Published online May 20, 2015. doi: 10.5321/wjs.v4.i2.56
Peer-review started: December 6, 2014
First decision: January 8, 2015
Revised: March 19, 2015
Accepted: April 10, 2015
Article in press: April 12, 2015
Published online: May 20, 2015
Salivary glands are complex in nature. They could be either tubulo acinar, merocrine or exocrine glands secreting mainly saliva. Salivary gland is one of the main soft tissue structures in the maxillofacial area. Saliva is a clear, slightly acidic muco serous fluid that coats the teeth, mucosa and thereby helps to create and maintain a healthy environment in the oral cavity. Salivary glands may be affected by a number of diseases: local and systemic and the prevalence of salivary gland diseases depend on various etiological factors. The glands may be infected by viral, bacterial, rarely fungal or its ductal obstruction which may cause painful swelling or obstruction, affecting their functions. The salivary gland may also be affected by a various benign and malignant tumours. This review article briefly describes about the various salivary gland disorders, diagnostic techniques and their management including the recent advances and the future perspective.
Core tip: The aim of this article was to analyse detailed aspects of various salivary gland disorders, their diagnostic and therapeutic advances in the prevention and management of salivary gland diseases of the oral cavity, including the recent developments and their future perspective.
- Citation: Krishnamurthy S, Vasudeva SB, Vijayasarathy S. Salivary gland disorders: A comprehensive review. World J Stomatol 2015; 4(2): 56-71
- URL: https://www.wjgnet.com/2218-6263/full/v4/i2/56.htm
- DOI: https://dx.doi.org/10.5321/wjs.v4.i2.56
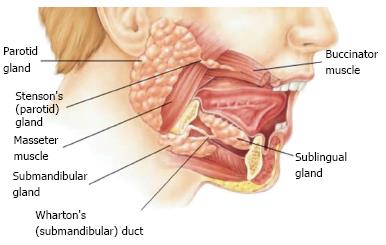
A gland consists of specialized type of cells, wherein they produce products which are used elsewhere in the body. Salivary glands are complex, tubulo acinar, exocrine or merocrine glands secreting mainly saliva. Saliva is the product of the major and minor salivary gland dispersed throughout the oral cavity. It is a complex mixture of organic, inorganic components and water, carrying out several functions. There are three pairs of major salivary glands namely parotid, sub mandibular and sublingual glands in addition to numerous minor salivary glands in the oral cavity[1].
The development of the parotid gland starts from 4-6th week, the submandibular gland at 6th week and the sublingual gland including minor salivary glands develops at 8-12 wk of embryonic life. The various developmental stages are: Bud formation, Epithelial cord formation, Branching and glandular differentiation, canalization and cyto differentiation. The parotid is ectodermal while the submandibular and sublingual glands are endodermal in their origins. The parotid represents the largest of the salivary gland which is situated between the external acoustic meatus between the ramus of the mandible and sternocleidomastoid muscle. Each gland is encapsulated and is composed of fat tissue and cells that secrete mainly the serous fluids. The major duct of each parotid gland is called Stensen’s duct which opens into the vestibule of the mouth opposite the crown of the upper second molar tooth. The parotid gland being primarily serous in secretion secretes watery serous saliva[2].
The submandibular glands are located along the side of the lower jawbone in the anterior part of digastric triangle. Each gland has a major duct called Wharton’s duct which opens on the floor of the mouth, on the summit of sublingual papilla at the side of frenulum of the tongue. Each of these glands is covered by a capsule which gives off mixed serous and mucous secretion in nature. The sublingual glands are the smallest of the major salivary glands which lies above mylohyoid and below the mucosa of the floor of the mouth. They are not covered by a capsule and are therefore more dispersed throughout the surrounding tissue. Their secretions are drained by many small ducts known as Rivinus’s ducts that exit along the sublingual fold at the floor of the mouth. Sometimes, few anterior ducts may join to form a common duct called Bartholin’s duct, their secretion being mixed in nature which empties into Wharton’s duct. The sublingual and minor salivary glands are primarily mucous in nature.
Salivary glands can be classified according to size as major and minor glands. The major salivary glands are of three pairs namely the parotid, submandibular and sublingual glands are shown in Figure 1. There are a numerous minor glands present in labial, buccal, glosso palatine, palatine and lingual areas in the oral cavity.
Based upon the type of secretion salivary glands may be predominantly serous, mucous or mixed depending on the type of secreting cells. Parotid and Von Ebners glands are purely serous while minor salivary glands like glosso palatine, palatine and anterior lingual glands are purely mucous. The mixed types of salivary glands are submandibular, sublingual, labial, buccal and posterior lingual glands.
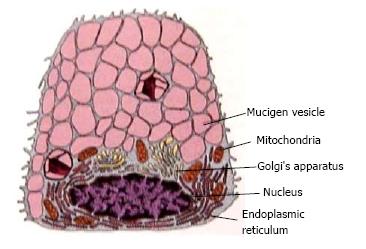
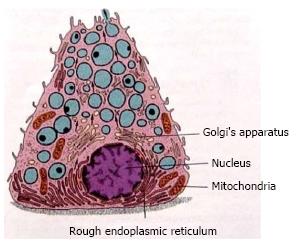
Each gland has the secretory unit which is mainly composed of acinus, myoepithelial cells, intercalated duct, striated and excretory ducts. The acinus could be serous, mucous or mixed. These acini contain amylase granules in serous and granules with mucin in mucous glands and are responsible for producing primary secretion is shown in Figures 2 and 3. The secretory granule in mixed salivary glands contains serous demilunes, capping mucous acinar cells (Demilunes of Gianuzzi or Heidenham) producing sero mucous saliva. The ductal system of the salivary gland has a varied network. The three classes of ducts are intercalated, striated, and excretory each with different structure and function.
Saliva: It is mainly secreted and produced by the salivary gland. The total volume of saliva secreted daily in an adult person is 600-1000 mL out of which 60% is secreted by the submandibular glands, 30% by the parotid, 5% by the lingual and 7% by the minor salivary glands with a pH in the range of 6.0-7.0. However, the salivary secretion is a reflex action arising from the salivary centres dependent on afferent stimulation. The sublingual and minor salivary glands spontaneously secrete saliva though the bulk of this secretion is nerve mediated. The normal average salivary flow rate ranges from 0.1-0.3 mL per minute[3].
Saliva is mainly composed of the following components. Electrolytes like sodium, potassium, chloride, bicarbonate, calcium, magnesium, phosphate, thiocynate, and fluoride. Secretory proteins/Peptides like Amylase, proline rich proteins, mucins, histatin, cystatin, peroxidase, lysozyme, lactoferrin, glycoproteins, lysozyme, defensins, and cathelicidin LL37. They also contain secretory immune globulins-(IgA), IgG, IgM, organic components like, glucose, amino acids, urea, uric acid, and lipid molecules. The other components that are present are epidermal growth factors, epithelial cells, insulin, cyclic adenosine monophosphate, binding proteins and serum albumin. In addition, biologically active peptides such as leptin, ghrelin and endothelin which are identified in saliva are of supreme importance to general health and also oral health in particular[4-9]. Functions: saliva mainly helps in lubrication for the movement of oral tissues against each other and the food, aids in digestion, in taste perception, neutralises by its buffering action the bacterial acids and thereby promotes remineralisation by reducing dissolution of enamel by inhibition of calcium phosphate precipitate. The saliva over all protects the teeth and the oral mucosa by the presence of immunoglobulin’s tissue repair factors and antibacterial system[4].
Oral diagnostic approaches to the patients with salivary gland disorders: (1) Past and Present History: to enquire about the history of patient who had undergone any surgery/radiotherapy, or have any underlying systemic problems/the patient is under any medications, etc. A thorough medical history and physical examination are also essential; and (2) Clinical Examination; a study by Navazesh suggests four clinical measures to diagnose the hypo function in the salivary gland. They are dryness of the lips and buccal mucosa, absence of saliva produced by the gland, bimanual palpation, and DMFT scores[5].
For evaluations of Dry mouth and a salivary mass or enlarged salivary gland, the following diagnostic approached may be applied: (1) Imaging of salivary glands; (2) Sialography; (3) Special imaging; (4) Sialochemistry; and (5) Biopsy and culture.
Salivary gland disorders: (1) Developmental-Aplasia, Atresia, Aberrancy; (2) Functional Disorders-Xerostomia, Sialorrhea (Ptyalism); (3) Inflammatory-infectious conditions; acute and chronic bacterial infection; Sialadenitis, Viral infection; Mumps, Human immunodeficiency virus associated salivary gland disorder; Post irradiation Sialadenitis, chronic sclerosing Sialadenitis, cheilitis glandularis; (4) Traumatic/Obstructive-Mucocele, salivary duct cyst (mucose retention cyst, Ranula), Nicotinic stomatitis, Sialolithiasis; (5) Autoimmune-Sarcoidosis, Sjogrens syndrome, Mikulicz’s disease; (6) Neurological-Frey’s syndrome; (7) Degenerative-idiopathic Sialolithiasis; (8) Non inflammatory non neoplastic-Sialadenosis; (9) Vascular-Necrotizing sialometaplasis; (10) Neoplastic-Benign: Papillary Cystadenoma Lymphomatosum, Pleomorphic Adenoma, BasalCell Adenomas, Oncocytoma, Canalicular Adenoma, Myoepithelioma, Sebaceous Adenoma, and Ductal Papilloma. Malignant: Adenoid Cystic Carcinoma, Hyalinising Clear Cell Carcinoma, Mucoepidermoid Carcinoma, Acinic Cell Carcinoma, Adeno carcinoma, Carcinoma[3,6]; and (11) Classification of Salivary Gland Tumours according to WHO 2005 is listed in Table 1.
| Epithelial tumors |
| Benign epithelial tumors |
| Pleomorphic adenoma |
| Myoepithelioma |
| Basal cell adenoma |
| Oncocytoma |
| Canalicular adenoma |
| Warthins tumors |
| Cystadenoma |
| Papillary cyst adenoma |
| Mucinous cyst adenoma |
| Benign sebaceous neoplasm |
| Sebaceous adenoma |
| Sebaceous lymphadenoma |
| Ductal papilloma |
| Intraductal |
| Inverted ductal |
| Sialadenoma papilliferum |
| Malignant epithelial tumors |
| Mucoepidermoid carcinoma |
| Acinic cell carcinoma |
| Adenoid cystic carcinoma |
| Polymorphous low grade adenocarcinoma |
| Epithelial myoepithelial carcinoma |
| Clear cell carcinoma |
| Basal cell adenocarcinoma |
| Oncocytic carcinoma |
| Myoepithelial carcinoma |
| Adenocarcinoma NOS |
| Carcinoma ex pleomorphic adenoma |
| Metastatising pleomorphic adenoma |
| Carcinosarcoma |
| Salivary duct carcinoma |
| Cyst adenocarcinoma |
| Low grade cribriform cystadenocarcinoma |
| Sialoblastoma |
| Malignant sebaceous tumor |
| Sebaceous adenocarcinoma |
| Sebaceous lymphadenocarcinoma |
| Squamous cell carcinoma |
| Mesenchymal tumors |
| Benign |
| Haemangioma |
| Haemangiopericytoma |
| Lipoma |
| Neurofibroma |
| Schwannoma |
| Malignant |
| Fibrosarcoma |
| Malignant fibrous histiocytoma |
| Liposarcoma |
| Malignant lymphoma |
| Metastatic tumor |
Atresia is the congenital occlusion or absence of salivary ducts which leads to xerostomia or mucous retention cyst.
Aplasia is the complete absence of one or more salivary gland which leads to xerostomia, and affected patients are more susceptible to dental caries. This condition could be an isolated finding or associated with other disorder like hemi facial microsomia or Treacher Collins syndrome. Recent studies suggest that mutation in fibroblast growth factor 10 (FGF10) affecting the FGF receptor signalling, has been linked with this condition[7]. Enamel hypoplasia, extensive occlusal wear of teeth or congenital absence of teeth are other oral manifestations of salivary agenesis. However the treatment is supportive.
Aberrancy: it is an anatomic variant wherein the normal salivary gland develops at an abnormal position. Sometimes they are found adjacent to lingual surface of the mandible within a depression. Ex: Staphne’s bone cyst or Staphne’s bone cavity: It is thought to be created by an ectopic portion of salivary gland tissue which causes remodelling of the mandibular bone. This creates an apparent cyst like radiolucent area seen on the radiographs[8]. It appears below the inferior alveolar nerve canal in the posterior region of the mandible.
This lesion is not discovered during routine examination, as it causes no symptoms and do not require intervention. However, surgical intervention is recommended in atypical regions in which the diagnosis is unclear and a tumor is suspected.
Xerostomia: It is defined as the subjective sensation of oral dryness that may or may not be associated with a reduction in salivary output. The condition may be transient, prolonged or permanent depending upon the duration of the condition.
Aetiology: Temporary causes are: (1) Psychological causes due to anxiety and depression; (2) Drug therapy-Drugs that exert anti-anticholinergic and decrease the volume of serous saliva are: anticholinergic ex:atropine, anti-hypertensive ex: reserpine, methyldopa, antihistamine ex: diphenhydramine, antidepressant: amitryptiline, antipsyschotics: diazepam, anti parkinsonian drugs: procyclidine, anti-emetics: hyoscine and antispasmodics: tizandine. Drugs that exert sympathomimetic action and produce more viscous mucinous saliva with less volume are: Nasal decongestants, appetite suppressants, bronchodilators, and amphetamines. Some drugs may also exert their neural effects in higher centres of the brain, by stimulation of adreno receptors in the frontal cortex that can produce inhibitory effects on salivary nuclei’s; (3) Duct calculi: a blockage of the duct of a major salivary gland (submandibular) can produce dryness on the affected side with pain and swelling in the gland on stimulation. If left untreated it can cause progressive fibrosis of the gland and permanent xerostomia; (4) infections; Sialadenitis is the inflammation of the salivary gland, acute infections like mumps and post-operative parotitis, chronic conditions like swellings related to nutritional deficiency, and iodine hypersensitivity, wherein in all these conditions causes hypo salivation[3,9].
Permanent causes: (5) Salivary gland aplasia, Sjogrens syndrome: causes dry eyes, dry mouth and often associated with rheumatoid arthritis. Other systemic disorders like diabetes mellitus, Parkinson’s disease, cystic fibrosis, sarcoidosis, vitamin A, riboflavin, nicotinic acid deficiencies and in anaemia’s; (6) Surgery or trauma to the ducts may also impair secretion; and (7) Radiotherapy: hypo salivation occurs on exposure of major salivary glands to radiation bilaterally in head and neck cancer. At radiation doses > 3000 cGy, the patient is at risk if all major glands are in the field of radiation. Irreversible effects occur at a dose of 6000 cGy for 5 wk. Radiation causes acinar cell atrophy and fibrosis, changes in vascular connective tissue and neurologic function. The degree of salivary gland alteration depends on dose volume factor, patient age, and time of exposure to radiation. Serous acini are affected before mucous acini resulting in thick viscous secretion. Depending on the amount of salivary tissue in the field, xerostomia may resolve within 6 mo and sometimes may be permanent. There can also be changes in salivary composition, decreased secretory IgA and buffering capacity with increased magnesium, calcium, potassium and sodium chloride in post radiotherapy cases[9-11].
Signs and symptoms: Lips are often cracked, peeling and atrophic; Buccal mucosa may be corrugated and pale: (1) Tongue may be smooth and reddened, cracked or fissured, with loss of papillation; (2) Increase in erosion and caries, particularly decay on root surfaces and even cusp tip involvement; (3) Erythematous form of candidiasis is frequent; (4) Lipstick sign: occurrence of shed epithelial cells on the labial surfaces of maxillary anterior teeth as the mucosa adheres to the teeth due to reduced saliva; (5) Tongue blade sign: when held against buccal mucosa, the tissue adheres to the tongue blade as it is lifted away; (6) Viscous sticky saliva with difficulty in speaking and swallowing; (7) Halitosis, altered taste and smell, gingivitis; (8) Complaint of burning mucosa, lips or tongue; (9) Ulceration of oral mucosa; (10) No accumulation of saliva in the floor of the mouth; (11) Poorly fitting prosthesis; and (12) Enlargement of salivary glands.
Xerostomia associated problems are: Dental Caries, Dry mouth, Dysgeusia, Dysphagia, oral Candidiasis, and Bacterial infections[12].
Treatment of xerostomia associated problems: Dental caries; use of fluorinated dentifrice (0.05% NaF)/fluoride gel in the concentration of 1% NaF, 0.4% Stannous fluoride application of 0.5% sodium fluoride varnish to teeth, regular use of re mineralising tooth paste. Dental examination every 6 mo and bitewing radiograph once a year for early diagnosis of dental caries. The recent advances in chair side diagnostics test kits are GC Salivary check-Buffer Kit that identifies, measures, and assesses patient for caries risk based on saliva conditions like hydration, consistency, pH of resting saliva and flow, and buffering capacity of stimulated saliva[13]. GC Saliva Check Mutans Kit is another chair side diagnostic kit used for rapid detection of high levels of S.mutans without the need for incubation is possible within 15 min. In a study, Gopinath et al[14] evaluated the effect of salivary testing in dental caries assessment using salivary testing kit (GC Asia Dental Pvt Ltd, Japan) and recommended adopting this test in patients with high caries risk.
A similar study conducted by Wennerholm et al[15] compared Saliva-Check Mutans and Saliva-Check IgA Mutans with the Cariogram for caries risk assessment and the data suggested that the combination of Saliva-Check Mutans and Saliva-Check IgA Mutans could be used for caries risk assessment.
Kanehire et al[16] aimed to develop a simple screening technique for the diagnosis of hypo salivation by estimation of capsaicin-stimulated salivary flow using filter paper. Five spots containing starch and potassium iodide on filter paper with or without capsaicin and a colouring reagent was designed in this assay system. The study suggested that this test would be useful for evaluating the retained functional ability of salivary glands and screening of hypo salivation with dry mouth.
Dry Mouth should be hydrated regularly using water or lozenges with citric acid to stimulate salivation, artificial salivary substitutes, lubricants such as lanolin based product Vaseline, olive oil, vitamin E or lip balm, oral gels such as oral balance, Dry mouth gel (GC Asia Dental Pvt Ltd, Japan) which can be applied on buccal and lingual surfaces of teeth and oral mucosa which can be applied any time during the day as needed. Even mouthwashes and sprays, sugar free gums, mints water or ice chips are recommended[6,12,17]. Sialogogues like pilocarpine 5 mg 3 times a day, cevimeline 30 mg 3 times a day, bromhexine, bethanecol, and anethole trithione are prescribed. Use of Salivary substitute’s solutions mainly containing electrolytes stimulates natural saliva, example Salivart, Oralube, Xerolube, Plax may also be recommended.
Application, in children 1 spray whereas in adult 2-3 sprays should be directed into the back of the mouth and tongue for the relief of dry mouth symptoms. The characteristics features that these substitutes possess are that they have electrolytes and pH similar to saliva and low viscosity allowing electrolytes particularly calcium to travel through matrix of saliva substitute which helps in remineralisation process. Mucin containing saliva orthana and Glycerate polymer are also suggested for xerostomia. There are studies suggesting the role of acupuncture therapy for improvement in salivation as a treatment option for patients responding to muscarinic agonists[17].
Measuring biofilm activity is possible by using recently introduced simple chair side adenosine triphosphate (ATP) bioluminescence test, CariScreen (Oral BioTech, Albany, Ore) the caries susceptibility test to assess cariogenic bacterial activity and their levels in caries free and caries active patients in about 15 s measurement with a meter[18].
Sialadenitis is an inflammation condition affecting the salivary glands. Parotid salivary glands are most commonly affected in adolescents and in children, debilitated adults, or patients with medication on tricyclic antidepressants and tranquilizers.
Aetiology: The main etiologic factors for sialadentis can be either infectious or non-infectious factors. Bacterial and viral agents can cause of sialadenitis. Bacterial sialadenitis is caused because of retrograde spread of infection secondary to decreased salivary flow or ductal obstruction. Decreased salivary flow can be secondary to medications, dehydration or debilitating conditions. Ductal obstruction can be due to sialolithiasis, strictures within the ductal system and common in submandibular salivary glands or due to pressure effect from adjacent tumors.
Staphylococcus aureus is the most common etiologic agent for acute bacterial parotitis in addition Staph.Pyogenes, Strep. Viridians and other microorganisms can also cause sialadenitis. Viruses causing sialadenitis include paromyxo viruses (mumps-most common), Coxsackie virus, cytomegalo virus, etc. The patient may present with fever and dehydration[19]. Clinical features: clinically there is sudden pain at the angle of the jaw which is unilateral with glandular enlargement and tender to palpation with purulent discharge over Stensens duct.
Treatment includes administration of salivary stimulants, antibiotics and surgical drainage.
Acute postoperative parotitis: Aetiology is a form of sialadenitis which occurs after a major surgical procedure where in the patient depends only on intravenous fluids. In addition these patients are on atropine which is a pre anaesthetic medication for drying the secretions and this may contribute to dryness of mouth and subsequent inflammation of parotid salivary glands. Non-infectious causes of salivary gland inflammation are sarcoidosis and Sjogrens syndrome.
Clinical features: Parotid gland is the most common salivary gland involved in acute bacterial sialadentitis. The patient presents with painful, usually bilateral swelling of the parotid salivary glands with low grade fever. In addition the patient may also complain of difficulty in opening the mouth. On clinical examination the skin over the parotid region may be inflamed and intra orally purulent discharge may be observed from parotid duct. Treatment-The condition usually resolves in about 48 h. However, symptomatic treatment is recommended.
Mumps is an acute paramyxo virus induced infection of parotid salivary glands. Aetiology- It is a contagious infection spreading through airborne droplets or direct contact of saliva. The peak incidence of mumps is reported during winter and spring season[20]. Clinical features: The infectivity of the mumps virus ranges from 3 to 4 d after the onset of the disease[21,22]. During the prodromal phase of the disease, the patient may complain of low grade fever, muscle pain, head ache and malaise[20] followed by unilateral or bilateral enlargement of parotid salivary glands associated with pain which is severe during mastication. The inflammation of the salivary gland starts reducing by the end of 1st week and the patient returns to normal by 10 d[21]. Epididymoorchitis, Oophoritis, pancreatitis and acute meningitis are the complications of mumps[21]. Treatment is symptomatic and Mumps vaccination (MMR) may decrease the incidence of this infection and considered as preventive measure.
Chronic recurrent parotitis: The proposed etio pathogenesis for this disorder include, congenital[23,24] and acquired factors like ductal obstruction secondary to inflammation infection and autoimmune diseases[25,26].
Chronic sclerosing sialadenitis: Also known as Kuttner’s tumour was identified by Kuttner in 1896. Aetiology-The condition is a chronic inflammatory reaction secondary to ductal obstruction and subsequent salivary stasis. However, salivary flow obstruction is proposed to be the main factor in the pathogenesis of this disorder[27].
Clinical feature: Clinically the condition presents as a painful, hard swelling of submandibular salivary gland. The pain and swelling may be present for a variable duration of time. The differential diagnosis includes chronic sialadenitis, sialolithiasis, and benign lymphoepithelial lesions. Treatment-The condition is managed by surgical excision of the involved gland and chances of recurrence of the lesion or changing into malignancy is found to be rare[27].
Hepatitis C virus associated sialadenitis: Aetiology-hepatitis C virus (HCV) is found to affect the salivary glands and cause the glandular inflammation. Clinical feature: The affected patients may present with mild swelling of the parotid gland with minimum or no symptoms of dry eyes and dry mouth[27,28]. The diagnosis of HCV is by the detection of HCV DNA and anti HCV antibodies. Treatment- Hepatitis associated sialadenitis is treated symptomatically[29].
Human immunodeficiency virus infection: In Human immunodeficiency virus (HIV) infected patients salivary gland lesions commonly occur which may be neo plastic or non-neoplastic in nature. AIDS related tumours such as lymphoma and Kaposis sarcoma and a Sjogrens syndrome like condition occurs in these patients and are described as “HIV salivary gland disease” (HIV-SGD) is considered to be due to reactivation of a latent virus. Various studies have expressed the strong association between salivary gland dysfunction seen in HIV affected patients and Human Cytomegalovirus (CMV) saliva. Clinical feature: The condition is characterised by xerostomia with unilateral or bilateral salivary gland enlargement with reduced tear production. Diagnosis is by biopsy of the major gland which shows the presence of hyperplastic lymph nodes with lymphocytes and cystic cavities obtained from patients affected from HIV-SGD[6,28]. Treatment-Administration of oral sialagogues/frequent sipping of water are recommended for xerostomia. Anti-retroviral therapy may be administered for the management of HIV. Rarely radiotherapy and parotidectomy may be beneficial in advanced condition.
Iodine 131 indiced sialadenitis: Aetiology-high dose of oral Iodine 131 in treatment of thyroid carcinomas can adversely affect the salivary glands leading to sialadenitis. The incidence of acute salivary gland inflammation range from 24%-67% and that of chronic salivary gland inflammation range from 11%-43%[29-31]. Clinical feature: The patients present with pain and swelling of the salivary glands with or without dry mouth condition[32]. Treatment- Administration of oral sialagogues/oral hydration, serotonin receptor blocker and dexamethasone are recommended.
Sialadenosis: Sialadenosis also known as sialosis is an enlargement of salivary glands which is non-inflammatory and non-neoplastic more commonly affecting the parotid salivary glands.
Etiology: This condition can be associated with: Endocrine disorders: (1) Diabetes mellitus and insipidus; (2) Accromegaly; (3) Hypothyroidism; and (4) Pregnancy. Nutritional status: (1) Anorexia nervosa; (2) Bulimia; (3) Chronic alchoholism; and (4) General malnutrition. Medication induced sialadenosis[33]: (1) Psychotropic medications; (2) Antihypertensive drugs; and (3) Sympathomimetic drugs. Clinical features- Patient presents with a slowly progressing bilateral (rarely unilateral) swelling of parotid salivary glands which may be asymptomatic[34]. Rarely patients may complain of reduced salivary flow. Treatment-Management of underlying systemic condition may help in reversing the sialdenosis.
Aetiology-They is caused due to rupture of a salivary gland duct mostly due to trauma resulting in spillage of mucin into the surrounding tissues. Clinical features: Clinically a mucocele appear as bluish thin walled lesion which is fluctuant, and the most common site of occurrence is on the lower lip. Ranula: is a special type of mucocele which grows in the floor of the mouth, usually unilateral and is called due to its similar appearance to enlarged abdomen region of a frog. Treatment-in case of superficial recurrent or deep mucoceles, surgical intervention is indicated while large ranulas are treated by marsupialization. A study by Wilcox et al[6] recommends intra lesional corticosteroids administration before surgery.
Nicotinic stomatitis: Aetiology-The long standing habits of tobacco and or alcohol/hot liquid consumption. Clinical feature: Exhibits whitened areas of the hard palate due to hyperkeratosis caused by the thermal irritation. This irritation also causes inflammation and dilatation of the duct openings of the minor salivary glands of the palate manifesting as red patches or spots on a white background[1]. Treatment- discontinuation of the habits reverses the condition back to normal.
Aetiology-is an autoimmune chronic granulomatous inflammatory condition which causes destruction of the tissue by T lymphocytic, mononuclear phagocytic infiltration and granuloma formation[6]. The parotid salivary glands are affected in 10%-30% of cases. Clinical feature: The patient presents with a hard, bilateral enlargement of the parotid gland usually asymptomatic in nature. Sarcoidosis of parotid glands along with uveitis and facial nerve paralysis is termed as Heerfordt’s syndrome or uveo parotid fever[35]. The patient may complain of dry mouth and minor salivary gland biopsy confirms the diagnosis. Treatment- palliative treatment primarily relieving of the symptoms of salivary component of sarcoidosis is advised.
Corticosteroid or with Chloroquine has been recommended. Immunosuppressive and immune modulatory medications are administered in patients who do not respond the corticosteroids.
Sjogrens syndrome: Aetiology-is an autoimmune disorder associated with HLA-DR3 AND HLA-B8. The disease was described by Henric Sjogren in 1933. Clinical feature: The primary Sjogren syndrome/sicca complex exhibit dry eyes and mouth. The secondary Sjogren syndrome develops SLE, polyarteritis nodosa, polymyositis, rheumatoid arthritis and in scleroderma.
This condition is most commonly seen in women over 40 years with male: female in the ratio of 1: 10.
Sjogrens syndrome case definition[6,34] requires at least 2 out of the following 3 criteria as mentioned in Table 2. Laboratory findings: Anti salivary duct antibodies, anti-nuclear antibodies, rheumatoid factor increased ESR, Lip biopsy-lymphocytes around salivary glands. The other tests are Schirmer test, Rose Bengal dye test, Sialography and sialochemistry[6]. Treatment-to limit the harmful effects of the disease especially the ocular and oral conditions, symptomatic relief of administration of artificial tears, saliva substitutes, fluoride applications and oral hygiene measures are suggested[6,34].
| Positive serum anti-SSA and/or anti-SSB or (positive rheumatoid factor and ANA ≥ 1:320) |
| Ocular staining score ≥ 3 |
| Presence of focal lymphocytic sialadenitis with focus score ≥ 1 focus/4 mm2 in labial salivary gland biopsies |
Mikulicz’s disease: Aetiology-Mikulicz’s disease of unknown aetiology was first reported by Johann von Mikulicz-Radecki in 1888. However, it has been demonstrated that autoimmune, viral, and genetic factors may contribute to the pathogenesis of the disease. Clinical feature: Patients suffering from Mikulicz’s disease present with asymptomatic, bilateral swelling of the parotid, and submandibular salivary glands along with lacrimal glands. This disease closely resembles Sjogren’s syndrome. However the lacrimal and salivary secretion depletion is very minimal in Mikulicz’s disease. Histologically the disease resembles Sjogren’s syndrome, but lacks the characteristic anti-SS-A and anti-SS-B antibodies of Sjogren’s syndrome. Studies have found increased levels of IgG4 antibodies in the serum of patients with Mikulicz’s disease. Treatment- Mikulicz’s disease is very much responsive for steroid therapy particularly to[35] methylprednisolone.
Frey’s syndrome also known as Auriculo temporal syndrome which is characterized by sweating in the pre auricular and temporal areas after gustatory stimulation.
Aetiology-the condition most commonly caused due to faulty regeneration of sympathetic and parasympathetic nerve fibres which were injured during parotid tumor surgery or ramus resection. Clinical feature: Post-surgery the parasympathetic fibres start innervating the sweat glands and vasculature of the skin around the parotid area. The symptoms usually appear within few minutes of the start of mastication or during stimulation of saliva and may remain up to 30 min after discontinuing mastication. The diagnosis of the syndrome can be confirmed by starch iodine test[36]. Treatment- Reassurance to the patient is advocated in most of the cases. Intra cutaneous injection of botulin toxin is found to be effective in severe condition and Tympanotomy[23] may be the treatment of choice with severe symptoms.
Sialolithiasis-is a condition of unknown aetiology. However, there could be several coexisting causes leading to the salivary stone formation. Some of these cofactors may be related to disturbed pH of saliva, abnormalities in the sphincter mechanism related to salivary duct opening and abnormal calcium metabolism[5,6]. Clinical Feature: This condition most often will not produce any signs and symptoms. Rarely, it may cause complete ductal obstruction, pain and swelling of the salivary glands. Treatment- Large salivary stone are managed by extracorporeal or intracorporal lithotripsy[6,17] procedure.
Sialadenosis is a non-infectious, non-inflammatory gland enlargement usually affecting the parotid bilaterally. This condition is most often seen in women causing salivary hypo salivation which can occur due to systemic disorders[6,12].
Necrotizing sialometaplasia: Aetiology-The probable cause could be due to vascular infarction of the salivary gland lobules and is often mistaken for oral cancer[37]. Vascular compression is caused by a necrotic myocutaneous reconstruction of the flap used in palatal surgeries and embolization from carotid endarterectomises, Berger’s disease, Raynaud’s phenomenon. Predisposing factors are dental injections, ill-fitting denture, traumatic injury, previous surgery and upper respiratory tract infections. Clinical feature: appears as a non-neoplastic lesion that usually arises from a minor salivary gland in the lips, posterior part of the palate, and retro molar regions. Treatment: The condition is self-limiting and the healing of the lesion normally takes about 6-8 wk.
Benign: Pleomorphic Adenoma, Papillary Cystadenoma Lymphomatosum (warthins tumor), Basal Cell Adenomas, Oncocytoma,Canalicular Adenoma, Myoepithelioma, Sebaceous Adenoma, Ductal Papilloma.
Malignant: Adenoid Cystic Carcinoma, Hyalinising Clear Cell Carcinoma, Mucoepidermoid Carcinoma, Acinic Cell Carcinoma, Adeno carcinoma, Pleomorphic Adenoma, Lymphoma[5,6,12].
Oral diagnostic approaches to the patients with salivary gland disorders: (1) Imaging of salivary glands: Salivary gland is one of the main soft tissue structures in the maxillofacial area. Imaging is useful in identifying the masses of salivary glands and also in differentiating them from the masses/pathologies of adjacent cervical spaces, especially para pharyngeal, masticator, submental spaces and mandibular lesions. Conventional radiography has a very limited role in the diagnosis of salivary gland pathology which includes plain radiography. It aids in identifying mainly salivary stones and calcifications. Gland plain radiography like in postero anterior skull projection with cheeks blown out to delineate the parotid duct and submandibular gland radiography includes lateral oblique radiograph with mouth wide open; and (2) Sialography was used as the sole imaging technique before the advent of advanced imaging techniques which include ultrasonography, elastography, computed tomography, scintigraphy, and magnetic resonance imaging. Sialography, an imaging technique of salivary gland, uses contrast medium to delineate the ductal system of salivary glands. Due to use of contrast medium this technique is not suitable and is contraindicated in acute conditions of salivary glands[38]. However sialography is found to be useful in assessment of salivary gland dysfunction secondary to obstructive disorders of the gland[39].
Studies have suggested other various diagnostic methods-magnetic resonance (MR) sialography is a non-invasive technique useful in evaluating the hypo functioning of salivary glands. Sialo endoscopy assist in detecting ductal anomalies that may not be[40] possible to detect by means of either traditional or new imaging techniques.
Sialography, Sialoendoscopy, and MR Sialography are indicated for evaluation of the ductal system of the salivary glands.
Ultrasonography, computed tomography, magnetic resonance imaging is helpful in assessment of the parenchyma of the salivary glands[41]. However; all these diagnostic aids have their own limitations in the diagnosis of salivary gland lesions.
Ultrasonography: Ultrasound examination of salivary glands with a high resolution transducer is found to be a highly sensitive, a non-invasive method for salivary gland evaluation[42]. It is a cost effective imaging tool which displays high definition images useful in evaluating the superficial structures particularly the peripheral areas of the affected salivary gland. High frequency linear probes of 7.5-12 MHz are used in imaging of salivary glands[43]. In acute conditions such as acute radiation induced sialadenitis, the gland appears swollen and show anoechic appearance on ultrasonography[44]. In a recent clinical study ultrasonography was found useful in diagnosing lymph node and salivary gland enlargement in submandibular region and suggested that it also helps in identifying the salivary glandular tissue in accessory salivary gland and salivary calculi[45].
Shock-wave lithotripsy: Shock-wave lithotripsy is a non-invasive diagnostic tool suggested for the management of sialolithiasis. Iro et al[46] in 1989 introduced the application of extracorporeal shock-wave lithotripsy (ESWL) in the management of salivary gland. Sialolithotripsy helps in removing salivary stones into smaller particles and thereby removal by flushing action is possible from the salivary duct system or after salivation induced by citric acid or other sialagogues. The shock-waves are generated extra-corporeally by using Piezoelectric and electromagnetic techniques or intra-corporeally using electro-hydraulic, pneumatic or laser endoscopic devices[46].
Sonoelastography: Elastography is an ultrasonography technique which measures the tissue elasticity in vivo. This imaging technique measures the elasticity of the glandular parenchyma and is useful in evaluating the hypo function of saliva especially in post radiation hypo function of salivary glands[41].
Computed tomography: Computed tomography (CT) scans of the salivary glands are useful in delineate the extent of the lesion and its relation to adjacent structures[47]. Multi detector CT scans help in characterizing tumours of salivary glands like Warthin tumor which demonstrates peak enhancement of signals after administration of contrast agents which is not found in other tumors of salivary glands. However CT scans perform poorly in characterizing the histopathologic nature of the tumors. CT scans help in differentiating the benign and malignant neoplasms of salivary glands. The irregular tumor margin and surrounding tissue infiltration is the characteristic feature of malignancy[48]. However studies have found overlap of CT scan characteristics between malignant and benign lesions. Apart from tumor identification CT scan also aids to view dystrophic calcifications in salivary glands.
CT sailography: Interpretation of sailography findings depend on the imaging technique used to acquire sailography images. Traditionally plain radiographs were used for assessment of salivary glands after injection of the contrast medium. Introduction of CT and MRI scans in maxillofacial imaging have shifted the focus from plain radiography to these advanced imaging techniques. However CT sialography may have limited applications due to the accessibility and cost factors. Moreover the prolonged image acquisition time of CT scans may jeopardize the viewing of CT contrast medium uptake[49]; (3) Special imaging; cone beam computed tomography (CBCT): some of the limitations of CT sialography have been addressed by use of CBCT technology with sialography. A study reported the usefulness of CBCT in demonstrating the secondary structures of submandibular salivary glands in comparison with plain radiography coupled with sialography. The same study reported that the effective dose from CBCT scans were comparable to that of plain radiography when a smaller field of view (FOV) was used[50].
Magnetic imaging resonance (MRI) scans are useful in assessment of salivary glands. The wide variety of soft tissue signals differences and multi planar image acquisition have made MRI an effective imaging modality for assessment of salivary gland tumors. This imaging modality is helpful in assessment of tumors affecting the deep lobes of parotid glands, skull base invasion of the tumours of salivary glands, evaluation of recurrent pleomorphic adenomas and much more[51]. Also high resolution MRI scans delineate the intra parotid course of facial nerve which is an important landmark for surgeons operating on parotid glands[52].
Magnetic resonance sialography-Major limitations of conventional sialography include use of iodine based contrast agents and inability of the contrast agent in overcoming the strictures within the ductal system of the salivary gland which in turn prevent the visualization. These limitations can be overcome by switching on to MR sialography which uses patients own saliva as a contrast medium. MR sialography also demonstrates the actual ductal diameter due to non-use of contrast agents[53].
Scintigraphy-Salivary gland scintigraphy uses Tc-99m pertechnetate which helps in assessment of salivary gland dysfunction in disorders like Sjogrens syndrome. This technique is valuable in assessment of xerostomia[54].
The minimally invasive techniques for preserving the glandular tissue which are currently being used in the management of obstructive salivary disease are sialoendoscopy, shockwave lithotripsy, interventional radiology, endoscopically video-assisted trans-oral and surgical retrieval of stones, and botulinum toxin therapy. Three dimensional reconstruction imaging (MR sialographic) and MR virtual endoscopy have recently been suggested for salivary gland ducts studies on par with their applications in medical field[55].
Emerging imaging based diagnostics: Positron emission tomography (PET) scan: A PET scan focuses for areas of high cellular activity suggesting a sign of cancer growth. It also helps to diagnosed cancer, and to assess its spread to lymph nodes or any other parts of the body. This test requires an injection of a very small quantity of radioactive substance usually a type of sugar known as FDG, which will be excreted by the body later in a day. As cancer cells growth is faster in the body, they absorb more of the radioactive sugar. After about an hour, the patient is moved onto a table and made to lie for about 30 min. Meanwhile a special camera captures a picture of areas of radioactivity in provide helpful information about whole body. It is also possible to take a PET and CT scan at the same time (PET/CT scan). This enables the doctor compare areas of higher radioactivity on the PET scan with the more detailed picture of that particular area on the CT scan[56]; (4) Sialochemistry and Sialometry: Sialochemistry deals with chemical analysis of saliva whereas Sialometry is concerned with measuring salivary flow rates and these two measurements of saliva helps in assessment of functioning of salivary glands. The normal volume of the saliva produced by both the major and minor salivary glands constitutes around 600 to 1000 mL per day[57]. This volume varies in different individuals and it may alter in different systemic conditions.
Sialometry can be in relation to whole saliva or gland specific saliva. Whole saliva is a mixture of salivary gland secretions, non salivary secretions including serum transudates, gingival crevicular fluid, food debris and oral microbes[58]. Most often clinicians assess the salivary gland functions through collection of whole saliva. This method is easy to perform and does not require any special equipment. However, whole saliva analysis is of limited value due to its low sensitivity in detecting gland specific dysfunction and gland specific changes in salivary chemical composition[59]; and (5) Salivary gland biopsy or fine needle aspiration (FNA) helps to determine whether the tumor is benign or malignant. In some cases this type of biopsy can help a clinician to avoid unnecessary surgery. Incisional biopsy; is a type of biopsy sometimes preferred if the FNA biopsy does not get a large enough sample to examine. For salivary gland tumors these types of biopsies are not done often. Surgery can both provide enough of a sample for a diagnosis and treat the tumor at the same time[6,17].
WHO classification of salivary glands neoplasm is listed in Table 1
Salivary gland neoplasm-Salivary gland cancers include tumors of different patho histologic characteristics and biological behaviour. The most prevalent salivary gland tumors[6,60-62] are: (1) Benign Condition: Pleomorphic Adenoma, Papillary Cystadenoma Lymphomatosum (Warthins Tumor), Basal Cell Adenomas, Oncocytoma; and (2) Malignant tumors: Muco Epidermoid Carcinoma, Adenoid Cystic Carcinoma. Salivary gland neoplasms according to study report represent less than 3% of all tumors.
Prevalence: The tumors can arise in about 80% in parotid gland, 15% in submandibular gland and 5% in the sublingual and minor salivary gland. 65% of submandibular, 50% of minor salivary gland and 20% of sublingual gland tumors are benign[6,12,60]. Aetiology: of the salivary gland neoplasm is not known. However, certain environmental factors and abnormalities are implicated. Environmental factors such as radiation, viruses, extensive use of tobacco and their products, molecular changes and genetic factors are considered as the causative factors. Clinical features: Subjects with benign tumor of parotid gland present with a unilateral, asymptomatic swelling of the involved gland and rarely suffer from pain, difficulty in swallowing and extrusion of fluid from the ears. The benign tumor of other types of salivary glands also present as asymptomatic mass of the affected gland without compromising the functions of the individual.
Malignant tumor of the salivary glands may also present as asymptomatic mass and in advanced stages may cause pain and mucosal/skin ulceration. One third of patients with parotid gland malignancy most often present with facial nerve paralysis[60]. The signs of malignancy in a previous benign tumor of parotid gland can be a sudden increase in the size of the mass, with facial nerve paralysis and shows ulceration of the skin overlying the parotid mass[61].
Pleomorphic adenoma: this tumor has many names-Mixed tumor, Endothelioma, etc. which was termed by Willis. In 90% of the cases the tumors affects the parotid gland, most often present in lower pole of superficial lobe of the gland. It occurs more frequently in females than in males between 4-6 decade with average of 43 years. Clinical features: The lesion presents as small, painless quiescent nodules which slowly begin to increase in size, sometimes showing intermittent growth. Surgical excision is the treatment of choice. Treatment: Based on factors like the high recurrence rate, the patient’s age, and extensiveness of resection, XRT may be a useful therapy for this type of tumor.
Papillary cystadenoma lymphomatosum (warthins tumor): is the most common tumor in salivary glands first recognised by Albrecht in 1910 and later in 1929 it was described by Warthins. Clinical features: The tumor occurs mainly in parotid, seen over 60 years of age with the sex prediction is male to female 5:1 ratio. Clinically seen bilateral in 6%-12% of patients as painless lesion unless it is secondarily affected[6,12]. Treatment is mostly by surgical excision.
Basal cell adenomas: Clinical features: a benign salivary gland adenoma constitute to about 1%-2% of the salivary adenomas occurring mostly in the parotid gland and upper lip of the minor salivary gland. The other types of fewer occurrences of benign salivary adenomas are Canalicular Adenoma, Myo Epithelioma, Ductal Papilloma, and Sebaceous Adenomas. Oncocytoma is another benign tumor particularly affecting the parotid bilaterally seen in both men and women[6,61-63]. Treatment is by conservative surgical excision.
Muco epidermoid carcinoma is the most common malignant tumor of the salivary gland mostly affecting the parotid gland and these accounts for 5% of salivary gland tumor. Clinical features: This tumor also affects minor salivary gland in 15% of these cases. They are seen in the age group of 40-50 years with female predilection. The tumor is classified as low grade or high grade depending on the ratio of epidermal cells to mucous cells. In this type of tumor the most common cytogenic abnormality is the recurrent translocation between chromosomes 11 and 19 to form CRTCI-MAML2 fusion protein[34,61]. Treatment is the surgical excision of the tumor with post-operative radiotherapy[61].
Adenoid cystic carcinoma accounts for 30% of tumors in minor salivary glands and 6% affecting the parotid gland. Clinical features: It occurs in the middle and older individuals. The tumor has the ability to infiltrate the nervous tissue and spread along the nerve pathways. Biomarkers of epithelial to mesenchymal transition (EMT) such as Snail and Slug appear to be helpful in the diagnosis of adenoid cystic carcinoma[62,64-68].
Treatment is the radical surgical excision followed by Photon beam radiotherapy has shown to be effective.
Adenocarcinoma is the tumor which takes its origin from epithelium of the salivary duct[62]. This group of salivary gland tumors includes specific lesions, like polymorphous low grade adenomacarcinoma, salivary duct carcinoma, Cribiform adenoma carcinoma, etc. These tumors present a painful swelling of the affected gland and are very rare in occurrence. Management of these tumors depends on the histologic type of the tumors.
Cribriform adenocarcinoma of the tongue and minor salivary glands (CATMSG) is a low grade salivary gland tumor affecting the minor salivary glands of the oral cavity. This tumor was earlier described by Michal et al[64] in 1999 under the name Cribriform Adenocarcinoma of the tongue. In later years studies suggested its origin to be renamed and considered as Cribriform Adenocarcinoma of minor salivary glands a distinct neoplasm[64].
Hyalinizing clear cell carcinoma (HCCC) is a rare, unique low grade tumor affecting minor salivary gland. Milchgrub et al[65] in 1994 first described this tumor which exhibited nests, cords, trabeculae and eosinophilic cells in a hyalinised stroma. Clinical features: It primarily arises in the oral cavity but has been described at all salivary gland and seromucous gland sites. Dardick extensively studied under electron microscope, and after re-examination the features of HCCC it was confirmed that it is a squamous lesion[65]. Treatment: the various salivary gland tumors exhibit different histo biological features. In benign tumors no other treatment is usually needed. But when the lesion spreads beyond, treatment of malignant salivary neoplasms depends on the appropriate diagnosis, histologic findings, the clinical stage/condition at presentation and the more recent is the considerations of genetic factor[66,67]. At present the treatment approach is towards conservative elective surgical procedures, combined with the application of postoperative irradiation and chemotherapy[6,17,48,60]. A clinician should have a thorough knowledge of the subject, also be aware of their recent advancements, and work with the group of associated specialists in the management of salivary gland disorders. By following the required, appropriate, systematic diagnostic procedures it helps the clinician to establish a definitive diagnosis and finally assesses the potential for treatment.
Metastatic malignant salivary gland neoplasms: Studies suggests that polymorphous low grade adenocarcinoma, adenoid cystic carcinoma and muco epidermoid carcinoma of the salivary glands[67] are found to have increased metastatic potential. Adenoid cystic carcinoma[68] has been found to metastasize to lungs, bones, skeletal muscles and skin.
Intensity modulated radiotherapy (IMRT) for head and neck cancer has partial parotid sparing effect which reduces the intensity of post radiotherapy xerostomia[66].
Exposure of salivary glands to Ionizing radiation cause damage to the secretory apparatus of the glands causing xerostomia which could be avoided by the use of any one of the presently available[67] techniques: (1) Shielding of one or more salivary glands form radiation-During radiotherapy for the tumors of parotid gland and areas outside the oral cavity, radio protecting shield can be used to protect the major salivary glands. Shielding may not be feasible in radiotherapy for midline lesions, cancer of oropharynx and larynx due to the position of the cancer and the alignment of the radiotherapy port. Use of conformational dose delivery techniques-The 3 dimensional imaging techniques like CT scans provide for accurate and precise delivery of radiation to the affected tissues with no or minimal damage to the surrounding normal structures. These radiotherapy techniques helps in minimizing the radiation induced xerostomia; (2) Stimulation of acinar cells prior to Radiotherapy-Administration of salivary stimulants like Pilocarpine before each radiotherapy session is found to reduce the complication of diminished salivary flow. However in radiation dose above 50 Gy this beneficial effect is reduced; (3) Use of salivary sparing agents during radiotherapy-Use of agents like Amifostine and heat shock proteins during radiotherapy for head and neck cancer helps in protecting the salivary glands against radiation induced damage; (4) Transplantation of the salivary gland away from the radiation filed-A few studies have reported the beneficial effects of transplanting the major salivary gland away from the radiation filed with maintenance of the ductal connection; and (5) Advanced methods like gene therapy for repairing the damaged acinar cells, injecting the stored pre radiotherapy salivary cells after the completion of radiotherapy, inducing the hematopoietic stem cells to differentiate into salivary acinar cells and thereby replacing the damaged cells and fabricate artificial salivary tissues from donor tissues and introducing them in place of damaged glands using tissue engineering techniques help in restoring the functions of salivary glands and reduce the complications of reduced salivary flow[68-75].
In the oral cavity the presence of multifarious microbial flora exhibits more than many hundreds of microbial species which have been identified so far. Advance microbial research has thrown open to much more new insights and saliva has become the major source to a library of information, and the biomarkers represent the disease and health status of the oral cavity[69-72].
Saliva is a fluid that can be easily collected and contains locally and systemically derived markers of oral disease[68]. The term “salivaomics” was coined in 2008 to reflect the rapid development of knowledge about the various “omics” constituents of saliva. Salivaomics includes five diagnostic alphabets proteins, mRNAs, miRNAs, metabolic compounds, and microbes offers substantial advantages because disease states may be accompanied by detectable changes in one, but not all, dimensions[69]. Human salivary proteome analysis is important for understanding oral health and disease pathogenesis.
Metabolomics is the global assessment and validation of endogenous small-molecule metabolites within a biologic system that has gained increasing popularity and significance in life sciences[70]. Analysis of these key metabolites in body fluids has become an important role to monitor the state of biological organisms and is a widely used diagnostic tool for disease. Metabolomics provides potential advantages that classical diagnostic approaches do not, based on the discovery of clinically relevant biomarkers that are affected by the disease[71-80].
Increase in the incidence of oral cancer has prompted research in salivary biomarkers for oral cancer. More than 100 different salivary biomarkers for oral cancer have been identified. A review on salivary biomarkers for oral cancer categorized this vast variety of salivary biomarkers under different groups which include: (1) Non-organic compound biomarkers, e.g., sodium, calcium, magnesium; (2) Peptide or protein biomarkers, e.g., P53 autoantibody, alpha amylase, etc.; (3) DNA, RNA and microRNA biomarkers, e.g., P53 gene codon 63, IL 8, miR-125a, etc.; (4) Metabolomic biomarkers, e.g., Valine, lactic acid, etc.; and (5) Miscellaneous biomarkers, e.g., Telomerase activity[63,81-95].
Salivary biomarkers are also used for assessment of caries risk. DNA based methods like DNA hybridization, mono clonal antibody (MAb) technique, 16S rRNA/ rDNA, gene cloning and genomic sequencing or T-RFLP methods of analysis help in identification and cariogenic microbial taxonomy using saliva without the need for culture methods[62,73,77,80].
PCR based identification techniques allow for accurate measurement of cariogenic microbiota. Salivary diagnostics suggests a new diagnostic tool for the detection and quantification of oral pathogens directly from its liquid state without the need for isolation of bacterial cells. In children low salivary levels of alpha defensins HNP1-3 may represent biological factor that contributes to caries susceptibility while salivary IgA antibody responses to streptococci mutants can be observed in early childhood[73].
Salivary epithelial cells are found to secrete proteins into blood stream which has led to research on the duacrine function of salivary glands. This function of salivary epithelial cells is being researched as a potential target site for in situ gene transfer producing proteins for treating several systemic disorders[74,82].
Saliva, the fluid bathing the oral cavity, is one of the important secretions in the human body. One of the main functions of saliva is digestion of complex carbohydrates and lipids. Technological advancements in the field of diagnostics have opened new avenues to understand the other important and far reaching functions of saliva. The constituents of saliva, also known as biomarkers, act as an index for underlying systemic disease ranging from infections to malignancies.
Salivary glands are surrounded by a rich network of vasculature allowing the biomarker constituents of blood to enter salivary acinus and finally into the salivary secretions. Biomarker is defined as an objectively measured and evaluated indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to therapeutic intervention.
Biomarkers can be in the form of proteins, carbohydrates, lipids or microorganisms. Change in the constitution of these biological molecules may reflect the status of underlying disease processes and can aid in diagnosis, management, evaluating the prognosis and monitoring the outcome of the condition[75]. Biomarkers in saliva have the potential to be used for screening purposes in epidemiological studies.
Matias I, Gatta-Cherifi B, in their study were able to quantify endocannabinoids in human saliva as potential and useful biomarker of obesity[76,96]. Two major forms of Ghrelin (GAH) a recently identified peptide hormone in saliva shows their decrease levels in salivary samples in obese type 2 diabetic patients.
The levels of mRNAs regulating the metabolism of endocannabinoids, N-acylethanolamines and of cannabinoid type 1 [CB (1)] receptor, were assessed in human salivary glands. The study helps in further understanding of the physiopathological mechanisms leading to type 2 diabetes and obesity.
There are numerous investigative tools to identify and quantify the type and load of microbes in the oral cavity. Most of these tools are based on microbial culture methods for identifying disease specific pathogens. Biomolecular microarray based diagnostics (quantitative 16S rRNA gene sequencing, terminal restriction fragment length polymorphism analysis, etc.) are advancements over the conventional culture methods. These methods, when combined with microbial culture techniques, help in enhancing the chances of accurate identification of pathogens[77]. Salivary fluid can also be used for detection of systemic infections. Saliva based enzyme-linked immunosorbent assay (ELISA) has shown promising results in detection of HIV pathogens with 99.3% sensitivity and 99.8% specificity. However positive test results are to be confirmed with western blot analysis. Other systemic infections which can be detected by salivary analysis include hepatitis A, B, C infections, malaria, Ebola, Dengue, CMV, EBV[77,92,93,97] and human herpes virus (HHV) infections. These infections are identified by assessing the viral load, viral antibodies and viral antigens in saliva. These diagnostic parameters are found to correlate well with their corresponding levels in serum. Leptin, is a cytokine identified in human saliva play a protective role in bacterial P. gingivalis infection[9,86] induced inflammatory responses. Another salivary component Ghrelin is found to have a countering effect on P. gingivalis induced impairment of mucin synthesis which plays a role in periodontal infections[61].
Molecular analysis of saliva employing next generation sequencing and human microbe identification micro array techniques have enabled the clinician to identify and characterize a large number of oral microbiota in diseases including Crohn’s disease, pancreatic cancer, oral cancer and obesity. In children suffering from Crohn’s disease, there is an overall decrease in diversity of oral microorganisms as compared to healthy children. Studies employing the advance microarray techniques report suggests overall significant reduction in Neisseria elongate and Streptococcus mitis species count in the saliva of patients with pancreatic cancer as compared to normal subjects[61,75].
Future research direction: Advances in the management of salivary gland tumours studies stress the need towards molecular targeted therapy of the unusual subpopulation of tumorigenic cancer cells which could arrest the recurrence and metastasis of the tumor. In this direction the cancer stem cell research needs to be further explored in the salivary gland tumors[78,98,99].
Recently a non-invasive, academic prototype chair side cancer diagnostic kit (GC America Inc.) has been devised by Wong DT for the early detection of cancer[79]. Newer field like Proteomics helps in the analysis of the salivary proteins which is extensively used in identification of a specific protein biomarker in saliva for diseases including AIDS, oral cancer, diabetes, periodontal disease and mammary gland carcinoma. The transudate of oral mucosa contains secretory immunoglobulin IgG, IgM and IgA, which serve as a valuable source for immunodiagnostic-based procedures. Using Point-of-care salivary diagnostic screening tests kit[79,92] it is possible to detect viruses in viral infectious diseases such as human papillomavirus (HPV), HCV and HIV.
Advanced Molecular Salivary tests for caries susceptibility may further aids in motivation and patient’s education, evidence based dentistry and also in determining effectiveness of anti-caries therapy or caries-control measures including community based services and caries vaccine[73,79]. Further advancements are now being focused at “Omic technologies”, which include genomics, proteomics, transcriptomics, and metabolomics have already set their mark in life science research studies[69,77,79]. These emerging technologies have shown to offer highly sensitive, specific, quick and affordable diagnostic test kits in future. Local drug delivery system is another interesting area with the advent of Nano medicine being used in pharmaceuticals industry and biomedical engineering field have shown promising results in future therapeutics. In cancer therapeutics, Nano particles, such as, semiconductor quantum dots, biodegradable micelles, iron oxide nano-crystals[78,81,82,94], are linked with bio targeting ligands, to aim at specific sites in malignant tumors, helpful in cancer therapeutics. Endothelin-1 is one of the probable salivary biomarkers for oral cancer has been reported[63,82,95] for early cancer detection. Dependability of saliva for early diagnosis of dengue disease especially useful in dengue endemic countries is awaited[96]. Salivary ghrelin plays an important protective role in chronic periodontitis and needs further research[86,97,98]. Salivaomics, the future of saliva-based techniques for early diagnosis of dental diseases, is promising. However, further long term studies are needed before these newer methods are adapted to routine clinical practice.
Conclusion: Saliva reflects the physiologic state of the body. Salivary gland diseases may be inflammatory, non-inflammatory, non-neoplastic or neoplastic lesions. Only when a definitive diagnosis is established, treatment depends upon the lesion size, cause, severity, extent and other clinical considerations of the disease. However, a thorough knowledge of the subject including their recent advancements together with a team of associated medical and dental specialists, it is possible to detect the diseases of salivary glands in their early stage and manage them more efficiently. Salivaomics, the future of saliva-based techniques for early diagnosis of dental diseases is promising. Saliva being readily available can be used as a diagnostic tool to help the clinicians for early detection of oral diseases like caries, periodontal disease, oral cancer, salivary gland disorders and non-oral diseases by adapting the advance noninvasive technique and technologies.
P- Reviewer: Ferri A, Kawamata H, Slomiany BL S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Balogh MB, Margret J, Fehrenbach . Dental Embryology, Histology and Anatomy. 2nd ed. Holland: Elsevier 2011; 161-168. [Cited in This Article: ] |
| 2. | Nanci A; Ten Cate’s Oral Histology: Development, Structure, and Function. 7th ed. USA: Mosby, 290-317. Holland: Elsevier; . [Cited in This Article: ] |
| 3. | Miletich I. Introduction to salivary glands: structure, function and embryonic development. Front Oral Biol. 2010;14:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (3)] |
| 4. | Schenkels LC, Veerman EC, Nieuw Amerongen AV. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med. 1995;6:161-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 222] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 5. | Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71:1363-1369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 209] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Margaret M. Grisius, Philip C. Fox Burkett’s Oral Medicine Diagnosis and treatment. 10th ed. London: Bc Decker Inc. Salivary Gland diseases 2003; 235-265. [Cited in This Article: ] |
| 7. | Entesarian M, Matsson H, Klar J, Bergendal B, Olson L, Arakaki R, Hayashi Y, Ohuchi H, Falahat B, Bolstad AI. Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat Genet. 2005;37:125-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Stene T, Pedersen KM. Aberrant salivary gland tissue in the anterior mandible. Oral Surg Oral Med Oral Pathol. 1977;44:72-75. [PubMed] [Cited in This Article: ] |
| 9. | Slomiany BL, Murty VL, Piotrowski J, Slomiany A. Salivary mucins in oral mucosal defense. Gen Pharmacol. 1996;27:761-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 100] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;8:25-47. [PubMed] [Cited in This Article: ] |
| 11. | Turner MD, Ship JA. Salivary secretion in health and disease-Salivary Diagnostics. 1st ed. USA: Wiley-Blackwell 2002; 60-68. [Cited in This Article: ] |
| 12. | Venkataraman BK, Iyengar AR. Diagnostic Oral Medicine. 1st ed. India: WoltersKluwer Pvt.Ltd 2013; 317-352. [Cited in This Article: ] |
| 13. | Maldupa I, Brinkmane A, Mihailova A. Comparative analysis of CRT Buffer, GC saliva check buffer tests and laboratory titration to evaluate saliva buffering capacity. Stomatologija. 2011;13:55-61. [PubMed] [Cited in This Article: ] |
| 14. | Gopinath VK, Arzreanne AR. Saliva as a Diagnostic Tool for Assessment of Dental Caries. AOS. 2006;1:57-59. [Cited in This Article: ] |
| 15. | Wennerholm K, Emilson CG. Comparison of Saliva-Check Mutans and Saliva-Check IgA Mutans with the Cariogram for caries risk assessment. Eur J Oral Sci. 2013;121:389-393. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 16. | Kanehira T, Yamaguchi T, Asano K, Morita M, Maeshima E, Matsuda A, Fujii Y, Sakamoto W. A screening test for capsaicin stimulated salivary flow using filter paper; a study for diagnosis of hypo salivation with a complaint of dry mouth. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2011;112:73-80. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 17. | Ship JA. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002;8:77-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Kutsch VK, Young DA. New directions in the etiology of dental caries disease. J Calif Dent Assoc. 2011;39:716-721. [PubMed] [Cited in This Article: ] |
| 19. | Ellies M, Laskawi R. Diseases of the salivary glands in infants and adolescents. Head Face Med. 2010;6:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (2)] |
| 20. | White SJ, Boldt KL, Holditch SJ, Poland GA, Jacobson RM. Measles, mumps, and rubella. Clin Obstet Gynecol. 2012;55:550-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Center for Disease Control and Prevention (CDC) Mumps. In: Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington DC: Public Health 2011; 205-214. [Cited in This Article: ] |
| 22. | Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella--vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1998;47:1-57. [PubMed] [Cited in This Article: ] |
| 23. | Chamisa I. Frey’s syndrome--unusually long delayed clinical onset post-parotidectomy: a case report. Pan Afr Med J. 2010;5:1. [PubMed] [Cited in This Article: ] |
| 24. | Grevers G. [Chronic recurrent parotitis in childhood]. Laryngorhinootologie. 1992;71:649-652. [PubMed] [Cited in This Article: ] |
| 25. | Zenk J, Constantinidis J, Kydles S, Hornung J, Iro H. [Clinical and diagnostic findings of sialolithiasis]. HNO. 1999;47:963-969. [PubMed] [Cited in This Article: ] |
| 26. | Hearth-Holmes M, Baethge BA, Abreo F, Wolf RE. Autoimmune exocrinopathy presenting as recurrent parotitis of childhood. Arch Otolaryngol Head Neck Surg. 1993;119:347-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 27. | Beriat GK, Akmansu SH, Kocatürk S, Ataoğlu O. Chronic Sclerosing Sialadenitis (Küttner’s tumour) of the Parotid Gland. Malays J Med Sci. 2010;17:57-61. [PubMed] [Cited in This Article: ] |
| 28. | Carrozzo M, Scally K. Oral manifestations of hepatitis C virus infection. World J Gastroenterol. 2014;20:7534-7543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 46] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Chow SM. Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid carcinoma. J HK Coll Radiol. 2005;8:127-135. [Cited in This Article: ] |
| 30. | Van Nostrand D, Neutze J, Atkins F. Side effects of “rational dose” iodine-131 therapy for metastatic well-differentiated thyroid carcinoma. J Nucl Med. 1986;27:1519-1527. [PubMed] [Cited in This Article: ] |
| 31. | Kita T, Yokoyama K, Higuchi T, Kinuya S, Taki J, Nakajima K, Michigishi T, Tonami N. Multifactorial analysis on the short-term side effects occurring within 96 hours after radioiodine-131 therapy for differentiated thyroid carcinoma. Ann Nucl Med. 2004;18:345-349. [PubMed] [Cited in This Article: ] |
| 32. | Silberstein EB. Reducing the incidence of 131I-induced sialadenitis: the role of pilocarpine. J Nucl Med. 2008;49:546-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Guggenheimer J, Close JM, Eghtesad B. Sialadenosis in patients with advanced liver disease. Head Neck Pathol. 2009;3:100-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Shiboski SC, Shiboski CH, Criswell L, Baer A, Challacombe S, Lanfranchi H, Schiødt M, Umehara H, Vivino F, Zhao Y. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken). 2012;64:475-487. [PubMed] [Cited in This Article: ] |
| 35. | Yamamoto M, Takahashi H, Ohara M, Suzuki C, Naishiro Y, Yamamoto H, Shinomura Y, Imai K. A new conceptualization for Mikulicz’s disease as an IgG4-related plasmacytic disease. Mod Rheumatol. 2006;16:335-340. [PubMed] [Cited in This Article: ] |
| 36. | Jahan-Tigh RR, Cohen PR. Frey syndrome in a patient with facial melanoma: auriculotemporal syndrome presenting with gustatory sweating following wide local excision, sentinel node biopsy, and superficial parotidectomy. J Clin Aesthet Dermatol. 2012;5:48-52. [PubMed] [Cited in This Article: ] |
| 37. | Hupp JR, Ellis E, Tucker MR. Contemporary oral and maxillofacial surgery. 5th ed. St. Louis, Mo: Mosby Elsevier 2005; 397-399. [Cited in This Article: ] |
| 38. | Rastogi R, Bhargava S, Mallarajapatna GJ, Singh SK. Pictorial essay: Salivary gland imaging. Indian J Radiol Imaging. 2012;22:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Jäger L, Menauer F, Holzknecht N, Scholz V, Grevers G, Reiser M. Sialolithiasis: MR sialography of the submandibular duct--an alternative to conventional sialography and US? Radiology. 2000;216:665-671. [PubMed] [Cited in This Article: ] |
| 40. | Kalinowski M, Heverhagen JT, Rehberg E, Klose KJ, Wagner HJ. Comparative study of MR sialography and digital subtraction sialography for benign salivary gland disorders. AJNR Am J Neuroradiol. 2002;23:1485-1492. [PubMed] [Cited in This Article: ] |
| 41. | Kałużny J, Kopeć T, Szczepanek-Parulska E, Stangierski A, Gurgul E, Ruchała M, Milecki P, Wierzbicka M. Shear wave elastography: a new noninvasive tool to assess the intensity of fibrosis of irradiated salivary glands in head and neck cancer patients. Biomed Res Int. 2014;2014:157809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Katz P, Hartl DM, Guerre A. Clinical ultrasound of the salivary glands. Otolaryngol Clin North Am. 2009;42:973-1000, Table of Contents. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 43. | Oeppen RS, Gibson D, Brennan PA. An update on the use of ultrasound imaging in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 2010;48:412-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Zenk JL. New therapy for the prevention and prophylactic treatment of acute radiation syndrome. Expert Opin Investig Drugs. 2007;16:767-770. [PubMed] [Cited in This Article: ] |
| 45. | Sridhar T, Gnanasundaram N. Ultrasonographic Evaluation of Salivary Gland Enlargements: A pilot Study. Inter J Dent Sci Res. 2013;1:28-35. [DOI] [Cited in This Article: ] |
| 46. | Iro H, Schneider T, Nitsche N, Waitz G, Ell C. [Extracorporeal piezoelectric lithotripsy of salivary calculi. Initial clinical experiences]. HNO. 1990;38:251-255. [PubMed] [Cited in This Article: ] |
| 47. | Casselman JW, Mancuso AA. Major salivary gland masses: comparison of MR imaging and CT. Radiology. 1987;165:183-189. [PubMed] [Cited in This Article: ] |
| 48. | Choi DS, Na DG, Byun HS, Ko YH, Kim CK, Cho JM, Lee HK. Salivary gland tumors: evaluation with two-phase helical CT. Radiology. 2000;214:231-236. [PubMed] [Cited in This Article: ] |
| 49. | Szolar DH, Groell R, Braun H, Preidler K, Stiskal M, Kern R, Kainz J, Moelleken S, Stammberger H. Ultrafast computed tomography and three-dimensional image processing of CT sialography in patients with parotid masses poorly defined by magnetic resonance imaging. Acta Otolaryngol. 1996;116:112-118. [PubMed] [Cited in This Article: ] |
| 50. | Jadu F, Yaffe MJ, Lam EW. A comparative study of the effective radiation doses from cone beam computed tomography and plain radiography for sialography. Dentomaxillofac Radiol. 2010;39:257-263. [PubMed] [Cited in This Article: ] |
| 51. | Freling NJ. [Imaging of the salivary glands. CT and MRI]. Radiologe. 1994;34:264-272. [PubMed] [Cited in This Article: ] |
| 52. | Chu J, Zhou Z, Hong G, Guan J, Li S, Rao L, Meng Q, Yang Z. High-resolution MRI of the intraparotid facial nerve based on a microsurface coil and a 3D reversed fast imaging with steady-state precession DWI sequence at 3T. AJNR Am J Neuroradiol. 2013;34:1643-1648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Karaca Erdoğan N, Altay C, Ozenler N, Bozkurt T, Uluç E, Dirim Mete B, Ozdemir I. Magnetic resonance sialography findings of submandibular ducts imaging. Biomed Res Int. 2013;2013:417052. [PubMed] [Cited in This Article: ] |
| 54. | Kang JY, Jang SJ, Lee WW, Jang SJ, Lee YJ, Kim SE. Evaluation of Salivary Gland Dysfunction Using Salivary Gland Scintigraphy in Sjögren’s Syndrome Patients and in Thyroid Cancer Patients after Radioactive Iodine Therapy. Nucl Med Mol Imaging. 2011;45:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Capaccio P, Torretta S, Ottavian F, Sambataro G, Pignataro L. Modern management of obstructive salivary diseases. Acta Otorhinolaryngol Ital. 2007;27:161-172. [PubMed] [Cited in This Article: ] |
| 56. | American Cancer Society Inc. How is salivary gland cancer diagnosed? Available from: http: //www.cancer.org/cancer/salivaryglandcancer/detailedguide/salivary-gland-cancer-diagnosis. [Cited in This Article: ] |
| 57. | de Almeida Pdel V, Grégio AM, Machado MA, de Lima AA, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9:72-80. [PubMed] [Cited in This Article: ] |
| 58. | Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, Floriano PN, Simmons G, Bhagwandin B, Jacobson JW. Current developments in salivary diagnostics. Biomark Med. 2010;4:171-189. [PubMed] [Cited in This Article: ] |
| 59. | Kalk WW, Vissink A, Spijkervet FK, Bootsma H, Kallenberg CG, Nieuw Amerongen AV. Sialometry and sialochemistry: diagnostic tools for Sjögren’s syndrome. Ann Rheum Dis. 2001;60:1110-1116. [PubMed] [Cited in This Article: ] |
| 60. | Lee WH, Tseng TM, Hsu HT, Lee FP, Hung SH, Chen PY. Salivary gland tumors: A 20-year review of clinical diagnostic accuracy at a single center. Oncol Lett. 2014;7:583-587. [PubMed] [Cited in This Article: ] |
| 61. | Adams A, Warner K, Nör JE. Salivary gland cancer stem cells. Oral Oncol. 2013;49:845-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Seethala RR. An update on grading of salivary gland carcinomas. Head Neck Pathol. 2009;3:69-77. [PubMed] [Cited in This Article: ] |
| 63. | Michal M, Kacerovska D, Kazakov DV. Cribriform adenocarcinoma of the tongue and minor salivary glands: a review. Head Neck Pathol. 2013;7 Suppl 1:S3-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Weinreb I. Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013;7 Suppl 1:S20-S29. [PubMed] [Cited in This Article: ] |
| 65. | Eisbruch A. Reducing xerostomia by IMRT: what may, and may not, be achieved. J Clin Oncol. 2007;25:4863-4864. [PubMed] [Cited in This Article: ] |
| 66. | Cardoso SV, Souza KC, Faria PR, Eisenberg AL, Dias FL, Loyola AM. Assessment of angiogenesis by CD105 antigen in epithelial salivary gland neoplasms with diverse metastatic behavior. BMC Cancer. 2009;9:391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Yurut-Caloglu V, Caloglu M, Ozyilmaz F, Saynak M, Cosar-Alas R, Karagol H, Bayir-Angin G, Uzal C. Lung, bone, skeletal muscles and cutaneous metastases from adenoid cystic carcinoma of the parotid gland: a case report and review of the literature. Med Oncol. 2007;24:458-462. [PubMed] [Cited in This Article: ] |
| 68. | Wong DT. Salivaomics. J Am Dent Assoc. 2012;143:19S-24S. [PubMed] [Cited in This Article: ] |
| 69. | Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76:2033-2041. [PubMed] [Cited in This Article: ] |
| 70. | Tonetti MS, D’Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911-920. [PubMed] [Cited in This Article: ] |
| 71. | Koneru S, Tanikonda R. Salivaomics-A promising future in early diagnosis of dental diseases. Dent Res J (Isfahan). 2014;11:11-15. [PubMed] [Cited in This Article: ] |
| 72. | Guo L, Shi W. Salivary biomarkers for caries risk assessment. J Calif Dent Assoc. 2013;41:107-109. [PubMed] [Cited in This Article: ] |
| 73. | Perez P, Rowzee AM, Zheng C, Adriaansen J, Baum BJ. Salivary epithelial cells: an unassuming target site for gene therapeutics. Int J Biochem Cell Biol. 2010;42:773-777. [PubMed] [Cited in This Article: ] |
| 74. | Yoshizawa JM, Schafer CA, Schafer JJ, Farrell JJ, Paster BJ, Wong DT. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26:781-791. [PubMed] [Cited in This Article: ] |
| 75. | Matias I, Gatta-Cherifi B, Tabarin A, Clark S, Leste-Lasserre T, Marsicano G, Piazza PV, Cota D. Endocannabinoids measurement in human saliva as potential biomarker of obesity. PLoS One. 2012;7:e42399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Wong DT, Salivary Diagnostics. 1st ed. USA: Wiley-Blackwell 2008; 111-156. [Cited in This Article: ] |
| 77. | Milano A, Longo F, Basile M, Iaffaioli RV, Caponigro F. Recent advances in the treatment of salivary gland cancers: emphasis on molecular targeted therapy. Oral Oncol. 2007;43:729-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 78. | Wei F, Wong DT. Point-of-care platforms for salivary diagnostics. Chin J Dent Res. 2012;15:7-15. [PubMed] [Cited in This Article: ] |
| 79. | Horgon RP, Kenny LC, SAC review: Omic technologies: genomics, transcriptomics, Proteomics and metabolomics. TOG. 2011;13:189-195. [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 80. | Rzymska-Grala I, Stopa Z, Grala B, Gołębiowski M, Wanyura H, Zuchowska A, Sawicka M, Zmorzyński M. Salivary gland calculi-contemporary methods of imaging. Pol J Radiol. 2010;75:25-37. [PubMed] [Cited in This Article: ] |
| 81. | Sreeja C, Shahela T, Aesha S, Satish MK. Taxonomy of salivary gland neoplasm. J Clin Diagn Res. 2014;8:291-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 82. | Sarath PV, Kannan N, Patil R, Manne RK, Swapna B, Suneel Kumar KV. Basal cell adenocarcinoma of the minor salivary glands involving palate and maxillary sinus. J Clin Imaging Sci. 2013;3:4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Redman RS. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem. 2008;83:103-130. [PubMed] [Cited in This Article: ] |
| 84. | Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71-109. [PubMed] [Cited in This Article: ] |
| 85. | Slomiany BL, Slomiany A. Role of leptin in modulation of Porphyromonas gingivalis lipopolysaccharide-induced up-regulation of endothelin-1 in salivary gland acinar cells. IUBMB Life. 2005;57:591-595. [PubMed] [Cited in This Article: ] |
| 86. | Slomiany BL, Slomiany A. Suppression by Ghrelin of Porphyromonas gingivalis-Induced Constitutive Nitric Oxide Synthase S-Nitrosylation and Apoptosis in Salivary Gland Acinar Cells. J Signal Transduct. 2010;2010:643642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Gibbins HL, Yakubov GE, Proctor GB, Wilson S, Carpenter GH. What interactions drive the salivary mucosal pellicle formation? Colloids Surf B Biointerfaces. 2014;120:184-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Gröschl M, Topf HG, Bohlender J, Zenk J, Klussmann S, Dötsch J, Rascher W, Rauh M. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin Chem. 2005;51:997-1006. [PubMed] [Cited in This Article: ] |
| 89. | Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57:675-687. [PubMed] [Cited in This Article: ] |
| 90. | Konturek JW, Bielanski W, Konturek SJ, Bogdal J, Oleksy J. Distribution and release of epidermal growth factor in man. Gut. 1989;30:1194-1200. [PubMed] [Cited in This Article: ] |
| 91. | Sarosiek J, Bilski J, Murty VL, Slomiany A, Slomiany BL. Role of salivary epidermal growth factor in the maintenance of physicochemical characteristics of oral and gastric mucosal mucus coat. Biochem Biophys Res Commun. 1988;152:1421-1427. [PubMed] [Cited in This Article: ] |
| 92. | Corstjens PL, Abrams WR, Malamud D. Detecting viruses by using salivary diagnostics. J Am Dent Assoc. 2012;143:12S-18S. [PubMed] [Cited in This Article: ] |
| 93. | Piacentini SC, Thieme TR, Beller M, Davidson SL. Diagnosis of hepatitis A, B, and C using oral samples. Ann N Y Acad Sci. 1993;694:334-336. [PubMed] [Cited in This Article: ] |
| 94. | Sarode SC. Aiming targeted therapy in oral cancer. J Dent Res Rev. 2014;2:1. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 95. | Cheng YS, Rees T, Jordan L, Oxford L, O’Brien J, Chen HS, Wong D. Salivary endothelin-1 potential for detecting oral cancer in patients with oral lichen planus or oral cancer in remission. Oral Oncol. 2011;47:1122-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 96. | Yap G, Sil BK, Ng LC. Use of saliva for early dengue diagnosis. PLoS Negl Trop Dis. 2011;5:e1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Aydin S. A comparison of ghrelin, glucose, alpha-amylase and protein levels in saliva from diabetics. J Biochem Mol Biol. 2007;40:29-35. [PubMed] [Cited in This Article: ] |
| 98. | Tang YL, Fan YL, Jiang J, Li KD, Zheng M, Chen W, Ma XR, Geng N, Chen QM, Chen Y. C-kit induces epithelial-mesenchymal transition and contributes to salivary adenoid cystic cancer progression. Oncotarget. 2014;5:1491-1501. [PubMed] [Cited in This Article: ] |
| 99. | Slomiany BL, Slomiany A. Ghrelin Protects against the Detrimental Consequences of Porphyromonas gingivalis-Induced Akt Inactivation through S-Nitrosylation on Salivary Mucin Synthesis. Int J Inflam. 2011;2011:807279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |