Pediatric Index of Mortality 2 (PIM2)[22] scores, and intensive care management conditions. The geographic area of 17 Canadian PICUs corresponded to a pediatric population of almost 6 millions children[23] and the 25 French PICUs cover a pediatric population of almost 11 millions children[24]. We also collected data on infection severity including acute respiratory distress syndrome (ARDS) that is characterized by an acute hypoxemia due to lung inflammation[1] in reaction to viral infection or secondary bacterial infection, nosocomial infection that could result from invasive treatments and seizures.
The study’s primary objective was to assess whether vaccination prior to hospitalization protects against critical illness. The secondary outcomes were A(H1N1)pdm09 incidence, the timing of the epidemic peak and the epidemic duration, PICU mortality, the incidence and duration of invasive mechanical ventilation, PICU length of stay between the two countries. Mechanical ventilation was considered invasive if delivered through an endotracheal tube or a tracheostomy. The duration of each episode of mechanical ventilation was defined as the time from intubation to final extubation or death. Mechanical ventilation was considered non-invasive if delivered through a nasal or facemask interface. Total duration of ventilation corresponded to the sum of the periods of both invasive and non-invasive ventilation.
Descriptive statistics included counts and proportions, means (and standard deviations), medians (and interquartile ranges) as appropriate. Incidence and incidence curves were calculated using as a denominator, the number of susceptible patients in the population in each country from Statistics Canada and the “Institut National de la Statistique et des Etudes Economiques” in France. We compared the two countries using bi-variate analysis including Pearson’s χ2 test or Fisher’s exact test for categorical variables. Student’s t-test, Wilcoxon rank-sum test or the log-rank test, were used for continuous variables. To assess associations between patient or country factors and outcomes, we performed a multivariate logistic regression for invasive ventilation risk and Cox proportional hazards modeling for time-dependent variables such as length of stay and invasive ventilation duration. Because data came from two different cohorts, there was heterogeneity in data distributions, requiring country-specific analyses for many variables. Variables used in final multivariate models met the following criteria: Factors of clinical interest or possibly associated with the outcomes (P < 0.1 in univariate analysis), more than 3 cases per group and per country, and with few (< 5%) missing values in each country. All variables were tested for excessive (> 0.80) co-linearity. For Cox regression modeling, variables respected the proportional hazards assumption. Analyses were considered statistically significant at α < 0.05. SPPS version 19 was used for all analyses. The statistical methods of this study were performed by a biomedical statistician (Thierry Ducruet from Sainte-Justine Hospital, co-author).
In total 285 children were included, 160 in Canada and 125 in France. The rate of admission to PICU due to A(H1N1)pdm09, calculated using the estimated population studied (see methods), was 2.63 per 100000 children in Canada and 1.15 per 100000 children in France (Table 1). The incidence curves showed a higher peak (41 vs 17 admissions per week, both during week 45) but shorter pandemic period (6 wk vs 11 wk) in Canada compared to France (Figure 1).
| Canada (n = 160) | France (n = 125) | OR (95%CI) Canada/France | P value | |
| Incidence rate (/100000 children) | 2.6 | 1·1 | 2.3 (1.8-2.9) | < 0.001 |
| Age, mean (SD), yr | 6.6 (0.40) | 5.5 (0.48) | NA | 0.09 |
| Weight, mean (SD), kg | 25.9 (1.62) | 20.1 (1.45) | NA | 0.01 |
| Female gender, n (%) | 68 (42) | 56 (45) | 0.91 (0.57-1.46) | 0.70 |
| Vaccination H1N1, n (%) | 34 (21) | 2 (2) | 16.6 (3.90-70.6) | < 0.001 |
| Underlying chronic conditions, n (%) | ||||
| Any underlying conditions | 102 (64) | 93 (74) | 0.60 (0.36-1.01) | 0.05 |
| Infant < 1 years old | 21 (13) | 32 (25) | 0.44 (0.24-0.81) | 0.007 |
| Lung disease | 65 (40) | 29 (23) | 2.26 (1.34-3.82) | 0.002 |
