Copyright
©The Author(s) 2016.
World J Clin Pediatr. Aug 8, 2016; 5(3): 311-318
Published online Aug 8, 2016. doi: 10.5409/wjcp.v5.i3.311
Published online Aug 8, 2016. doi: 10.5409/wjcp.v5.i3.311
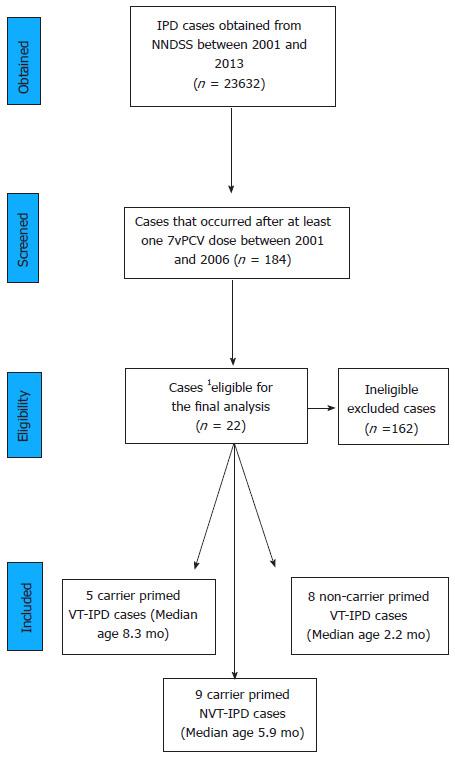
Figure 1 Flowchart showing the selection process of analysed cases and their median ages.
1Australian non-Indigenous immunocompetent infants with receipt of first dose of 7vPCV after diphtheria, tetanus and acellular pertussis vaccine. NNDSS: National Notifiable Diseases Surveillance System; 7vPCV: 7-valent pneumococcal conjugate vaccine; VT-IPD: Vaccine type invasive pneumococcal disease; NVT-IPD: Non-vaccine type invasive pneumococcal disease.
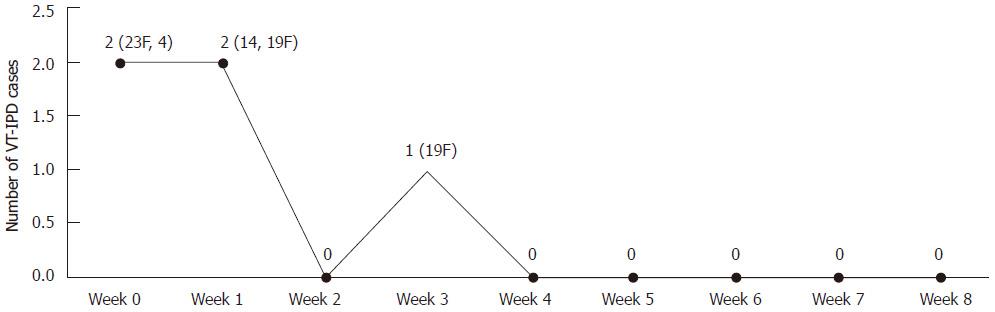
Figure 2 Number of vaccine type invasive pneumococcal disease cases 9 wk after a single carrier primed dose of the 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006).
VT-IPD: Vaccine type invasive pneumococcal disease.
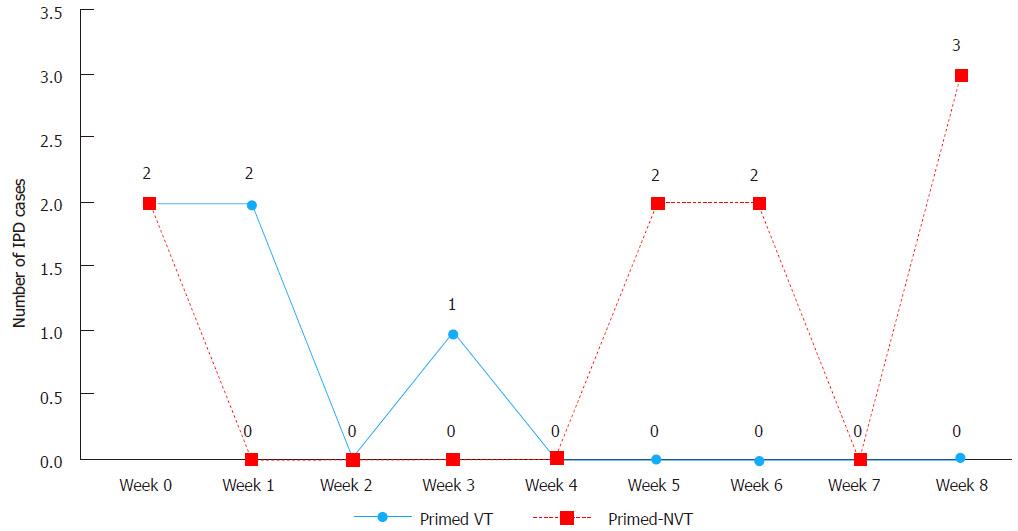
Figure 3 Number of vaccine type invasive pneumococcal disease compared to non-vaccine type invasive pneumococcal disease cases 9 wk after single carrier primed dose of 7-valent pneumococcal conjugate vaccine among non-Indigenous Australian infants (2001-2006).
