Published online Feb 28, 2016. doi: 10.5319/wjo.v6.i1.13
Peer-review started: September 1, 2015
First decision: November 27, 2015
Revised: December 18, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: February 28, 2016
Intratemporal facial nerve schwannoma (FNS) are rare benign tumors of the skull base. Many of these tumors will be detected during evaluation for symptoms suggestive of vestibular schwannoma. However, there are several signs and symptoms which can suggest the facial nerve as the origin of the tumor. Intratemporal FNS can be multiple, like “beads on a string”, or solitary lesions of the internal auditory canal. This variable tumor morphology necessitates multiple treatment options to allow patients the best chance of preservation of facial nerve function. Historically FNS were managed with resection of the nerve with cable grafting. However this leaves the patient with permanent facial weakness and asymmetry. Currently most patients find this outcome unacceptable, especially when they present with good to normal facial nerve function. Facial paralysis has a significantly negative impact on quality life, so treatment regimens that spare facial nerve function have been used in patients who present with moderate to good facial nerve function. Nerve sparing options include tumor debulking, decompression of the bony facial canal, radiosurgery, and observation. The choice of management depends on the degree of facial nerve dysfunction at presentation, hearing status in the affected ear, medical comorbidities and patient preference. Each treatment option will be discussed in detail and suggestions for patient management will be presented.
Core tip: The management of intratemporal facial nerve schwannoma (FNS) has changed over the past 15 years. Current management strategies involve tumor stripping, bony decompression, radiosurgery, and observation. Each of these treatment options are designed to minimize the risk of injury to a functional facial nerve. Complete surgical excision and cable grafting are reserved for tumors which have already resulted in severe facial weakness. Each management strategy will be discussed in detail with a management algorithm will be presented. Intratemporal FNS are unusual benign tumors affecting the facial nerve as it passes through the bony canal of the temporal bone. Previous management paradigms involved complete resection of the tumor and nerve with simultaneous cable grafting; however, patients were left with long term facial paresis. Newer treatment strategies resulting in less facial nerve morbidity have become more popular in the last 15 years including: Surgical debulking, stereotactic radiosurgery, bony decompression and observation. Each of these strategies will be discussed with emphasis on facial nerve outcomes and tumor control rates.
- Citation: Makadia L, Mowry SE. Management of intratemporal facial nerve schwannomas: The evolution of treatment paradigms from 2000-2015. World J Otorhinolaryngol 2016; 6(1): 13-18
- URL: https://www.wjgnet.com/2218-6247/full/v6/i1/13.htm
- DOI: https://dx.doi.org/10.5319/wjo.v6.i1.13
Facial nerve schwannoma (FNS) are rare benign tumors which can affect any part of the facial nerve (FN). Classically these tumors were treated with complete tumor resection and cable grafting of the residual nerve. However within the last 15 years the emphasis on preservation of FN function has become paramount. Various treatment modalities are available to this end: Observation, radiosurgery, debulking/tumor stripping surgery, or decompression of the facial canal. Physicians who manage these patients must understand the risks and benefits of the different treatment approaches and to understand their efficacy in managing FNS patients.
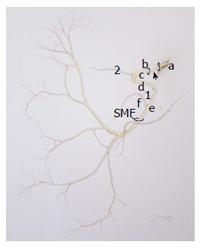
The FN, cranial nerve VII, has a complex anatomy and path. The FN can be divided into four segments: The brainstem nucleus and tracts, cisternal segment, intratemporal segment, and peripheral segment. The FN nucleus comprises the motor component whereas the nucleus ambuugus is the first order synapse for the sensory division of the nervus intermedius which runs with the main trunk of the FN. The cisternal segment begins as the nerve exits the brainstem and ends as the nerve enters the internal auditory canal (IAC). This segment has no epineurium. The intratemporal segment begins at the porus acousticus and travels through the bony facial canal to end at the stylomastoid foramen. The intratemporal nerve can be divided into 5 discrete segments: Fundal (IAC), labyrinthine, geniculate ganglion, tympanic and descending (mastoid) segments (Figure 1). The peripheral segment refers to the nerve as it exits the stylomastoid foramen and continues on to the face, where it innervates muscles of facial expression[1].