| Asthma | 42 (26) | 16 (13) | 2.40 (1.29-4.56) | 0.005 |
| Chronic lung disease | 33 (20.6) | 14 (11.2) | 2.06 (1.05-4.05) | 0.03 |
| Cystic fibrosis | 0 (0) | 2 (2) | NA | NA |
| BPD | 4 (2) | 4 (3) | 0.78 (0.19-3.16) | 0.731 |
| Tracheostomy | 5 (3) | 1 (1) | 4.00 (0.46-33.3) | 0.241 |
| Congenital heart disease | 24 (15) | 3 (2) | 7.18 (2.11-24.4) | < 0.001 |
| Neurological disease | 31 (19) | 19 (15) | 1.33 (0.71-2.50) | 0.36 |
| Seizure disorder | 19 (12) | 5 (4) | 3.23 (1.18-9.09) | 0.02 |
| Immunosuppressive disorder | 11 (7) | 9 (7) | 0.95 (0.38-2.37) | 0.91 |
| Diabetes mellitus | 6 (3.8) | 0 (0) | NA | 0.041 |
| Renal insufficiency | 7 (4) | 1 (1) | 5.56 (0.69-50.0) | 0.081 |
| Others diseases | 32 (20) | 28 (22) | 0.87 (0.95-1.54) | 0.62 |
| PELOD score, mean (SD)2 | 6.67 (0.82) | 7.80 (1.47) | NA | 0.47 |
| PIM2 score, mean (SD)3 | 8.47 (1.05) | 9.74 (2.77) | NA | 0.67 |
| Clinical presentation at admission | ||||
| Lower respiratory infection, n (%) | 101 (63) | 90 (72) | 0.67 (0.40-1.10) | 0.11 |
| CNS infection | 2 (1) | 7 (6) | 0.21 (0.04-0.99) | 0.04 |
| Shock | 13 (8) | 6 (5) | 1.75 (0.65-4.76) | 0.26 |
| Other | 48 (30) | 35 (29) | 1.10 (0.67-1.85) | 0.90 |
| Bacterial infection at admission | 22 (14) | 27 (22) | 0.58 (0.31-1.07) | 0.08 |
The sex ratios and age distribution of critically ill children were similar in Canada and in France. After vaccination program start (Figure 1), vaccination coverage prior to hospitalization of children admitted to PICU was higher in Canada than in France (21% vs 2% of children respectively, P < 0.001), and in both countries, this vaccination coverage was substantially lower than that of the general pediatric population (P < 0.001, using conservative estimates of 54% in children in Canada and 18% in France[7-10]). Co-morbid conditions were common in both Canada and France but individual distributions were different.
The most common reason for PICU admission was lower respiratory infection in both Canada (63%) and France (72%) and clinical presentations at admission were similar between the two countries (Table 1). The mean organ dysfunction score (PELOD score) at day one and mean predicted mortality score (PIM2 score) were similar. During hospitalization, there was a higher rate of severity of illness in France: ARDS, nosocomial infection, nosocomial pulmonary infection, and seizures (Table 2).
| Canada (n = 160) | France (n = 125) | OR (95%CI), difference | P value | |
| Time-dependent variables, median (25th 75th percentile), d | ||||
| PICU length of stay | 2.9 (2.1-3.6) | 3.0 (1.8-4.2) | 0.1 | 0.03 |
| Duration of mechanical ventilation | 4.0 (2.8-5.2) | 5.0 (3.2-6.8) | 1 | 0.07 |
| Duration of invasive ventilation | 4.0 (2.9-5.1) | 6.0 (4.6-7.4) | 2 | 0.02 |
| Categorical variables, n (%) | ||||
| Mortality | 7 (4.4) | 8 (6.5) | 0.67 (0.24-1.90) | 0.45 |
| Respiratory dysfunction | ||||
| ARDS | 29 (18) | 40 (32) | 0.48 (0.27-0.81) | 0.007 |
| Mechanical ventilation | 86 (54) | 66 (53) | 1.04 (0.67-1.67) | 0.87 |
| Invasive ventilation | 78 (49) | 50 (40) | 1.43 (0.91-2.50) | 0.14 |
| Pneumothorax | 19 (12) | 10 (8) | 1.17 (0.67-3.33) | 0.32 |
| ECMO | 3 (2) | 8 (6) | 0.28 (0.07-1.07) | 0.05 |
| Neurologic dysfunction | ||||
| Seizures | 2 (1) | 9 (7) | 0.16 (0.03-0.13) | 0.01 |
| ADEM | 3 (2) | 7 (6) | 0.32 (0.08-1.26) | 0.09 |
| Renal dysfunction | ||||
| Dialysis/hemofiltration | 10 (6) | 4 (3) | 2.00 (0.63-6.67) | 0.24 |
| Nosocomial infections | ||||
| Nosocomial infection | 15 (9) | 26 (21) | 0.39 (0.20-0.78) | 0.006 |
| Ventilator-associated pneumonia | 9 (6) | 21 (17) | 0.29 (0.13-0.67) | 0.002 |
| Antiviral treatment | ||||
| Oseltamivir | 148 (93) | 111 (89) | 1.55 (0.69-3.49) | 0.28 |
| Oseltamivir within 48 h | 102 (63) | 99 (79) | 0.46 (0.27-0.79) | 0.004 |
Mortality rate (4.4% vs 6.5%, P = 0.45) and rate of invasive mechanical ventilation (49% vs 40%, P = 0.14) were similar in Canada and France (Table 2). The duration of invasive mechanical ventilation (median, 4 d vs 6 d, P = 0.02) and total (invasive and non-invasive) mechanical ventilation (4 d vs 5 d, P = 0.07) was shorter in Canada than in France (Table 2). The mean PICU length of stay was shorter in Canada (5.7 d vs 8.2 d, P = 0.03) but median PICU length of stay was not different (3 d vs 2.9 d).
Among Canadian patients, independent multivariate analyses showed that H1N1 vaccination and asthma were associated with an almost four-fold decrease risk of invasive ventilation: (OR = 0.3, 95%CI: 0.11-0.83, P = 0.02) and (OR = 0.23, 95%CI: 0.09-0.64, P = 0.004), respectively (Table 3). This multivariate analysis did not include French patients because there were only 2 children in the vaccine group (Table 1).
| Included variables | n = 157 | OR | 95%CI | P value |
| PIM2 > 7.5 | 39 | 6.26 | 2.43-16.4 | < 0.001 |
| Age, years < 1 | 21 | 1.88 | 0.51-6.94 | 0.35 |
| 1-4 | 52 | 1.50 | 0.51-4.35 | 0.46 |
| 5-9 | 46 | 2.42 | 0.45-6.93 | 0.10 |
| > 10 | 38 | 1 | (Ref) | |
| H1N1 vaccine | 32 | 0.30 | 0.11-0.83 | 0.02 |
| Asthma | 41 | 0.23 | 0.09-0.64 | 0.004 |
| Lung diseases (not asthma) | 22 | 0.99 | 0.32-3.08 | 0.99 |
| Neurologic diseases | 31 | 2.51 | 0.92-6.90 | 0.07 |
| Cardiologic diseases | 28 | 1.13 | 0.43-2.97 | 0.76 |
| Others diseases | 47 | 0.87 | 0.37-2.05 | 0.76 |
| Oseltamivir within 48 h | 102 | 1.02 | 0.47-2.24 | 0.95 |
In this bi-national observational study of pandemic in–fluenza A(H1N1)-associated critically illness in children, we found that pandemic influenza A(H1N1) vaccination prior to hospitalization was less common among critically ill children when compared to the general paediatric population, and that history of vaccination was not associated with a clinically relevant difference in PICU length of stay (0.1 d). However, in Canada, with higher vaccine coverage among critically ill patients, the PICU course seems less severe (shorter duration of invasive mechanical ventilation and PICU stay, lesser development of ARDS, and fewer subsequently acquired bacterial infections) (Table 2).
Despite a higher vaccine coverage and potential previous exposure to the virus in Canada during the first pandemic wave in the Spring of 2009[1], the incidence of admission of critically ill children to intensive care due to Influenza A(H1N1)pdm09 during the Fall of 2009 was twice as high in Canada as in France (2.6 per 100000 children vs 1.1 per 100000 children). However, the mortality rate for these critically ill children was similar between the two countries.
We originally hypothesized that the higher child vaccination coverage in Canada (> 50% vs 18% in France) and previous exposure to influenza A(H1N1)pdm09 would have protected Canadian children from critical illness in the Fall of 2009. We did not observed such a protection. This hypothesis was based on the following arguments: (1) previous exposure to influenza A(H1N1)pdm09 would have increased herd immunity; (2) adjuvant pandemic vaccine has an efficacy up to 97%[25-27]; (3) an influenza vaccination coverage rate above 45% reduces influenza transmission[28]; and (4) modeling studies suggested that the vaccination campaign was associated with a decrease in mortality and morbidity of 20% and 18% respectively[29]. Other factors previously identified as contributing to outbreak spread such as proximity to the first infectious focus, human mobility, reproduction number, generation time, population susceptibility, age pyramid, school calendar, and climate[30] were similar between the two countries and the underlying characteristics of the children were similar (Table 1). Given that the difference in incidence of PICU admission was the opposite of what was expected, our study suggests that additional national, geography-specific, and/or further unappreciated factors likely exhibit substantial residual influence on the incidence of pandemic influenza in differing regions of the world.
It has also been shown that the virulence of influenza A(H1N1)pdm09 strains virulence can vary considerably in animals and in humans[31-35]. Some specific strains were associated with severe disease in Canada and France but the proportion of these virulent strains in Canada and France is incompletely reported. Differing virulence could have contributed to the increased incidence of critical illness in Canada, as well as to the higher mortality observed in Argentina and Turkish pediatric cohorts when compared with those in North America, Europe and Australia and New Zealand[36-39].
Despite the higher incidence of critical illness in Canada when compared to France, our study provides some arguments on the positive impact of vaccine on influenza critical illness in children, even when the vaccine is given when pandemic second wave has already started (Figure 1). Our study showed that: (1) the second wave ended earlier than in France, which had a lower vaccine coverage; (2) vaccination coverage was substantially lower in the PICU population than in the general pediatric population; (3) total duration of mechanical ventilation was shorter in Canada; and (4) vaccination was associated with a decreased risk of invasive mechanical ventilation (Table 3). As expected, asthma was also associated with a decreased risk of invasive ventilation. This is consistent with previous findings of a low rate (4.6%) of invasive mechanical ventilation in PICU patients admitted for acute asthma[40]. The significant association between vaccination coverage and reduction in invasive mechanical ventilation is remarkable considering that the rate of invasive mechanical ventilation in children without a diagnosis of asthma diagnosis in this study was > 40%.
This study has several strengths: (1) It represents the largest pediatric cohort of critically ill H1N1 infection yet described in Canada and France; (2) the evolution of new H1N1 cases per week in PICUs (Figure 1) was similar to the consultations rates for influenza-like illness in the general population of Canada and France[41,42]; and (3) there was a large difference in vaccine coverage. This difference in coverage may be attributed to differences in perception of risk amongst the population such as awareness of the public health issues, the risk of being infected by the virus, the risk of severe illness if infected, and the risk of harm from a pandemic vaccine[43,44].
Our study has several limitations that should be noted. First, the suspected difference in virulence between the two countries could have created a bias on the analysis of pandemic vaccine impact. However, the analysis of critically ill children in Canada only provided an association between vaccine delivery and reduction in the risk of invasive ventilation (Table 3); second, admission criteria in PICUs are not standardized across countries and this can impact the incidence of PICU admission and inferred critical illness. However, several arguments suggest that admission criteria between Canada and France are similar, including: (1) the similar number of PICU beds per capita; and (2) patients displayed similar organ failure score (PELOD score) and predicted risk of mortality (PIM2) on admission to PICU (Table 1). Interestingly, this difference in ICU admission rate was also observed in adult intensive care units, with a rate of A(H1N1)pdm09-associated admission of 3.5/100000 population in Canada and 2.1/100000 population in France (OR = 1.7)[45,46]. Another limitation is that the two national cohorts used similar but not identical case report forms. Therefore, we needed to compare similar variables that may have been collected in slightly different ways in order to compare the two cohorts. In order to address this point for future outbreaks and pandemics, a number of national critical care research consortia initiated the International Forum of Acute Care Trialists which seeks to improve the care of acutely ill patients around the world by harmonizing case report forms and definitions[47]. This goal has been further advanced by the creation of International Severe Acute Respiratory and Emerging Infection Consortium.
In conclusion, the critical illness due to H1N1pdm09 had a higher incidence in Canada than in France. In both Canada and France, critically ill children were much less likely to have received vaccination against influenza A(H1N1)pdm09 prior to hospitalization when compared with children in the general population. In Canada, with higher vaccine coverage among critically ill patients, the PICU course seems less severe and the risk of invasive mechanical ventilation was lower amongst Canadian critically ill children receiving prior vaccination. There is a need for further studies to confirm our observations as numerous and still uncertain factors influence differences in pandemic influenza incidence and severity in different regions of the world, even in countries with similar population characteristics, access to health care resources and response systems.
The authors thank the healthcare professionals who delivered exemplary care to our patients, and research assistants who worked tirelessly, in the face of uncertain risks. The authors also thank all the following site investigators who contributed to this work: Pediatric Canadian Critical Care Trials Group pH1n1 Collaborative. Ari Joffe MD, Stollery Children’s Hospital (Edmonton); Marc André Dugas MD, Centre Hospitalier de l’Université Laval - CHUL (Québec); Davinia Withington MD, Montreal Children’s Hospital (Montreal); Miriam Santschi MD, Centre Hospitalier Universitaire de Sherbrooke - CHUS (Sherbrooke); Jill Barter MD, Janeway Children’s Health and Rehabilitation Centre (St-John’s); Chris Soder MD, IWK Health Centre (Halifax); Kusum Menon MD, Children’s Hospital of Eastern Ontario - CHEO (Ottawa); Basem Alsaati MD, Kingston General Hospital (Kingston); Jamie Hutchison MD, Hospital for Sick Children (Toronto); Karen Choong, Hamilton Health Sciences (Hamilton); Alik Kornecki MD, London Health Sciences Centre (London); Murray Kesselman MD and Stasa Veroukis MD, Winnipeg Children’s Hospital (Winnipeg); Tanya Holt MD, Royal University Hospital (Saskatoon); Elaine Gilfoyle MD, Alberta Children’s Hospital (Calgary); Peter Skippen MD, BC Children’s Hospital (Vancouver); Jeff Burzynski MD, Vancouver Island Health Authority (Victoria). Groupe Francophone de Réanimation et Urgences Pédiatriques. Astrid Botte MD - François Dubos MD,PhD, Hôpital Jeanne de Flandre, Centre Hospitalier Régional Universitaire de Lille (Lille); Gérard Krim MD, Centre Hospitalier Universitaire Amiens (Amiens); Odile Noizet MD, Centre Hospitalier Universitaire de Reims (Reims); Mikael Jokic MD, PhD, Hôpital Femme-Enfant-Hématologie, Centre Hospitalier Universitaire de Caen (Caen); Stéphane Dauger MD, PhD - François Angoulvant MD, Hopital Robert Debré - Assistance Publique - Hôpitaux de Paris (Paris); Laurent Dupic MD - Gérard Chéron MD,PhD, Hopital Necker Enfant-Malades - Assistance Publique - Hôpitaux de Paris (Paris); Sylvain Renolleau MD,PhD, Hopital Trousseau - Assistance Publique - Hôpitaux de Paris (Paris); Jean Bergougnoux MD, Hopital Kremlin-Bicêtre - Assistance Publique - Hôpitaux de Paris (Paris); Isabelle Bunker MD - Nicolas Joram MD, Centre Hospitalier Universitaire Nantes (Nantes); Armelle Garenne MD, Centre Hospitalier Universitaire de Brest (Brest); Jean-Claude Granry MD, PhD, Centre Hospitalier Universitaire Angers (Angers); Antoine Bouissou MD, Hôpital Clocheville, Centre Hospitalier Universitaire de Tours (Tours); Paul Nolent MD - Olivier Richer MD, Hôpital Pellegrin, Centre Hospitalier Universitaire de Bordeaux (Bordeaux); Marie-Odile Marcoux MD - Isabelle Claudet MD, Hôpital des Enfants, Centre Hospitalier Universitaire de Toulouse (Toulouse); Jean-Pascal Saulnier MD, Centre Hospitalier Universitaire de Poitiers (Poitiers); Sophie Keterer MD, Hôpital de la mère et de l’Enfant, Centre Hospitalier Universitaire Limoges (Limoges); Benoit Bœuf MD, Hôpital Estaing, Centre Hospitalier Universitaire Clermont-Ferrand (Clermont-Ferrand); Etienne Javouhey MD, PhD - Robin Pouyau MD - Hôpital Femme Mère Enfant, Centre Hospitalier Universitaire Lyon (Lyon); Isabelle Wrobleski MD, Hôpital Couple Enfant, Centre Hospitalier Universitaire de Grenoble (Grenoble); Jean-Bernard Gouyon MD, Hôpital Femme-Enfant, Centre Hospitalier Universitaire Dijon (Dijon); Gérard Thiriez MD, PhD, Centre Hospitalier Universitaire de Besançon (Besançon); Christophe Milesi MD, Hôpital Arnaud de Villeneuve, Centre Hospitalier Universitaire de Montpellier (Montpellier); Serge Le Tacon MD, Hôpital d’Enfants, Centre Hospitalier Universitaire Nancy-Brabois (Nancy); Philippe Desprez MD, Centre Hospitalier Universitaire Hautepierre (Strasbourg).
By March 2009, pandemic influenza A(H1N1)pdm09 had begun to spread from Mexico across the globe. The epidemiology of the first pandemic wave in Canada revealed that A(H1N1)pdm09 affected both young healthy patients and patients with underlying conditions. To limit the impact of the pandemic influenza A (H1N1)pdm0 especially on children, a vaccination campaign started when the second wave occurred. A lot of discussions criticized the vaccination campaign policy.
Nowadays, Bird flu could combine with human flu to create a virulent kind of super-flu that can spread worldwide. The information gathered from previous pandemic (including the authors’ study) are helpful to predict the spread and severity of such a risk.
This study report data on: (1) the incidence of critically ill children with pandemic influenza A (H1N1)pdm09 infection that was not known in Europe and Canada; (2) on mortality rate were higher in South American and Turkish studies; and (3) a positive impact of vaccination, even if started at second wave start, was not previously described in critically ill children.
According to the results, in case of pandemic, it is recommended to perform the flu vaccination as soon as the vaccine is available to potentially decrease disease severity.
H1N1pdm09 infection: Flu pandemic; PICU: Pediatric intensive care units; ARDS: An acute hypoxemia due to lung inflammation.
The study is well designed with detailed methodology to assess the impact of vaccination status on severity of infection and mortality rates.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: France
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Durandy YD, Pavlovic M, Sergi CM, Toyoda T S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Jouvet P, Hutchison J, Pinto R, Menon K, Rodin R, Choong K, Kesselman M, Veroukis S, André Dugas M, Santschi M. Critical illness in children with influenza A/pH1N1 2009 infection in Canada. Pediatr Crit Care Med. 2010;11:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Torres SF, Iolster T, Schnitzler EJ, Farias JA, Bordogna AC, Rufach D, Montes MJ, Siaba AJ, Rodríguez MG, Jabornisky R. High mortality in patients with influenza A pH1N1 2009 admitted to a pediatric intensive care unit: a predictive model of mortality. Pediatr Crit Care Med. 2012;13:e78-e83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925-1934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 695] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 4. | Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605-2615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2118] [Cited by in RCA: 2219] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 5. | ECDC working group on influenza A(H1N1)v. Preliminary analysis of influenza A(H1N1)v individual and aggregated case reports from EU and EFTA countries. Euro Surveill. 2009;14:19238. [PubMed] |
| 6. | World Health Organization. Epidemiological summary of pandemic influenza A (H1N1) 2009 virus - Ontario, Canada, June 2009. Wkly Epidemiol Rec. 2009;84:485-491. [PubMed] |
| 7. | Sociaux MdlSedS. Statistiques descriptives de la grippe pandèmique A (H1N1). Quèbec (Canada): Ministère de la Santè et des Services Sociaux. 2010. [accessed 2013 Oct 24]. Available from: http://www.msss.gouv.qc.ca/extranet/pandemie/etat_situation/. |
| 8. | Bone A, Guthmann JP, Nicolau J, Lévy-Bruhl D. Population and risk group uptake of H1N1 influenza vaccine in mainland France 2009-2010: results of a national vaccination campaign. Vaccine. 2010;28:8157-8161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Dauger S. Regard d’un pédiatre sur l’enseignement de la réanimation pédiatrique. Paris (France): Groupe Francophone de Réanimation Pédiatrique. 2010. [accessed 2013 Oct 24]. Available from: http://gfrup.sfpediatrie.com/sites/default/files/u12548/cnerm2010_dauger.pdf. |
| 10. | Weil-Olivier C, Lina B. Vaccination coverage with seasonal and pandemic influenza vaccines in children in France, 2009-2010 season. Vaccine. 2011;29:7075-7079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | The World Bank ed. World Development Indicators database, Washington DC: Communications development incorporated, 2011: 1-215. |
| 12. | World Health Organization. 2012 ed. World Health Statistics 2012, Geneva: WHO Press 2012; 1-180. |
| 13. | Stiff D, Kumar A, Kissoon N, Fowler R, Jouvet P, Skippen P, Smetanin P, Kesselman M, Veroukis S. Potential pediatric intensive care unit demand/capacity mismatch due to novel pH1N1 in Canada. Pediatr Crit Care Med. 2011;12:e51-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Jamieson B, Jain R, Carleton B, Goldman RD. Use of oseltamivir in children. Can Fam Physician. 2009;55:1199-1201. [PubMed] |
| 15. | Ministère de la Santé et des Sports. Information sur la grippe A(H1N1) 2009 (données épidémiologiques et cliniques, diagnostic, vaccination, traitement). Paris (France): Ministère de la Santé et des Sports. 2009. [accessed 2013; Oct 24] Available from: http://www.sante.gouv.fr/IMG/pdf/Diaporama_d_information_sur_la_grippe_A_H1N1_2009_donnees_epidemiologiques_et_cliniques_diagnostic_vaccination_traitement_.pdf. |
| 16. | World Health Organization. Pandemic (H1N1) 2009 briefing note 2: WHO recommendations on pandemic (H1N1) 2009 vaccines. [accessed 2013 Oct 24]. Available from: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/. |
| 17. | Brien S, Kwong JC, Charland KM, Verma AD, Brownstein JS, Buckeridge DL. Neighborhood determinants of 2009 pandemic A/H1N1 influenza vaccination in Montreal, Quebec, Canada. Am J Epidemiol. 2012;176:897-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Ministère de la Santé et des Sports. Nouvelle recommandations sur la prise en charge des patients grippés (10 décembre 2009). Paris (France). [accessed 2013 Oct 24]. Available from: http://sante.gouv.fr/nouvelles-recommandations-sur-la-prise-en-charge-des-patients-grippes-10-decembre-2009.html. |
| 19. | Ministère de la Santé et des Sports. Lancement de la campagne vaccinale contre la grippe A(H1N1) dans les centres de vaccination. Paris (France), 2009. [accessed 2013 Oct 24]. Available from: http://www.sante.gouv.fr/dossier-de-presse-du-20-octobre-2009-lancement-de-la-campagne-de-vaccination-dans-les-etablissements-de-sante.html. |
| 20. | World Health Organization. WHO information for laboratory diagnosis of new influenza A(H1N1) virus in humans. Geneva (Switzerland): 2009. [accessed 2013; Oct 24] Available from: http://apps.who.int/iris/bitstream/10665/44518/1/9789241548090_eng.pdf. |
| 21. | Leteurtre S, Duhamel A, Grandbastien B, Lacroix J, Leclerc F. Paediatric logistic organ dysfunction (PELOD) score. Lancet. 2006;367:897; author reply 900-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 818] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 23. | Canada Statistiques. 2006 Census: portrait of the Canadian Population in 2006, by age and sex: national portrait: more seniors, fewer children. Ottawa (Canada). [accessed 2013 Oct 24]. Available from: http://www12.statcan.ca/census-recensement/2006/as-sa/97-551/p2-eng.cfm. |
| 24. | Insee. La pyramide des âges au premier Janvier 2006. Insee Résultats: La situation démographique en 2005; Available from: http://www.insee.fr/fr/ppp/bases-de-donnees/irweb/sd2005/dd/pdf/sd2005_pyra2006.pdf. |
| 25. | Wichmann O, Stocker P, Poggensee G, Altmann D, Walter D, Hellenbrand W, Krause G, Eckmanns T. Pandemic influenza A(H1N1) 2009 breakthrough infections and estimates of vaccine effectiveness in Germany 2009-2010. Euro Surveill. 2010;15:pii: 19561. [PubMed] |
| 26. | Yin JK, Chow MY, Khandaker G, King C, Richmond P, Heron L, Booy R. Impacts on influenza A(H1N1)pdm09 infection from cross-protection of seasonal trivalent influenza vaccines and A(H1N1)pdm09 vaccines: systematic review and meta-analyses. Vaccine. 2012;30:3209-3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Van Buynder PG, Dhaliwal JK, Van Buynder JL, Couturier C, Minville-Leblanc M, Garceau R, Tremblay FW. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respir Viruses. 2010;4:171-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Grijalva CG, Zhu Y, Simonsen L, Mitchel E, Griffin MR. The population impact of a large school-based influenza vaccination campaign. PLoS One. 2010;5:e15097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Conway JM, Tuite AR, Fisman DN, Hupert N, Meza R, Davoudi B, English K, van den Driessche P, Brauer F, Ma J. Vaccination against 2009 pandemic H1N1 in a population dynamical model of Vancouver, Canada: timing is everything. BMC Public Health. 2011;11:932. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Fléchelles O, Fowler R, Jouvet P. H1N1 pandemic: clinical and epidemiologic characteristics of the Canadian pediatric outbreak. Expert Rev Anti Infect Ther. 2013;11:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Rousset D, Bouscambert-Duchamp M, Enouf V, Valette M, Grog I, Caro V, van der Werf S, Lina B. Épidémie de grippe A(H1N1)2009 en France: les paramètres virologiques. Bulletin Epidémiologique Hebdomadaire. 2010;24-25-26:272-274. |
| 32. | Meunier I, Embury-Hyatt C, Stebner S, Gray M, Bastien N, Li Y, Plummer F, Kobinger GP, von Messling V. Virulence differences of closely related pandemic 2009 H1N1 isolates correlate with increased inflammatory responses in ferrets. Virology. 2012;422:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Song MS, Pascua PN, Choi YK. Virulence of pandemic (H1N1) 2009 influenza A polymerase reassortant viruses. Virulence. 2012;2:422-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Camp JV, Chu YK, Chung DH, McAllister RC, Adcock RS, Gerlach RL, Wiemken TL, Peyrani P, Ramirez JA, Summersgill JT. Phenotypic differences in virulence and immune response in closely related clinical isolates of influenza A 2009 H1N1 pandemic viruses in mice. PLoS One. 2013;8:e56602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Antón A, Marcos MA, Martínez MJ, Ramón S, Martínez A, Cardeñosa N, Godoy P, Torner N, De Molina P, Isanta R. D225G mutation in the hemagglutinin protein found in 3 severe cases of 2009 pandemic influenza A (H1N1) in Spain. Diagn Microbiol Infect Dis. 2010;67:207-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Kendirli T, Demirkol D, Yildizdas D, Anil AB, Asilioğlu N, Karapinar B, Erkek N, Sevketoğlu E, Dursun O, Arslanköylü AE. Critically ill children with pandemic influenza (H1N1) in pediatric intensive care units in Turkey. Pediatr Crit Care Med. 2012;13:e11-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Yung M, Slater A, Festa M, Williams G, Erickson S, Pettila V, Alexander J, Howe BD, Shekerdemian LS. Pandemic H1N1 in children requiring intensive care in Australia and New Zealand during winter 2009. Pediatrics. 2011;127:e156-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M. Critically ill children during the 2009-2010 influenza pandemic in the United States. Pediatrics. 2011;128:e1450-e1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Farias JA, Fernández A, Monteverde E, Vidal N, Arias P, Montes MJ, Rodríguez G, Allasia M, Ratto ME, Jaén R. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 2010;36:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Shibata S, Khemani RG, Markovitz B. Patient origin is associated with duration of endotracheal intubation and PICU length of stay for children with status asthmaticus. J Intensive Care Med. 2014;29:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Government of Canada Publications. Fluwatch. Ottawa (Canada). 2010. [accessed 2016 Feb 1]. Available from: http://publications.gc.ca/site/eng/9.507424/publication.html. |
| 42. | Bulletin des Groupes Régionaux d’observation de la grippe 2010. Paris (France). 2010. [accessed 2016 Feb 1]. Available from: http://www.grog.org/cgi-files/db.cgi?code=330&action=bulletin_grog. |
| 43. | Brien S, Kwong JC, Buckeridge DL. The determinants of 2009 pandemic A/H1N1 influenza vaccination: a systematic review. Vaccine. 2012;30:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 44. | Nguyen T, Henningsen KH, Brehaut JC, Hoe E, Wilson K. Acceptance of a pandemic influenza vaccine: a systematic review of surveys of the general public. Infect Drug Resist. 2011;4:197-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 45. | Vaux S, Brouard C, Fuhrman C, Turbelin C, Cohen JM, Valette M, Enouf V, Caillère N, George S, Fonteneau L. Dynamique et impact de l’épidémie A(H1N1)2009 en France métropolitaine, 2009-2010. Numéro thématique - Épidémie de grippe A(H1N1)2009: premiers éléments de bilan en France. Bulletin Epidémiologique Hebdomadaire. 2010;24-25-26:259-264. |
| 46. | Helferty M, Vachon J, Tarasuk J, Rodin R, Spika J, Pelletier L. Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009. CMAJ. 2010;182:1981-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | InFACT a global Initiative. Toronto (Canada): Canadian Critical Care Trials Groups. 2010. [accessed 2013 Oct 24]. Available from: http://www.infactglobal.org/. |