IPD: Invasive pneumococcal disease; VT: Vaccine type; NVT: Non VT.
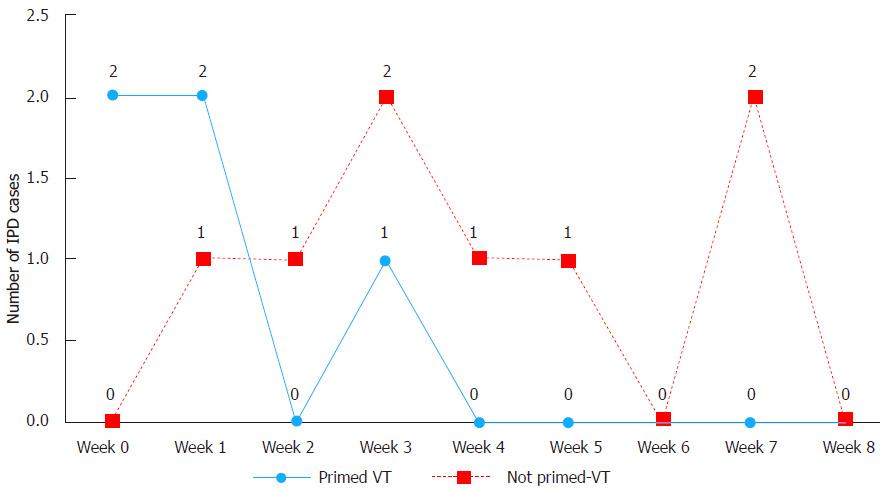
Figure 4 Number of vaccine type invasive pneumococcal disease cases 9 wk after a single carrier primed dose of 7-valent pneumococcal conjugate vaccine compared to the vaccine type invasive pneumococcal disease cases after non-carrier primed single 7-valent pneumococcal conjugate vaccine dose among non-Indigenous Australian infants (2001-2006).
IPD: Invasive pneumococcal disease; VT: Vaccine type.
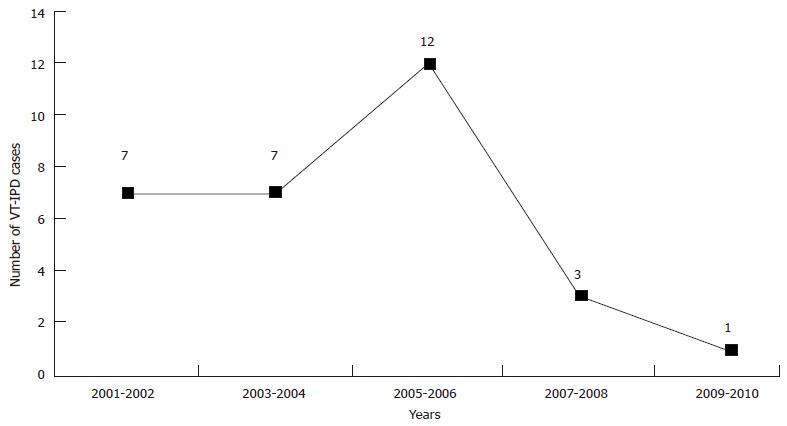
Figure 5 Number of vaccine type invasive pneumococcal disease cases among non-Indigenous Australian in children aged < 2 mo (2001-2010).
VT-IPD: Vaccine type invasive pneumococcal disease.
- Citation: Tashani M, Jayasinghe S, Harboe ZB, Rashid H, Booy R. Potential carrier priming effect in Australian infants after 7-valent pneumococcal conjugate vaccine introduction. World J Clin Pediatr 2016; 5(3): 311-318
- URL: https://www.wjgnet.com/2219-2808/full/v5/i3/311.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v5.i3.311