Tumors involving the intratemporal FN can be challenging to identify preoperatively. These tumors may have several symptoms in common with the more common vestibular schwannoma (VS) including sensorineural hearing loss, vertigo and imbalance, and tinnitus. While FN dysfunction is relatively uncommon in patients with VS (2%) this symptom is much more common in primary FN tumors. Symptoms suggesting possible FN origin also include lacrimal gland dysfunction and taste disturbance[2,3]. The classic motor symptoms for a FN tumor are recurrent FN weakness (frequently misdiagnosed as bell palsy), hemifacial spasm or slowly progressive facial weakness. These symptoms are not specific to FNS but common to all FN tumors including hemangioma.
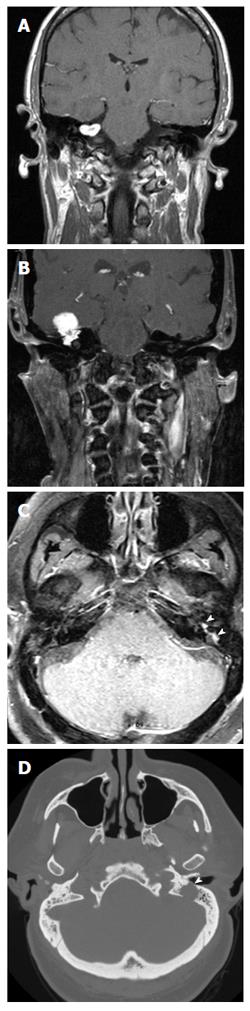
However, many FN tumors do not present with FN dysfunction at all. It is common for the diagnosis of FNS to be made intra-operatively; this is particularly true for tumors limited to the CPA/IAC (2) (Figure 2A). As imaging technology has improved over time, it has become much easier to diagnose a FNS before going into surgery. High-resolution computer tomography (HRCT), magnetic resonance imaging (MRI), audiometry and vestibular testing, electroneurography, and electromyography became tools to help make the diagnosis[2,4]. Full discussion of the diagnostic modalities for the FNS is beyond the scope of this article and the reader is referred to other references for more in-depth discussions[5].
FNSs are classically described as a contrast enhancing mass in the course of the FN, on MR. On T1-contrast enhanced MRI, FNSs present as smoothly circumscribed fusiform enhancing mass along the intratemporal FN. Usually, intratemporal FNSs involve multiple segments of the FN and appear as “beads on a string” on MR[6] (Figure 2C).
Other distinct appearances of the FNS are seen along the various segments of the FN. Those limited to the cerebellopontine angle or IAC (CPA-IAC) are very hard to distinguish from VS. Occasionally a labyrinthine “tail”, the schwannoma’s extension into the labyrinthine segment of the FN canal, can be seen as an indication of a FNS rather than VS. A large CPA-IAC FNS may present with a “dumbbell” imaging appearance, due to the extension of the FNS from the IAC fundus to the labyrinthine segment and to the geniculate fossa[3]. FNSs centered in the geniculate fossa or those that extend along the greater superficial petrosal nerve present with a mass in the middle cranial fossa (MCF)[3] (Figure 2B). These lesions may enhance along the course of the nerve or be discrete, well-defined extradural lesions arising along the floor of the middle fossa. FNSs located in the tympanic segment often lobulate into the middle ear cavity[3]. FNSs arising from the mastoid segment may spread into nearby mastoid air cells, demonstrating unusual, irregular tumor margins as the tumor expands through the air cells along the pathways of least resistance. HRCT images of these lesions reveal a widening or even erosion of the bony canal[3] (Figure 2D).
As the approach to medicine has changed over time, the goals of treatment have evolved. For many benign pathologies, medicine now focuses on maximizing the patients’ quality of life (QOL), in addition to treating the disorder. This changing pattern is evident in the treatment patterns for intratemporal FNSs.
Complete surgical resection and grafting were very popular up until the mid-1990s[7]. Many physicians offer tumor resection with cable grafting if the patient presents with facial palsy and facial dysfunction, House-Brackmann (HB) Grade 4 or worse (Table 1)[8]. Grafting provides the possibility that some degree of function will resume, however, full symmetric facial function is never achievable.
| Grade | Description | Gross function | Resting appearance | Dynamic appearance |
| 1 | Normal | Normal | Normal | Normal |
| 2 | Mild dysfunction | Slight weakness with effort, may have mild synkinesis | Normal | Mild oral and forehead asymmetry; complete eye closure with minimal effort |
| 3 | Moderate dysfunction | Obvious asymmetry with movement, noticeable synkinesis or contracture | Normal | Mild oral asymmetry, complete eye closure with effort, slight forehead movement |
| 4 | Moderately severe dysfunction | Obvious asymmetry, disfiguring asymmetry | Normal | Asymmetrical mouth, incomplete eye closure, no forehead movement |
| 5 | Severe dysfunction | Barely perceptible movement | Asymmetric | Slight oral/nasal movement with effort, incomplete eye closure |
| 6 | Flaccid paralysis | None | Asymmetric | No movement |
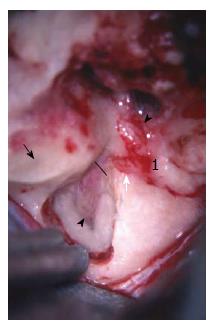
Depending on the location of the tumor and the degree of hearing loss at the time of presentation, the surgical approach will vary[6,9]. For tumors involving the geniculate and more medially, the MCF or subtemporal approach is offered to the patients if the tumor does not extend significantly (less than 1.5 cm) into the CPA and the hearing is serviceable (hearing is defined as at least serviceable if it is either Class A or B on the AAO-HNS scale[10]) (Figure 3).
The MCF approach can be combined with a transmastoid approach for tumors involving larger portions of the nerve but with serviceable hearing. This combined approach allows for access to multiple segments of the nerve while maintaining the auditory apparatus. When hearing is non-serviceable a translabyrinthine approach is appropriate and can be used for any size or location of tumor[6]. This can be carried out using a nerve graft (greater auricular nerve, sural nerve or cadaveric donor cable graft) or through direct anastomosis[9,11]. Graft length has not been shown to correlate to the degree of facial function, but graft location has been shown to be a factor; the more proximal to the brainstem the first neurorraphy is placed, the worse the FN outcome[6]. Pre-operative FN function is one predictor of ultimate FN function after grafting; those with better pre-operative function do better with grafting than those who present with poorer FN function and have a graft. In patients undergoing total tumor resection, tumor recurrence is not expected[6].
Many studies have shown the negative effects of facial paralysis on psychosocial function and QOL using evaluation tools such as the Glasgow Benefit Inventory, the short-form 36, the Derriford Appearance, the FaCE Scale, and the Facial Disability Index[12,13]. Facial paralysis affects QOL is by limiting the patient’s ability to express emotion through facial motor movements, thus affecting their ability to form social relationships and have successful social interactions. This leads to feelings of social isolation[14]. Facial paralysis alters self-perception of facial appearance. People with a disfigured facial appearance are often looked at differently by society and valued less because they do not look “normal”, affecting their ability to form relationships and affecting their psychosocial well-being[15]. Because QOL factors are more heavily considered in current treatment algorithms, management techniques that preserve FN function while still successfully managing the tumor may be preferred[7].
The main alternative management modalities for intratemporal FNSs are tumor debulking, bony decompression, stereotactic radiation, and observation.
Debulking or stripping surgery refers to the removal of as much of the tumor as possible while leaving the main trunk of the FN intact[6,16]. The goal of a tumor debulking is to remove as much of the tumor mass as possible while maintaining the anatomic and functional integrity of the nerve. This surgical method can achieve near-total tumor removal. However, by definition, some of the tumor is left on the FN, which can result in tumor regrowth. Debulking surgery is carried out under high magnification microdissection between nerve fibers and the actual tumor. In some cases however, the nerve fibers are scattered in the tumor and thus not suitable for debulking; this cannot be determined until the tumor-nerve interface is assessed intraoperatively[6]. Continuous electromyographic FN monitoring is used during debulking. Short bursts of activity may be present but the microdissection is stopped if fibrillation potentials (trains) are produced[6]. The percentage of tumor removal is then estimated by the end of the operation and can also be assessed via volumetric analysis of post-operative MRI scans. As in tumor resection, an MCF or a TL approach can be used, taking into consideration the hearing factors previously mentioned.
Debulking surgery is generally a choice of treatment in patients that do not present with facial dysfunction[16]. This surgical technique can be very useful for tumors of the CPA/IAC, which are generally presumed to be VSs preoperatively. If a FNS is found intra-operatively instead of a VS, the physician may choose to debulk the FNS until fibrillation potentials are encountered, at which point they will leave the rest of the tumor on the nerve. Post-operative FN function is expected to be maintained (a Grade 1 or 2 HB). In one study, the immediate and long-term postoperative FN function after debulking was evaluated. Out of 11 patients who underwent debulking, only 2 patients had worse than a HB score of 3 upon immediate post-operative evaluation. Results for the long-term follow-up were even better. Only one patient had a score of HB 3; all other patients scored HB 1 or 2[6]. However, patients should be informed of the risk of FN damage and functional decline. There is also a risk of regrowth, because a portion of the tumor is left on the nerve. Therefore, it is imperative to continue following the tumor with serial MRI imaging[6].
Bony decompression refers to the removal of the bony facial canal along the course of the FN to relieve the intrafascicular pressure created by the tumor[17-19]. Bony decompression should be considered as a first-line option in patients that are pre-operatively diagnosed with a facial schwannoma and present with normal facial function (HB 1 or 2) and small tumors[6,7,17]. Decompression is beneficial in that it preserves facial function, and may even improve facial function, while also preserving hearing ability[2,6,17-19]. However, because decompression does not treat the underlying tumor, risk of tumor growth does exist[13,17]. Therefore, it is important to continually monitor the tumor post-op using a serial MRI imaging[17]. Wilkinson et al[7] reported that in 78.9% of patients undergoing decompression, FN function either remained the same or improved. Furthermore, three patients (15.8%) in the decompression group showed improvement whereas only one patient (11.1%) in the observation group showed improvement. No patients had hearing loss[7].
Stereotactic radiosurgery (SRS) is a less invasive approach in which external beam radiation is directed at the tumor while minimizing damage to surrounding tissue[20,21]. Ionizing radiation acts at the cellular level to causing DNA damage to the rapidly dividing cells. In rapidly dividing malignant cells, this results in apoptosis and tumor resolution. In benign, slowly growing tumors (such as FNS), SRS does not result in resolution of the tumor; however, tumor growth is controlled and the volume of the tumor may even be reduced over time[21]. Recent evidence suggests that schwannoma cells are relatively radioresistant and tumor control may be more related to radiation induced fibrosis of the tumor vasculature[22].
SRS is typically used for benign tumors less than 3.5 cm in size[20]. A major benefit of SRS is that pre-treatment FN function is preserved and in rare cases, may be improved[23-29]. SRS should only be used in tumors that are demonstrating growth on serial MRI. This may include residual tumors that remain after debulking surgery or partial resection[23,25,27,29].
Large series of FNSs alone are lacking due to the rarity of this lesion[23,27]. Some inferences about effectiveness and long-term effects of SRS for FNS can be taken from the body of literature for VS; however these VS data must be used with caution when counseling FNS patients. Hearing ability in patients undergoing SRS for VS seems to be maintained up to about 5 years post-treatment. However, evidence suggests that beginning 6 years after VS SRS, patients may experience hearing loss, especially in tumors limited to the IAC[23,27,28]. Another risk is potential damage to the FN resulting in worse FN function than pre-treatment levels[23,27,28].
Observation with serial imaging techniques is now becoming a popular first-line management choice for FNSs. Patients presenting with small FNSs and without facial dysfunction (present with HB 1 or 2) or other neurologic deficit can be followed with MRI, audiograms, and other imaging techniques[2,25,26,30]. This conservative approach may allow the patient to maintain their functional FN for a long period of time, up to 10 years, without having to intervene with a more destructive or invasive approach[25,26,30,31]. It is possible to take a conservative approach because of the slow-growing characteristic of these tumors, allowing the patient to avoid intervention for many years[4,25]. Observation with imaging should be maintained until facial function deteriorates (HB Grade 3 or worse), or the tumor grows significantly in size[2,4,25,26]. At this point, the physician should consider a more aggressive approach[2,4,25,26]. It has been shown that observation for a period of time before doing surgery does not result in worse outcomes, when compared to cases in which surgery was the initial treatment of choice[4]. Observation is especially recommended in elderly patients or those patients who have significant comorbidities[27]. Observation is not an appropriate choice for tumors causing significant brainstem deformation or compression[26].
Poor FN function (HB IV-VI) and poor hearing (AAO-HNS class C-D) at presentation a translabyrinthine resection with cable grafting is recommended; Poor FN function (HB IV-VI) and serviceable hearing (AAO-HNS class A-B) consider middle fossa ± transmastoid resection; Moderate facial function (HB III) and serviceable hearing consider FN decompression via MCF and mastoid or consider observation; Good facial function (HB I-II) and serviceable hearing consider observation or FN decompression via MCF and mastoid; Poor surgical candidate or refuses surgery consider radiosurgery if growth demonstrated or observation.
In summary, there are currently many options for management of intratemporal facial schwannomas, and the modern practitioner should be familiar with these various treatment options when counseling patients. A high index of suspicion for FNS will allow appropriate diagnosis and treatment when these patients do present. Non-surgical options are also appropriate management choices for a select group of patients. Good facial function and hearing preservation are possible with a number these surgical techniques.
P- Reviewer: Lichtor T, Ralli G S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
| 1. | Phillips CD, Bubash LA. The facial nerve: anatomy and common pathology. Semin Ultrasound CT MR. 2002;23:202-217. [PubMed] [Cited in This Article: ] |
| 2. | Marzo SJ, Zender CA, Leonetti JP. Facial nerve schwannoma. Curr Opin Otolaryngol Head Neck Surg. 2009;17:346-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Wiggins RH, Harnsberger HR, Salzman KL, Shelton C, Kertesz TR, Glastonbury CM. The many faces of facial nerve schwannoma. AJNR Am J Neuroradiol. 2006;27:694-699. [PubMed] [Cited in This Article: ] |
| 4. | McMonagle B, Al-Sanosi A, Croxson G, Fagan P. Facial schwannoma: results of a large case series and review. J Laryngol Otol. 2008;122:1139-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Mowry S, Gantz B. Surgery for Bell’s Palsy. Slattery W, editors. New York: Thieme International 2014; . [Cited in This Article: ] |
| 6. | Mowry S, Hansen M, Gantz B. Surgical management of internal auditory canal and cerebellopontine angle facial nerve schwannoma. Otol Neurotol. 2012;33:1071-1076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wilkinson EP, Hoa M, Slattery WH, Fayad JN, Friedman RA, Schwartz MS, Brackmann DE. Evolution in the management of facial nerve schwannoma. Laryngoscope. 2011;121:2065-2074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146-147. [PubMed] [Cited in This Article: ] |
| 9. | Minovi A, Vosschulte R, Hofmann E, Draf W, Bockmühl U. Facial nerve neuroma: surgical concept and functional results. Skull Base. 2004;14:195-200; discussion 200-201. [PubMed] [Cited in This Article: ] |
| 10. | Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma). American Academy of Otolaryngology-Head and Neck Surgery Foundation, INC. Otolaryngol Head Neck Surg. 1995;113:179-180. [PubMed] [Cited in This Article: ] |
| 11. | Nadeau DP, Sataloff RT. Fascicle preservation surgery for facial nerve neuromas involving the posterior cranial fossa. Otol Neurotol. 2003;24:317-325. [PubMed] [Cited in This Article: ] |
| 12. | Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007;133:56-60. [PubMed] [Cited in This Article: ] |
| 13. | Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clin Otolaryngol. 2015;Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 14. | Coulson SE, O’dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004;25:1014-1019. [PubMed] [Cited in This Article: ] |
| 15. | Ho AL, Scott AM, Klassen AF, Cano SJ, Pusic AL, Van Laeken N. Measuring quality of life and patient satisfaction in facial paralysis patients: a systematic review of patient-reported outcome measures. Plast Reconstr Surg. 2012;130:91-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Lee JD, Kim SH, Song MH, Lee HK, Lee WS. Management of facial nerve schwannoma in patients with favorable facial function. Laryngoscope. 2007;117:1063-1068. [PubMed] [Cited in This Article: ] |
| 17. | Bacciu A, Medina M, Ben Ammar M, D’Orazio F, Di Lella F, Russo A, Magnan J, Sanna M. Intraoperatively diagnosed cerebellopontine angle facial nerve schwannoma: how to deal with it. Ann Otol Rhinol Laryngol. 2014;123:647-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ross L, Drazin D, Eboli P, Lekovic GP. Atypical tumors of the facial nerve: case series and review of the literature. Neurosurg Focus. 2013;34:E2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Shirazi MA, Leonetti JP, Marzo SJ, Anderson DE. Surgical management of facial neuromas: lessons learned. Otol Neurotol. 2007;28:958-963. [PubMed] [Cited in This Article: ] |
| 20. | Lindquist C. Gamma Knife Radiosurgery. Semin Radiat Oncol. 1995;5:197-202. [PubMed] [Cited in This Article: ] |
| 22. | Yeung AH, Sughrue ME, Kane AJ, Tihan T, Cheung SW, Parsa AT. Radiobiology of vestibular schwannomas: mechanisms of radioresistance and potential targets for therapeutic sensitization. Neurosurg Focus. 2009;27:E2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Kida Y, Yoshimoto M, Hasegawa T. Radiosurgery for facial schwannoma. J Neurosurg. 2007;106:24-29. [PubMed] [Cited in This Article: ] |
| 24. | Litre CF, Gourg GP, Tamura M, Mdarhri D, Touzani A, Roche PH, Régis J. Gamma knife surgery for facial nerve schwannomas. Neurosurgery. 2007;60:853-859; discussion 853-859. [PubMed] [Cited in This Article: ] |
| 25. | Madhok R, Kondziolka D, Flickinger JC, Lunsford LD. Gamma knife radiosurgery for facial schwannomas. Neurosurgery. 2009;64:1102-1105; discussion 1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Jacob JT, Driscoll CL, Link MJ. Facial nerve schwannomas of the cerebellopontine angle: the mayo clinic experience. J Neurol Surg B Skull Base. 2012;73:230-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Moon JH, Chang WS, Jung HH, Lee KS, Park YG, Chang JH. Gamma Knife surgery for facial nerve schwannomas. J Neurosurg. 2014;121 Suppl:116-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | McRackan TR, Wilkinson EP, Brackmann DE, Slattery WH. Stereotactic radiosurgery for facial nerve schwannomas: meta-analysis and clinical review. Otol Neurotol. 2015;36:393-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Sheehan JP, Kano H, Xu Z, Chiang V, Mathieu D, Chao S, Akpinar B, Lee JY, Yu JB, Hess J. Gamma Knife radiosurgery for facial nerve schwannomas: a multicenter study. J Neurosurg. 2015;123:387-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Bacciu A, Nusier A, Lauda L, Falcioni M, Russo A, Sanna M. Are the current treatment strategies for facial nerve schwannoma appropriate also for complex cases. Audiol Neurootol. 2013;18:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Yang W, Zhao J, Han Y, Yang H, Xing J, Zhang Y, Wang Y, Liu H. Long-term outcomes of facial nerve schwannomas with favorable facial nerve function: tumor growth rate is correlated with initial tumor size. Am J Otolaryngol. 2015;36:163-165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |