Published online Nov 28, 2015. doi: 10.5319/wjo.v5.i4.93
Peer-review started: February 7, 2015
First decision: March 28, 2015
Revised: August 30, 2015
Accepted: September 25, 2015
Article in press: September 28, 2015
Published online: November 28, 2015
The most dreaded complication in head and neck surgery is the development of fistula. Fistulas are common and devastating. The prevalence and the risk factors that contribute to fistula formation after head and neck procedures were discussed briefly. The main goal of this manuscript is to discuss current management of head and neck fistula. We believed that the best management strategy for head and neck fistulas is prevention. We recommend a holistic preventive approach during the perioperative period. The roles of different types of wound products and hyperbaric oxygen therapy were also discussed and highlighted. We also discussed the operative repair of fistulas, which relies on the tenet of providing well-vascularized tissue to an area of poor wound healing. Most often, the surgeon’s preference and range of operative skills dictate the timing and the type of repair. We highlighted the use of the pectoralis major, a well-known flap, as well as a novel technique in the surgical repair of complex, difficult-to-heal head and neck fistula.
Core tip: Fistulas after head and neck surgery is the most dreaded complication for good reasons: they are common, they occur in the sickest group of patients, and they are devastating physically and psychologically. The best treatment for fistulas is prevention. We discussed in details steps that can be taken to optimize patients and to reduce the risk of fistula formation. As we discussed up-to-date evidence on the conservative and surgical managements of fistula, we highlighted areas that need improvements and benefit from further researches.
- Citation: Khanh NT, Iyer NG. Management of post-operative fistula in head and neck surgery: Sweeping it under the carpet? World J Otorhinolaryngol 2015; 5(4): 93-104
- URL: https://www.wjgnet.com/2218-6247/full/v5/i4/93.htm
- DOI: https://dx.doi.org/10.5319/wjo.v5.i4.93
The most significant complication in oncologic surgery of the head and neck is the development of fistula. Fistula is an aberrant connection between two epithelial surfaces that permitting the passage of fluids and secretions. Most fistulas in the head and neck (H and N) are external fistulas in which the fistulas drain onto the skin. This article will focus on the two types of fistula that arise as complications after head and neck surgery: Orocutaneous (OCF) and pharyngocutaneous fistula (PCF). Asides from the anatomical differences, these two types of fistula share many common features in their epidemiology, risk factors, morbidity and managements and thus, will be discussed together.
In high-quality series of patients undergoing total laryngectomy (TL), a highly standardized head and neck operation, the rate of fistula was reported from 3% to 65%[1-5]. The true mean was between 10%-40% according to a meta-analysis by Paydarfar and Birkmeyer. In hypopharyngectomy, where the surgical approach is more diverse owing much to the multitude of flap options, the frequency of fistula distributed widely. Fistula developed in 43% to 59% hypopharyngeal defect closed with a radial forearm flap[6]. The fistula rate for hypopharyngeal defect closed with an anterolateral thigh flap was reported favorably at 0%-9%[7-9]. A recent retrospective study looking at 368 consecutive cases of reconstruction using jejunal free flap from 1977 to 2010 revealed an overall fistula rate of 8.2%[10]. Collectively, with the increasing use of concurrent chemo RT as first line treatment, more and more H and N cancer patients present with high risks of developing fistulas postoperatively[11,12]. While the frequency of fistula may vary, its morbidity is uniformly damaging. In the immediate postoperative period, when the fistula tract involves and exposes the carotid to the enzymatic action of saliva, patient is at an increased risk of devastating carotid blowout. In a subgroup analysis with matched pair control group, Upile et al[13], reported a pre-blowout salivary fistula in all patients with carotid artery rupture. Furthermore, fistula prolongs functional recovery of oral intake and speech, increases hospitalization time and cost. More importantly, fistula delays the initiation of adjuvant therapy[14]. The total treatment package time of surgery and postoperative radiotherapy (RT) of < 100 d was shown to improve local control and overall survival[15]. However, whether fistula formation affects survival rate remains unclear. A recent retrospective study of 217 patients in the Netherlands found no statistical difference in the survival rate in patients with PCF vs patients without PCF[16]. In contrast, a recent study of 232 patients underwent surgery for HNSCC at Asan Medical Center, South Korea reported decreased disease-free survivals and decreased overall survival in patients who developed fistula[17]. Last but not least, abnormal tissue granulation and wound contracture due to the presence of fistula may contribute to late complication such as the development of stricture in as high as 50% of patients[18].
Given the significant morbidity and sequelae of fistula formation, the best management calls for prevention. Unfortunately, the 21st century head and neck surgeons are more likely to face patients with multiple comorbidities and operate on irradiated tissues than ever before[11,12]. Both historical and recent data have repeatedly demonstrated that preoperative RT significantly increases the risk of fistula formation[1,2,19-24]. In a study of 177 patients, McLean et al[25] demonstrated that the mean time to spontaneous fistula closure in the irradiated group is 50 d vs 24 d in non-irradiated group. Unsurprisingly, there is a dose dependent effect with a fistula percentage reported at 43% in patients who received more than 60 Gy to the neck compared to 25% in patients who received < 60 Gy[26]. Irradiated patients are also more likely to fail conservative management for their fistula. The risk of fistula formation does not differ whether the patient received RT less than or more than three months prior to surgery[20]. This finding supports the theory that irradiated tissue is chronically hypoxic, hypoperfused, and hypocellular[27]. Concurrent chemotherapy further increases the risk of fistula[28] as well as concurrent neck dissection[20]. In a study of 110 patients undergoing salvage laryngectomy, the rate of PCF is as high as 57.2% in patients with neck dissection vs 13.4% in patients without neck dissection[29].
Another well-established risk factor exposing patient to fistula formation is the presence of surgical site infection (SSI). A recent series of 504 free flap head and neck reconstruction analyzed the oral microbiome found in SSI post head and neck surgery. Remarkably, 40% of patients colonized with MRSA developed an SSI. Moreover, this series also showed that normal oral microbiota is only found in 25% of wound culture. Gram-negative bacilli are found in 44% of wound cultures, MRSA in 20% and MSSA in 16%[30]. MRSA was shown to be associated with higher postoperative fistula formation[31,32]. More recently, a small study established MRSA as the causative agent of PCF following laryngectomy[33]. Poor oral hygiene is also a significant factor for the development of wound complications postop in a prospective study of 186 H and N patients[34]. Patients are two times more likely to have an SSI, dehiscence and fistula when they experienced perioperative weight loss[35]. Incidence of PCF after TL is also significantly higher in patients with lower albumin level[16]. Besides poor nutritional status, other predictive factors for fistula formation have also been identified: Preoperative hypothyroidism, tumor site and stage, prior tracheostomy, low perioperative hemoglobin, perioperative blood transfusion, concurrent neck dissection in salvage surgery, and duration of surgery[16,20,34,36-38]. Interestingly, in the above studies, systemic comorbid, with the exception of hypothyroidism, was not found to be a major risk factor. While the heterogeneity for the analyzed population is certainly implicated, perhaps a more intuitive answer lies in the fact that irradiated tissue is so hypoxic, hypoperfused and hypocellular that it is not possible to observe the consequences of the added insults from systemic illness.
In summary, the risk factors that predispose the patient to fistula formation have been well described in the literature. Certainly, there is extensive interplay and overlap between these risk factors; for instance, concurrent neck dissection may predispose the patient to fistula formation due to the increased blood lost, increased operative time and extensive dissection. Some risk factors are outside the surgeon’s ability to influence; however, we maintain that the best treatment of fistula is prevention. Indeed, at every stage of patient care whether pre-, intra-, or post-operatively, there are measures that can help us avoid this dreaded complication.
Firstly, the indication and extend of surgery must be carefully reviewed. Especially in the salvage setting when there are multiple risk factors at play, the decision for neck dissection in a node-negative neck must be considered judiciously. Concurrent neck dissection, as previously mentioned, increases the risk of fistula development. Recent data that recommend against concurrent neck dissection begin to emerge, although they suffer from small sample size, selection bias, and are limited to laryngeal tumor[29,39]. At this time junction, the negative predictive value of CT-PET is not high enough to safely guide conservative management. Novel techniques such as MRI-PET or diffusion-weighted MRI, have shown good sensitivity and specificity but further data are needed in this field. Another surgical consideration to be made at this time is deciding on how to close the defect. A thorough discussion about flap choice is beyond the scope of this paper. However, we would like to offer a caveat that whilst the desire to choose a flap with the lowest fistula rate is strong, long-term morbidity, such as stricture, must also be put in contention. For example, while the fistula rate for ALT flap and jejunal flap for a circumferential hypopharyngeal defect were reported to be less than 10% in several series[7-10,40], objective assessment by esophagoscope and esophagram showed that jejunal flap had a much lower stricture rate than compare to ALT flap[41]. The jejunal flap is a superior choice for patients with good long-term prognoses. Finally, another consideration is whether to use or not to use a prophylactic flap to reinforce the suture line. Incorporation of well-vascularized and healthy tissue into an anastomosis that is at high risk of breakdown can theoretically improve wound healing. Recent and robust data support prophylactic flap reconstruction. A recent multisite retrospective review of closure technique in 359 patients showed reduced overall complications and fistula in patients who received vascularized tissue[42]. Another systematic review of 591 patients by Paleri et al[43] reported a consistent lower rate of PCF across the 7 studies when vascularized tissue is used to buttress the anastomosis. Thus, if the procedure is within the surgeon’s operative range and the patient can tolerate the added anesthesia time, a prophylactic flap should be considered[43].
Once the scope of surgery has been decided, nutrition status should be assessed and optimized early, since this requires the most amount of time to adjust. Malnutrition is a hallmark of head and neck cancer patient due to a combination of factors: Lifetime of tobacco and alcohol abuse, cancer biology, and dysphagia. A multidisciplinary approach with a speech therapist, a dietician and a nursing coordinator should be a matter of course. The timeless study done by Haydock et al[44] in 1987 reported remarkably better wound healing when nutritional status was improved at least one week preoperatively. Additionally, the question of preoperative gastrostomy placement may emerge, especially if the patient requires adjuvant therapy at a later stage. Although gastrostomy has a good safety profile[45], the usefulness of prophylaxis gastrostomy is hotly debated due largely to the lack of randomized control trials (RCT) comparing different modes of enteral feedings[46]. Some data suggest that the nutritional status appeared to be the same between prophylaxis G-tube, reactive G-tube and nasogastric tube and that a large proportion of prophylaxis G-tube was unused and unnecessary[45,47,48]. On the other hand, newer data suggest that patients benefit from prophylactic G-tube and experienced a lower rate of complications overall[49-51]. Thus, given the lack of consensus, we recommend a case-by-case consideration, a multidisciplinary approach and thorough patient counseling to this contentious issue of G-tube placement. However, two things are clear: Firstly, enteral feeding is superior to parenteral feeding and secondly, percutaneous endoscopic gastrostomy (PEG) is safer than radiologically inserted gastrostomy[52]. Complication rate of PEG in patients with H and N cancer is the same as patients with other pathologies requiring PEG[52]. Lastly, G-tube has been shown to aid closure of PCF in multiple series[53-56] and thus should be strongly considered once a fistula has developed.
As with any other major surgeries, attention should be directed to optimize the patient’s psychological and physiological states. Of note, thyroid function should be tested and titrated for correction. This is especially important in patients undergoing salvage surgery and patients who are severely hypoalbuminemic. Previous RT may have damaged the thyroid and a hypoalbuminemia state may alter the metabolism of largely protein-bound thyrosine. It is critical to titrate thyrosine as soon as possible because of its long half-life.
A recent survey of antibiotic prophylaxis choice in the United Kingdom showed that there is little consensus and evidence-based practice on choice and duration of antibiotic prophylaxis in patients undergoing laryngectomy[57]. For reason previously discussed, we strongly recommend MRSA decolonization. MRSA decolonization has been shown to reduce the risk of SSIs in patients undergoing head and neck operations[58,59]. Evidence also support a short course of prophylaxis antibiotic targeting Gram-negative bacilli and MSSA such as ampicillin/sulbactam within an hour of incision[60]. Paradoxically, antimicrobial prophylaxis exceeding 48 h increases the risk of SSI[61]. An experienced dentist, practicing as part of the multidisciplinary team, should prescribe mouthwash, perform simple restorative procedures to the teeth including calculi removal as well as remove seriously damaged teeth or teeth that is within the operative field[62] (Table 1).
| Preoperativeconsiderations | Level of evidence | Ref. |
| Extend of surgery | ||
| Concurrent neck dissection | III | [29] |
| Sentinel lymph node biopsy | II | [75] |
| Extend of resection | - | - |
| Choice of flap | ||
| Short-term vs long-term complications | - | - |
| Use of prophylactic flap | III | [42,43] |
| Nutrition | ||
| For prophylactic gastrostomy | II, III | [45-48] |
| Against prophylactic gastrostomy | II, III | [49-51] |
| PEG is preferred over RIG | II | [52] |
| Improve preoperative nutritional status | - | - |
| Optimization of comorbidities | ||
| Treat comorbidities (hypothyroidism, diabetes, anemia) | - | - |
| MRSA decolonization | III | [58,59] |
| Antibiotics prophylaxis | III | [60] |
| Dental care | IV | [62] |
Although radiation can obliterate tissue planes and render tissues fibrotic, every attempt should be made to strictly obey the Halstedian principles of meticulous dissection and gentle tissue handling. Suture materials and charred tissues from excessive electrocautery are considered foreign bodies and must be limited as much as possible. To this end, modern energy devices may theoretically help reduce the risks of SSIs and fistula formation; additionally, one study suggested that ultrasound-based energy scalpel creates less collateral endothelial damage during vascular dissection, which theoretically improves flap survivability and thus, decreases fistula formation. However, initial data show no difference in fistula frequency[63,64]. Use of mechanical staple is also controversial with conflicting evidence: some series, including a systematic review, reported decreased PCF incidence[65,66]; however, a recent series from John Hopkins H and N unit described an overwhelmingly increased rate of PCF when mechanical staple was used[38].
Technology is also permitting a new and minimally invasive protocol to achieve goal-directed volume expansion in head and neck surgery to optimize hemodynamics[67]. Traditionally, intraoperative goal-directed volume expansion is achieved with an esophageal Doppler ultrasound, which is difficult in major head and neck resection. Intraoperative cardiac output (CO) monitoring and optimization have been shown to shorten postoperative stay and reduce complications in different types of major surgeries[68-70]. Unfortunately, as recent as 2012, in the United Kingdom, only 9% of head and neck units routinely monitor CO intraoperatively[71]. Certainly, this is an area where further researches, adoption of new technology, and improvements are needed.
Another area in which further researches and adaptation are needed is the application of sentinel lymph node biopsy (SLNB) in a clinically negative neck. SLNB may reduce unnecessary neck dissection, amount of blood lost, and operative time, all of which are aforementioned risk factors for fistula formation. From a prospective series of 59 patients with T1/T2 oral squamous cell carcinoma undergoing SLNB, a NPV of 97.5% was reported[39]. Other series, including a multicenter prospective trial, have also reported excellent outcomes of SLNB for oral cancer[72,73] even for a previously treated neck[74]. A recent meta-analysis of 23 studies reported the sensitivity and negative predictive value of SLNB as 95% and 96%, respectively. Although 82.4% of the data are comprised of oral cavity tumors, the authors concluded that SLNB is worth considering in laryngeal, hypopharyngeal and oropharyngeal tumors[75]. Thus, with the growing body of evidence and the desire to avoid unnecessary neck dissection and its associated risks, the impetus is shifting toward applying SLNB more regularly.
Prevention of infection, early enteral feeding, and adequate tissue perfusion in the immediate post-operative period are important protective factors against fistula formation. Since most SSIs develop more than 1 wk postoperatively[30], theoretically, patients may benefit from a short pulse of prophylactic antibiotics during this period. Nutrition is of paramount importance to combat the postoperative catabolic state. Although Hb < 12.6 is a known RF for fistula development, we do not suggest routine transfusion to keep Hb above this threshold. A more rational approach is to maintain stable hemodynamic to maintain adequate tissue oxygen tension. IV Iron with or without rHuEPO has been shown to reduce transfusion requirements in surgical patients[76] and may be a useful adjunct in head and neck cancer patients. We also recommend aggressive prophylaxis against post-operative nausea and vomiting although the evidence that post-operative vomiting increases the risk of post-operative fistula is rather limited[77]. Attention to oral hygiene is often neglected during this stage, partly due to poor patient compliance but also difficulty in execution. The healthcare team should be vigilant and take measures include frequent suctioning, chlorhexidine gargling, mechanical cleaning of the oral cavity with gauze and cotton buds and even oscillating electric toothbrush to minimize the risk of gingivitis, mucositis, SSIs, and fistulas[62].
The mainstay of conservative management of OCF and PCF is wound care with frequent wound inspections and application of wound products. The goal of conservative management of fistula is to promote healing by secondary intention. Thus, a holistic approach must be commenced prior to any attempt at addressing the fistula itself: Necrotic tissues must be debrided; infections must be treated; enteral feeding must be started; hemodynamics must be optimized and comorbid must be controlled. We also suggest paying attention to the care of perifistular skin integrity because macerated and infected skin will eventually lead to ulceration and ultimately enlargement of the fistula. To this end, prior to applying wound products and topical agents, a patient allergy history must be reviewed because the development of contact dermatitis will preclude the wound from healing and the fistula from closing.
Conventional, simple wound dressing by applying gauze soaked in solutions such as saline, water, chlorhexidine or Dakin’s solution is usually not appropriate in the setting of fistula due to the high and constant output of both saliva and exudate. Furthermore, conventional dressing requires constant dressing change, which is especially time consuming in an already very difficult and complex 3-dimensional wound. Unsurprisingly, no data on simple wound dressing in the head and neck setting was found on PubMed Central. Other surgical specialties are also moving away from using simple, wet-to-dry dressing due to its nonselective mechanical debridement[78]. Modern wound dressings were invented with the aim of absorbing exudates while providing a matrix upon which granulation can take place as well as enhance the principles of moist wound healing[79,80]. Hydrocolloid (HC) dressings come in a form of a polyurethrane film or foam combined with a gel-forming agent. These products include Granuflex® (ConvaTec, United States), Tegasorb® (3M Healthcare, United Kingdom), etc. Although these products are good at achieving moist wound healing by promoting uniform and robust granulation[80], their utility in managing head and neck fistula is again restricted by their limited capacity to absorb exudate. Even in less exudative wounds such as leg ulcers or burnt wounds, the range of exudate between 4-12 g/10 cm2 per 24 h[81,82] is already beyond the capacity of most hydrocolloid dressings, which can absorb about 10 g/10 cm2 per 24 h[83]. Although an old study, there has been no newer evidence on this topic since this study. In our anecdotal experience, a combined output of exudates and saliva in most PCF or OCF will overwhelm the absorbent capacity of even the newest hydrocolloid dressing. Thus, HC is only applicable when the fistula output can be controlled or diverted. Another area where hydrocolloid dressings have shown promise in the head and neck setting is when it was applied to defect with exposed bones prior to skin grafts[84]. Last but not least, the use of hydrocolloids dressing is further hampered by their restricted conformability, malodor that could confuse assessment of infection and further reduce quality of life[85].
Another alternative to hydrocolloid products are the hydrogel products. They share a common basic structure of absorbent polymers such as alginate, starch, pectins, or sodium carboxymethylcellulose and water. Examples of hydrogel products are Intrasite Gel® (Smith and Nephew Medical, United States), Granugel® (ConvaTec, United States), Nugel® (Johnson and Johnson Medical, United States). These products have improved absorbent capacity due to their absorbent polymer component. In fact, a retrospective study of 47 head and neck cancer patients with wound complications and fistula in France showed improved wound healing with a mean of 33.53 d in the group using seaweed-based products vs 72.94 d in the control group[86]. Theoretically, the efficacy of intense absorbent wound product can be improved with antimicrobial properties. This can be achieved with nanosilver-impregnated dressings[87] or with honey-coated bandage[88]. Despite its established popularity in treating wound dehiscence in abdominal surgery, there is little evidence of silver-based dressing use in head and neck wound. The utility and effectiveness of silver dressings in the treatment of chronic wounds have been established in several studies and a meta-analysis[89]. However, what was not mentioned in this meta-analysis is the cost. In a recent study of wounds rated to have healing potential between 7 to 21 d, the cost of Aquacel® Ag was 4814.08 Euro’s vs a cost of 13249.46 Euro’s for Acticoat[90,91]. Perhaps the dearth of data regarding silver-based dressing in the management of head and neck fistula is down to cost. A more affordable alternative may be readily available in the form of honey-based wound products. Although we were not able to find any trial comparing silver-coated dressing to honey-coated dressing to other forms of dressing in the H and N setting, an RCT in Denmark compared honey-coated bandage to silver-coated bandage in treatment of malignant wounds of the breast and showed no difference between the two regiments. Patients in both group reported improve satisfaction with quality of life (QOL) and the wound size also reduced significantly[92]. The reduction in size is encouraging because these are malignant wounds, which the law of nature forbids from healing. It is important to point out that the antimicrobial effect of honey can be 11-fold higher than compared to application of sugar solutions due to enzymatic activities of compounds such as bee defensin-1 in addition to osmotic effects[92]. Honey-impregnation also has the added benefits of anti-odor, anti-exudation, and analgesia[93]. Anecdotal experience from France and Britain described positive and promising results in treating a series of 3000 non-healing surgical wound dehiscence a salivary fistulas with topical and regular oral ingestion of honey several times a day[94]. Despite initial enthusiasm, a recent Cochrane review failed to find clear benefits of honey as a topical treatment[95]. Given the safety of honey-based wound therapy, the readiness availability and affordability of honey, we believe that the application of honey-based wound products to H and N wound in general and H and N fistula in particular worth further researches, especially in the forms of prospective, randomized controlled trials. Other forms of affordable antimicrobial dressing such as the recently reported hydrogel product with polyvinyl alcohol, polyvinylpyrrolidone and seaweed formulation[96] warrant further investigation and application in our specialty.
Negative pressure wound therapy (NPWT) in treating complex wound and fistula in H and N surgery is in vogue. Regrettably, head and neck surgeons joined the party rather late. In the early 90’s, NPWT was first reported by Fleischmann et al[97] in the treatment of open fracture; only until 2005, the first successful application of NPWT in 23 H and N patients was reported by Rosenthal et al[98]. Of note, four PCFs were treated with the mean duration of treatment of 6.25 d. Three of these four patients had previous RT. Other series followed and quickly validated the utility of NPWT in managing H and N wounds, including combat wounds[99-103]. A result of meta-analysis in 2011 firmly placed NPWT as an integral modality of modern wound treatments[104]. Even for wounds with high levels of complexity such as wounds with great vessel exposure, or peristomal application, NPWT is efficacious and safe with a reported complication rate of 3.5%[105] (Table 2).
| Wound products | Advantages | Disadvantages |
| Simple wet-to-dry/wet-to-moist dressing | Affordable Readily available No special training/dedicate wound nurse | Require frequent changes, sometimes multiple times a day Cannot be used when wound is high output or exudative Traumatize tissue and disrupt granulation |
| Hydrocolloid based | Provide microdebridement Maintain moist wound bed Promote granulation and epithelization Readily available Evidence supported use on bone | Limited absorbent capacity Malodourous Rigid form factor made it difficult to apply; limited use in wound with deep tracts, undermining May irritate/dessicate perifistular skin May adhere to wound bed and cause pain when removed |
| Hydrogel based | May be applied to moderately exudative wound Easy to remove, can be changed daily Maintain moist wound bed Promote granulation and epithelization Mildly analgesic | May not work in highly exudative/high output wound May irritate/macerate perifistular skin Malodorous Require secondary dressing |
| Silver-coated | Provide autolytic debridment Proven anti-microbial efficacy and decreases bioburden Promote granulation and epithelization May be applied to highly exudative wound | Costly Different silver products have different properties; no reliable evidence supporting one product over another May cause discoloration or dermatitis of perifistular skin |
| Honey-impregnated | Can be changed every other day or longer depends on need Affordable Anti-odor Anti-microbial Mildly analgesic Moisturize and maintain perifistular skin | Conflicting evidence Only one brand formally approved for Medical use: MedihoneyTM (United States) Different honey from different bees and/or flower species have different efficacy |
| Negative wound pressure therapy | May be applied to highly exudative wound Little data on safety profile and side effects, especially in diabetic Proven efficacy Promote granulation Promote vascularization Can be used in highly exudative wound Can be combined with other products (Dakin’s solution, octanidine…) to add anti-microbial effect | Technically challenged to achieve airtight seal, especially when fistula is in communication with the aerodigestive tract May require bedside procedure to divert fistula if near tracheostomy Moderate cost Controversy surrounding use directly on vessels Require experience/training and dedicated wound team Dressing change can be painful to patients |
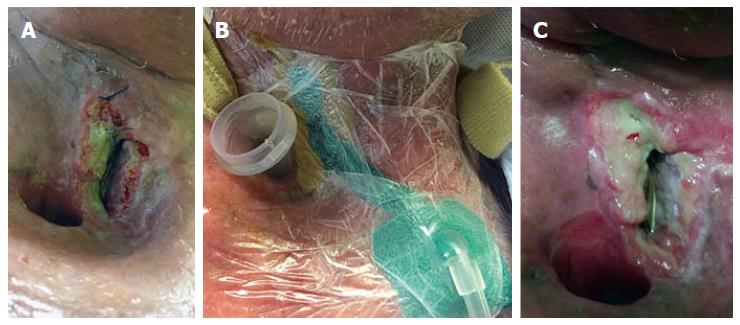
During the early days, applications of NPWT were plaque with difficulties in applying the device to a 3-dimensional wound in a topographically complex area. Communication between the aerodigstive tract and the skin adds another layer of immense complexity when NPWT is attempted in treating fistulas. Intriguing solutions have been described in the literature recently. Honey can also be used as an adjunct to maintain air-tightness for NPWT[95]. Medical silicone can bolster and form an airtight seal in a leaking NPWT dressing[106]. Our own data supported the use of hydrogum dental paste to form an airtight seal for NPWT treatment of OCF[102]. NPWT for PCF can be further complicated by the proximity of the tracheostomy site. Figure 1 illustrates a NPWT dressing applied at a challenging location for a peristomal fistula using a combination of hydrogum dental paste and medical silicone. Other options exist. In their series, Loaec et al[103] describes the use of a Tulle Gras (Smith and Nephew, Australia) to maintain air-tightness. Counter-incision can also be used to divert the fistula away from the tracheostomy with great effectiveness[104]. Intraluminal negative pressure dressing, which allows intraoperative and prophylactic placement of NPWT, has also shown great potentials[106]. Certainly, the intraluminal device itself is intriguing; but the possibility of using NPWT as a prophylaxis against fistula in high risks patients merits further attention. Additionally, reported success of adjunct combination of other wound products such as silver, octenidine, and Dakin’s solution with NPWT in treatment of chronic, difficult-to-heal wounds[107-109] justify future application of these products in the HandN setting (Figure 1).
In our experience, hyperbaric oxygen therapy is used when we are unable to take the patients back to theatre, when other conventional treatments have failed, and our back is against the wall. The cost is nearly prohibitive; and the treatment regime, cumbersome. Despite these drawbacks, HBOT has a long and successful history of usage in managing complicated H and N wounds. The first study in 1973 reported complete healing in all four patients with fistulas and wound infections after undergoing HBOT. Remarkably, a landmark RCT in 1993 investigated prophylactic HBOT in high-risk patients showed significantly fewer wound-related complications in the HBOT group: 11% dehiscence in HBOT vs 48% in control group, 6% infection in HBOT vs 24% in control group[110]. Subsequent trials investigating the utilities of HBOT prophylaxis also showed improved QOL, improved wound healing, and survivability of skin grafts and free flaps[27,111]. Moreover, a comprehensive review by Feldmeier et al[112] dismissed concern of HBOT enhancing the rate of tumor growth, clearing the way for future use in a prophylactic setting prior to resection. Many other studies reported improved healing in patients with radionecrosis of the larynx, mandible, or soft tissue after undergoing HBOT[113-115]. HBOT has been shown to improve wound healings and facilitate fistula closure in H and N wounds that failed to respond to conventional therapies[116-118]. The therapeutic effect of HBOT is three-pronged. HBOT improves the oxygen tension in tissue, promotes angiogenesis and cellular synthesis, and generates oxygen free radicals that are important in oxygen-dependent killing of bacteria. Although there are data that dispute the use of HBOT in treating complicated H and N wounds, most notably, the prospective RCT by Annane et al[118] that showed no difference in wound healings between the HBOT group and the placebo group of pressurized Nitrogen, a Cochrane review in 2012 validated the overall favorable outcome of HBOT in difficult-to-heal wound[119]. Finally, data from patients with osteoradionecrosis undergoing HBOT prior to surgery may support the use of HBOT as a bridge prior to surgical repair of the fistula.
We also would like to point out that there might exist a psychosocial benefit to the act of changing dressing and tending to the wound of H and N patients. Psychosocial support offered during dressing changes in breast patients with stubborn malignant wounds have been shown to improve the women’s sense of well-being and self-confidence[91]. H and N cancer and surgical treatment for H and N cancer significant alter the physical appearance and patient’s body image, not unlike what happen to women receiving treatment for breast cancer. Arguably, the development of a devastating complication such as fistula puts the patient at a higher risk for psychosocial issues. Unfortunately, unlike breast cancer, researches in body image and psychosocial issues in H and N cancer patients are lacking and have only started gaining minor tractions recently[120]. Until further evidence become available, we maintain that wound inspection and wound dressing change provide the unique opportunity for the health care providers to deliver much needed psychosocial care, especially when the patients are suffering from a complication as devastating as fistula.
Once conservative management failed to promote healing, surgical repair is required. The timing for the surgical management of postoperative fistula is worth considering. The common practice is to defer surgery until conservative treatment options have been exhausted. However, conventional surgical wisdoms support early repair to avoid scarring and fibrosis. Iteld and Yu also recommended early surgical repair within 3 to 28 d for PFC larger than 5 mm and in patients with high risk factors. Late surgical repair also runs the risk of allowing the fistula to convert a small defect into a larger one that requires extensive reconstruction[121]. The obvious downside to early surgical repair is the lack of time given for the wound to heal. We are unable to find further evidence on the optimal timing of surgical intervention. Thus, due to the lack of evidence, timing of surgery remains a matter of surgeon’s and also, patient’s discretion, with a caveat that fistula is unlikely to close with conservative management after 4-8 wk[122].
The choice of flap in H and N surgery is ever expanding. It is beyond the scope of this paper to discuss each flap in details and its potential benefits in preventing and/or treating fistula. We however would like to call attention to some very new data that could help guide future practice and research. The Pectoralis Major (PM) flap, which was and still is a workhorse in H and N reconstruction, was previously shown to reliably decrease the rate of fistula formation[123,124]. However, newer data reported an interesting observation: although PM flap was associated with increased incidence of PCF, PCFs reinforced by PM flap were less severe[38]. The authors in this study suggest that this result might have been confounded due to the concurrent use of mechanical stapled device; however, this suggests that with evolving epidemiology and surgical practices, it is necessary to review old-timed practice. In contrast, a very recent study from Taiwan, describing a unique double-layer design in the surgical treatment of large, complex fistula, offered a new direction in flap design. Double-layer techniques have been described with successful results in the past[125,126]. However, this newer design is structurally more secured, requires less local tissue to be available to create the double layers, and is compatible with a variety of flaps[127]. The long-term outcome of this new technique was also reported as favorable, with a mean follow up of 28 mo. In conclusion, in the surgical repair of fistula, from the time of surgery to the type of surgery, much depend on the preference of the surgeon. While it is important to continue the surgical tradition of innovation, the surgical management of H and N fistula demands future researches in revising and standardizing practice.
Fistula of the H and N is dreaded for good reasons: They are common, they occur in the sickest group of patients, and they are devastating physically and psychologically. But perhaps, the most dreaded part about managing this complication is the lack of consensus and well-defined guidelines. We are armed with a myriad of wound products, treatment options, and choices of flap; yet, as we have described above, many crucial questions remain unanswered. Unfortunately, at the time of writing, to the best of the authors’ knowledge, there is no risk stratification system to predict fistula formation in surgery of the H and N. A scoring system of risk factors is a foundation upon which our specialty can start designing trials with well-defined populations and good controls to objectively compare treatment modalities and outcomes. Until such a stratification system exists, the crack in our collective knowledge in the optimal prevention and treatment of fistula will continue to exist. Without knowing what is the best treatment and when is the best time to apply it, postoperative H and N fistula will continue to be swept under the carpet of novel wound products, and innovative surgical techniques.
P- Reviewer: Deganello A, Mostafa BE, Rogers SN S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Thawley SE. Complications of combined radiation therapy and surgery for carcinoma of the larynx and inferior hypopharynx. Laryngoscope. 1981;91:677-700. [PubMed] [Cited in This Article: ] |
| 2. | Bresson K, Rasmussen H, Rasmussen PA. Pharyngo-cutaneous fistulae in totally laryngectomized patients. J Laryngol Otol. 1974;88:835-842. [PubMed] [Cited in This Article: ] |
| 3. | Johansen LV, Overgaard J, Elbrønd O. Pharyngo-cutaneous fistulae after laryngectomy. Influence of previous radiotherapy and prophylactic metronidazole. Cancer. 1988;61:673-678. [PubMed] [Cited in This Article: ] |
| 4. | Redaelli de Zinis LO, Ferrari L, Tomenzoli D, Premoli G, Parrinello G, Nicolai P. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Head Neck. 1999;21:131-138. [PubMed] [Cited in This Article: ] |
| 5. | Mäkitie AA, Irish J, Gullane PJ. Pharyngocutaneous fistula. Curr Opin Otolaryngol Head Neck Surg. 2003;11:78-84. [PubMed] [Cited in This Article: ] |
| 6. | Chepeha DB. Chapter 105: Reconstruction of the hypopharynx and esophagus. Cummings Otolaryngology – Head & Neck Surgery. 5th ed. Philadelphia, PA: Mosby 2012; 1448-1461. [Cited in This Article: ] |
| 7. | Murray DJ, Gilbert RW, Vesely MJ, Novak CB, Zaitlin-Gencher S, Clark JR, Gullane PJ, Neligan PC. Functional outcomes and donor site morbidity following circumferential pharyngoesophageal reconstruction using an anterolateral thigh flap and salivary bypass tube. Head Neck. 2007;29:147-154. [PubMed] [Cited in This Article: ] |
| 8. | Yu P, Hanasono MM, Skoracki RJ, Baumann DP, Lewin JS, Weber RS, Robb GL. Pharyngoesophageal reconstruction with the anterolateral thigh flap after total laryngopharyngectomy. Cancer. 2010;116:1718-1724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 9. | Lewin JS, Barringer DA, May AH, Gillenwater AM, Arnold KA, Roberts DB, Yu P. Functional outcomes after circumferential pharyngoesophageal reconstruction. Laryngoscope. 2005;115:1266-1271. [PubMed] [Cited in This Article: ] |
| 10. | Perez-Smith D, Wagels M, Theile DR. Jejunal free flap reconstruction of the pharyngolaryngectomy defect: 368 consecutive cases. J Plast Reconstr Aesthet Surg. 2013;66:9-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098. [PubMed] [Cited in This Article: ] |
| 12. | Sewnaik A, Keereweer S, Al-Mamgani A, Baatenburg de Jong RJ, Wieringa MH, Meeuwis CA, Kerrebijn JD. High complication risk of salvage surgery after chemoradiation failures. Acta Otolaryngol. 2012;132:96-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Upile T, Triaridis S, Kirkland P, Archer D, Searle A, Irving C, Rhys Evans P. The management of carotid artery rupture. Eur Arch Otorhinolaryngol. 2005;262:555-560. [PubMed] [Cited in This Article: ] |
| 14. | Qureshi SS, Chaturvedi P, Pai PS, Chaukar DA, Deshpande MS, Pathak KA, D’cruz AK. A prospective study of pharyngocutaneous fistulas following total laryngectomy. J Cancer Res Ther. 2005;1:51-56. [PubMed] [Cited in This Article: ] |
| 15. | Rosenthal DI, Liu L, Lee JH, Vapiwala N, Chalian AA, Weinstein GS, Chilian I, Weber RS, Machtay M. Importance of the treatment package time in surgery and postoperative radiation therapy for squamous carcinoma of the head and neck. Head Neck. 2002;24:115-126. [PubMed] [Cited in This Article: ] |
| 16. | Timmermans AJ, Lansaat L, Theunissen EA, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. Predictive factors for pharyngocutaneous fistulization after total laryngectomy. Ann Otol Rhinol Laryngol. 2014;123:153-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 17. | Kim DY, Roh JL, Choi JW, Choi SH, Nam SY, Kim SY. Risk factors and survival outcomes for patients with anastomotic leakage after surgery for head and neck squamous cell carcinoma. Clin Exp Otorhinolaryngol. 2014;7:36-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Varvares MA, Cheney ML, Gliklich RE, Boyd JM, Goldsmith T, Lazor J, Baron JC, Montgomery WW. Use of the radial forearm fasciocutaneous free flap and montgomery salivary bypass tube for pharyngoesophageal reconstruction. Head Neck. 2000;22:463-468. [PubMed] [Cited in This Article: ] |
| 19. | Chee N, Siow JK. Pharyngocutaneous fistula after laryngectomy--incidence, predisposing factors and outcome. Singapore Med J. 1999;40:130-132. [PubMed] [Cited in This Article: ] |
| 20. | Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta-analysis of postlaryngectomy pharyngocutaneous fistula. Arch Otolaryngol Head Neck Surg. 2006;132:67-72. [PubMed] [Cited in This Article: ] |
| 21. | Dassonville O, Poissonnet G, Chamorey E, Vallicioni J, Demard F, Santini J, Lecoq M, Converset S, Mahdyoun P, Bozec A. Head and neck reconstruction with free flaps: a report on 213 cases. Eur Arch Otorhinolaryngol. 2008;265:85-95. [PubMed] [Cited in This Article: ] |
| 22. | McDonald MW, Lawson J, Garg MK, Quon H, Ridge JA, Saba N, Salama JK, Smith RV, Yeung AR, Yom SS. ACR appropriateness criteria retreatment of recurrent head and neck cancer after prior definitive radiation expert panel on radiation oncology-head and neck cancer. Int J Radiat Oncol Biol Phys. 2011;80:1292-1298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Bourget A, Chang JT, Wu DB, Chang CJ, Wei FC. Free flap reconstruction in the head and neck region following radiotherapy: a cohort study identifying negative outcome predictors. Plast Reconstr Surg. 2011;127:1901-1908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Mücke T, Rau A, Weitz J, Ljubic A, Rohleder N, Wolff KD, Mitchell DA, Kesting MR. Influence of irradiation and oncologic surgery on head and neck microsurgical reconstructions. Oral Oncol. 2012;48:367-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | McLean JN, Nicholas C, Duggal P, Chen A, Grist WG, Losken A, Carlson GW. Surgical management of pharyngocutaneous fistula after total laryngectomy. Ann Plast Surg. 2012;68:442-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Benatar MJ, Dassonville O, Chamorey E, Poissonnet G, Ettaiche M, Pierre CS, Benezery K, Hechema R, Demard F, Santini J. Impact of preoperative radiotherapy on head and neck free flap reconstruction: a report on 429 cases. J Plast Reconstr Aesthet Surg. 2013;66:478-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 27. | Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283-288. [PubMed] [Cited in This Article: ] |
| 28. | Klozar J, Cada Z, Koslabova E. Complications of total laryngectomy in the era of chemoradiation. Eur Arch Otorhinolaryngol. 2012;269:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Deganello A, Meccariello G, Bini B, Paiar F, Santoro R, Mannelli G, Gallo O. Is elective neck dissection necessary in cases of laryngeal recurrence after previous radiotherapy for early glottic cancer? J Laryngol Otol. 2014;128:1089-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Durand ML, Yarlagadda BB, Rich DL, Lin DT, Emerick KS, Rocco JW, Deschler DG. The time course and microbiology of surgical site infections after head and neck free flap surgery. Laryngoscope. 2015;125:1084-1089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 31. | Watters K, O’dwyer TP, Rowley H. Cost and morbidity of MRSA in head and neck cancer patients: what are the consequences? J Laryngol Otol. 2004;118:694-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Shiomori T, Miyamoto H, Udaka T, Okochi J, Hiraki N, Hohchi N, Hashida K, Fujimura T, Kitamura T, Nagatani G. Clinical features of head and neck cancer patients with methicillin-resistant Staphylococcus aureus. Acta Otolaryngol. 2007;127:180-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Jeannon JP, Orabi A, Manganaris A, Simo R. Methicillin Resistant Staphylococcus Aureus Infection as a causative agent of fistula formation following total laryngectomy for advanced head & amp; neck cancer. Head Neck Oncol. 2010;2:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Chaukar DA, Deshmukh AD, Majeed T, Chaturvedi P, Pai P, D’cruz AK. Factors affecting wound complications in head and neck surgery: A prospective study. Indian J Med Paediatr Oncol. 2013;34:247-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Gourin CG, Starmer HM, Herbert RJ, Frick KD, Forastiere AA, Eisele DW, Quon H. Short- and long-term outcomes of laryngeal cancer care in the elderly. Laryngoscope. 2015;125:924-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | White HN, Golden B, Sweeny L, Carroll WR, Magnuson JS, Rosenthal EL. Assessment and incidence of salivary leak following laryngectomy. Laryngoscope. 2012;122:1796-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Belusic-Gobic M, Car M, Juretic M, Cerovic R, Gobic D, Golubovic V. Risk factors for wound infection after oral cancer surgery. Oral Oncol. 2007;43:77-81. [PubMed] [Cited in This Article: ] |
| 38. | Benson EM, Hirata RM, Thompson CB, Ha PK, Fakhry C, Saunders JR, Califano JA, Arnaoutakis D, Levine M, Tang M. Pharyngocutaneous fistula after total laryngectomy: a single-institution experience, 2001-2012. Am J Otolaryngol. 2015;36:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 39. | Pezier T, Nixon IJ, Gurney B, Schilling C, Hussain K, Lyons AJ, Oakley R, Simo R, Jeannon JP, McGurk M. Sentinel lymph node biopsy for T1/T2 oral cavity squamous cell carcinoma--a prospective case series. Ann Surg Oncol. 2012;19:3528-3533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Clark JR, Gilbert R, Irish J, Brown D, Neligan P, Gullane PJ. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116:173-181. [PubMed] [Cited in This Article: ] |
| 41. | Tan NC, Lin PY, Kuo PJ, Tsai YT, Chen YC, Nguyen KT, Kuo YR. An objective comparison regarding rate of fistula and stricture among anterolateral thigh, radial forearm, and jejunal free tissue transfers in circumferential pharyngo-esophageal reconstruction. Microsurgery. 2015;35:345-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Patel UA, Moore BA, Wax M, Rosenthal E, Sweeny L, Militsakh ON, Califano JA, Lin AC, Hasney CP, Butcher RB, Flohr J, Arnaoutakis D, Huddle M, Richmon JD. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA Otolaryngol Head Neck Surg. 2013;139:1156-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 43. | Paleri V, Drinnan M, van den Brekel MW, Hinni ML, Bradley PJ, Wolf GT, de Bree R, Fagan JJ, Hamoir M, Strojan P. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. Laryngoscope. 2014;124:1848-1853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 44. | Haydock DA, Hill GL. Improved wound healing response in surgical patients receiving intravenous nutrition. Br J Surg. 1987;74:320-323. [PubMed] [Cited in This Article: ] |
| 45. | Paleri V, Patterson J. Use of gastrostomy in head and neck cancer: a systematic review to identify areas for future research. Clin Otolaryngol. 2010;35:177-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Nugent B, Lewis S, O’Sullivan JM. Enteral feeding methods for nutritional management in patients with head and neck cancers being treated with radiotherapy and/or chemotherapy. Cochrane Database Syst Rev. 2013;1:CD007904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 47. | Madhoun MF, Blankenship MM, Blankenship DM, Krempl GA, Tierney WM. Prophylactic PEG placement in head and neck cancer: how many feeding tubes are unused (and unnecessary)? World J Gastroenterol. 2011;17:1004-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 50] [Cited by in F6Publishing: 53] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 48. | Kramer S, Newcomb M, Hessler J, Siddiqui F. Prophylactic versus reactive PEG tube placement in head and neck cancer. Otolaryngol Head Neck Surg. 2014;150:407-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 49. | Baschnagel AM, Yadav S, Marina O, Parzuchowski A, Lanni TB, Warner JN, Parzuchowski JS, Ignatius RT, Akervall J, Chen PY. Toxicities and costs of placing prophylactic and reactive percutaneous gastrostomy tubes in patients with locally advanced head and neck cancers treated with chemoradiotherapy. Head Neck. 2014;36:1155-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 50. | Shinozaki T, Hayashi R, Miyazaki M, Tomioka T, Zenda S, Tahara M, Akimoto T. Gastrostomy dependence in head and neck carcinoma patient receiving post-operative therapy. Jpn J Clin Oncol. 2014;44:1058-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Pulkkinen J, Rekola J, Asanti M, Grénman R. Prophylactic percutaneous endoscopic gastrostomy in head and neck cancer patients: results of tertiary institute. Eur Arch Otorhinolaryngol. 2014;271:1755-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 52. | Grant DG, Bradley PT, Pothier DD, Bailey D, Caldera S, Baldwin DL, Birchall MA. Complications following gastrostomy tube insertion in patients with head and neck cancer: a prospective multi-institution study, systematic review and meta-analysis. Clin Otolaryngol. 2009;34:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | Anghel AG, Anghel I, Dumitru M, Cristian D, Burcos T. The use of gastrostomy procedures in HNC patients. Chirurgia (Bucur). 2013;108:341-345. [PubMed] [Cited in This Article: ] |
| 54. | Bohannon IA, Carroll WR, Magnuson JS, Rosenthal EL. Closure of post-laryngectomy pharyngocutaneous fistulae. Head Neck Oncol. 2011;3:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Palomar-Asenjo V, Sarroca Capell E, Tobías Gómez S, Pérez Hernández I, Palomar-García V. [Pharyngocutaneous fistula following total laryngectomy. A case-control study of risk factors implicated in its onset]. Acta Otorrinolaringol Esp. 2008;59:480-484. [PubMed] [Cited in This Article: ] |
| 56. | Sarra LD, Rodríguez JC, García Valea M, Bitar J, Da Silva A. [Fistula following total laryngectomy. Retrospective study and bibliographical review]. Acta Otorrinolaringol Esp. 2009;60:186-189. [PubMed] [Cited in This Article: ] |
| 57. | Harris R, Ofo E, Cope D, Nixon I, Oakley R, Jeannon JP, Simo R. Current trends in antibiotic prophylaxis for laryngectomy in the UK - a national survey. J Laryngol Otol. 2015;129:63-67. [PubMed] [Cited in This Article: ] |
| 58. | Richer SL, Wenig BL. The efficacy of preoperative screening and the treatment of methicillin-resistant Staphylococcus aureus in an otolaryngology surgical practice. Otolaryngol Head Neck Surg. 2009;140:29-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Miyake M, Ohbayashi Y, Iwasaki A, Ogawa T, Nagahata S. Risk Factors for Methicillin-Resistant Staphylococcus aureus (MRSA) and Use of a Nasal Mupirocin Ointment in Oral Cancer Inpatients. J Oral Maxillofac Surg. 2007;65:2159-2163. [PubMed] [Cited in This Article: ] |
| 60. | Yarlagadda BB, Deschler DG, Rich DL, Lin DT, Emerick KS, Rocco JW, Durand ML. Head and neck free flap surgical site infections in the era of the Surgical Care Improvement Project. Head Neck. 2015;Jan 12; Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 61. | Lotfi CJ, Cavalcanti Rde C, Costa e Silva AM, Latorre Mdo R, Ribeiro Kde C, Carvalho AL, Kowalski LP. Risk factors for surgical-site infections in head and neck cancer surgery. Otolaryngol Head Neck Surg. 2008;138:74-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Chandu A, Stulner C, Bridgeman AM, Smith AC. Maintenance of mouth hygiene in patients with oral cancer in the immediate post-operative period. Aust Dent J. 2002;47:170-173. [PubMed] [Cited in This Article: ] |
| 63. | Erkut B, Unlu Y, Karapolat S, Ugur Kocogullari C, Ceviz M, Becit N, Kocak H. Comparison of harmonic scalpel and high-frequency electrocautery in radial artery harvesting. J Cardiovasc Surg (Torino). 2008;49:371-379. [PubMed] [Cited in This Article: ] |
| 64. | Dean NR, Rosenthal EL, Morgan BA, Magnuson JS, Carroll WR. Harmonic Scalpel versus electrocautery and surgical clips in head and neck free-flap harvesting. Ear Nose Throat J. 2014;93:E36-E39. [PubMed] [Cited in This Article: ] |
| 65. | Calli C, Pinar E, Oncel S. Pharyngocutaneous fistula after total laryngectomy: Less common with mechanical stapler closure. Ann Otol Rhinol Laryngol. 2011;120:339-344. [PubMed] [Cited in This Article: ] |
| 66. | Aires FT, Dedivitis RA, Castro MA, Bernardo WM, Cernea CR, Brandão LG. Efficacy of stapler pharyngeal closure after total laryngectomy: A systematic review. Head Neck. 2014;36:739-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Abdel-Galil K, Craske D, McCaul J. Optimisation of intraoperative haemodynamics: early experience of its use in major head and neck surgery. Br J Oral Maxillofac Surg. 2010;48:189-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 68. | Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634-642. [PubMed] [Cited in This Article: ] |
| 69. | Noblett SE, Snowden CP, Shenton BK, Horgan AF. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg. 2006;93:1069-1076. [PubMed] [Cited in This Article: ] |
| 70. | Green D, Paklet L. Latest developments in peri-operative monitoring of the high-risk major surgery patient. Int J Surg. 2010;8:90-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Chalmers A, Turner MW, Anand R, Puxeddu R, Brennan PA. Cardiac output monitoring to guide fluid replacement in head and neck microvascular free flap surgery-what is current practice in the UK? Br J Oral Maxillofac Surg. 2012;50:500-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Rigual N, Loree T, Frustino J, Jayaprakash V, Cohan D, Sullivan M, Kuriakose MA. Sentinel node biopsy in lieu of neck dissection for staging oral cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:779-782. [PubMed] [Cited in This Article: ] |
| 73. | Flach GB, Bloemena E, Klop WM, van Es RJ, Schepman KP, Hoekstra OS, Castelijns JA, Leemans CR, de Bree R. Sentinel lymph node biopsy in clinically N0 T1-T2 staged oral cancer: the Dutch multicenter trial. Oral Oncol. 2014;50:1020-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 74. | Flach GB, Broglie MA, van Schie A, Bloemena E, Leemans CR, de Bree R, Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma in the previously treated neck. Oral Oncol. 2012;48:85-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Thompson CF, St John MA, Lawson G, Grogan T, Elashoff D, Mendelsohn AH. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: a meta-analysis. Eur Arch Otorhinolaryngol. 2013;270:2115-2122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 76. | Muñoz M, Gómez-Ramírez S, Cuenca J, García-Erce JA, Iglesias-Aparicio D, Haman-Alcober S, Ariza D, Naveira E. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54:289-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Tomkinson A, Shone GR, Dingle A, Roblin DG, Quine S. Pharyngocutaneous fistula following total laryngectomy and post-operative vomiting. Clin Otolaryngol Allied Sci. 1996;21:369-370. [PubMed] [Cited in This Article: ] |
| 78. | Dale BA, Wright DH. Say goodbye to wet-to-dry wound care dressings: changing the culture of wound care management within your agency. Home Healthc Nurse. 2011;29:429-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Agren M. The cytocompatibility of hydrocolloid dressings. J Wound Care. 1997;6:272-274. [PubMed] [Cited in This Article: ] |
| 80. | Downs EC, Robertson NE, Riss TL, Plunkett ML. Calcium alginate beads as a slow-release system for delivering angiogenic molecules in vivo and in vitro. J Cell Physiol. 1992;152:422-429. [PubMed] [Cited in This Article: ] |
| 81. | Lamke LO, Nilsson GE, Reichner HL. The evaporative water loss from burns and water vapor permeability of grafts and artificial membranes used in the treatment of burns. Burns. 1977;3:159-165. [Cited in This Article: ] |
| 82. | Thomas S, Fear M, Humphreys J. The effect of dressings on the production of exudate from venous leg ulcers. Wounds. 1996;8:145-150. [Cited in This Article: ] |
| 83. | Thomas S. A comparative study of the properties of twelve hydrocolloid dressings. Philadelphia, PA: Mosby 1997; July. [accessed 2015 Jan 20] Available from: http: //www.worldwidewounds.com/1997/july/Thomas-Hydronet/hydronet.html. [Cited in This Article: ] |
| 84. | von Lindern JJ, Niederhagen B, Appel T, Bergé S. Treatment of soft tissue defects with exposed bone in the head and face region with alginates and hydrocolloid dressings. J Oral Maxillofac Surg. 2002;60:1126-1130. [PubMed] [Cited in This Article: ] |
| 85. | Brown-Etris M, Milne C, Orsted H, Gates JL, Netsch D, Punchello M, Couture N, Albert M, Attrell E, Freyberg J. A prospective, randomized, multisite clinical evaluation of a transparent absorbent acrylic dressing and a hydrocolloid dressing in the management of Stage II and shallow Stage III pressure ulcers. Adv Skin Wound Care. 2008;21:169-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Diallo BK, Lacher-Fougere S, Baltazart B, Traissac L, Houliat T. [Results of alginate and hypertonic solution in wound healing of head and neck cancers]. Rev Laryngol Otol Rhinol (Bord). 2008;129:289-292. [PubMed] [Cited in This Article: ] |
| 87. | Fong J, Wood F. Nanocrystalline silver dressings in wound management: a review. Int J Nanomedicine. 2006;1:441-449. [PubMed] [Cited in This Article: ] |
| 88. |
Israili ZH; Antimicrobial properties of honey. |
| 89. | Lo SF, Chang CJ, Hu WY, Hayter M, Chang YT. The effectiveness of silver-releasing dressings in the management of non-healing chronic wounds: a meta-analysis. J Clin Nurs. 2009;18:716-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 90. | Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel(®) Ag dressing versus Acticoat™ dressing in partial thickness burns: a prospective, randomized, controlled study in 100 patients. Part 1: burn wound healing. Burns. 2014;40:416-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Lund-Nielsen B, Adamsen L, Kolmos HJ, Rørth M, Tolver A, Gottrup F. The effect of honey-coated bandages compared with silver-coated bandages on treatment of malignant wounds-a randomized study. Wound Repair Regen. 2011;19:664-670. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 92. | Kwakman PH, te Velde AA, de Boer L, Speijer D, Vandenbroucke-Grauls CM, Zaat SA. How honey kills bacteria. FASEB J. 2010;24:2576-2582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 93. | Jull AB, Walker N, Deshpande S. Honey as a topical treatment for wounds. Cochrane Database Syst Rev. 2013;2:CD005083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 94. | Ganacias-Acuna EF. Active Leptospermum honey and negative pressure wound therapy for nonhealing postsurgical wounds. Ostomy Wound Manage. 2010;56:10-12. [PubMed] [Cited in This Article: ] |
| 95. | Cooper R. Impact of honey as a topical treatment for wounds remains unclear. Evid Based Med. 2014;19:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Tan SP, McLoughlin P, O’Sullivan L, Prieto ML, Gardiner GE, Lawlor PG, Hughes H. Development of a novel antimicrobial seaweed extract-based hydrogel wound dressing. Int J Pharm. 2013;456:10-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Unfallchirurg. 1993;96:488-492. [PubMed] [Cited in This Article: ] |
| 98. | Rosenthal EL, Blackwell KE, McGrew B, Carroll WR, Peters GE. Use of negative pressure dressings in head and neck reconstruction. Head Neck. 2005;27:970-975. [PubMed] [Cited in This Article: ] |
| 99. | Schuster R, Moradzadeh A, Waxman K. The use of vacuum-assisted closure therapy for the treatment of a large infected facial wound. Am Surg. 2006;72:129-131. [PubMed] [Cited in This Article: ] |
| 100. | Andrews BT, Smith RB, Goldstein DP, Funk GF. Management of complicated head and neck wounds with vacuum-assisted closure system. Head Neck. 2006;28:974-981. [PubMed] [Cited in This Article: ] |
| 101. | McDonald TJ, Lopez MA. Management of facial trauma: lessons of war. Facial Plast Surg. 2010;26:482-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 102. | Tian B, Khoo D, Tay AC, Soo KC, Tan NC, Tan HK, Iyer NG. Management of orocutaneous fistulas using a vacuum-assisted closure system. Head Neck. 2014;36:873-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Loaec E, Vaillant PY, Bonne L, Marianowski R. Negative-pressure wound therapy for the treatment of pharyngocutaneous fistula. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131:351-355. [PubMed] [Cited in This Article: ] |
| 104. | Palm HG, Hauer T, Simon C, Willy C. [Vacuum-assisted closure of head and neck wounds]. HNO. 2011;59:819-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Asher SA, White HN, Illing EA, Carroll WR, Magnuson JS, Rosenthal EL. Intraluminal negative pressure wound therapy for optimizing pharyngeal reconstruction. JAMA Otolaryngol Head Neck Surg. 2014;140:143-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Hendricks N, Hendricks J, Hoffmann K, Hemprich A, Halama D. Using medical silicone to ensure an airtight negative pressure wound therapy dressing seal in challenging wounds: a case series. Ostomy Wound Manage. 2014;60:40-46. [PubMed] [Cited in This Article: ] |
| 107. | Karr JC, de Mola FL, Pham T, Tooke L. Wound healing and cost-saving benefits of combining negative-pressure wound therapy with silver. Adv Skin Wound Care. 2013;26:562-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 108. | Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty. 2014;14:e41. [PubMed] [Cited in This Article: ] |
| 109. | Matiasek J, Djedovic G, Mattesich M, Morandi E, Pauzenberger R, Pikula R, Verstappen R, Pierer G, Koller R, Rieger UM. The combined use of NPWT and instillation using an octenidine based wound rinsing solution: a case study. J Wound Care. 2014;23:590, 592-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 110. | Marx RE. Radiation injury to tissue. In: Kindwall EP, ed. Hyperbaric Medicine Practice. Flagstaff, Best Publishing 1995; 464-503. [Cited in This Article: ] |
| 111. | Teguh DN, Levendag PC, Noever I, Voet P, van der Est H, van Rooij P, Dumans AG, de Boer MF, van der Huls MP, Sterk W. Early hyperbaric oxygen therapy for reducing radiotherapy side effects: early results of a randomized trial in oropharyngeal and nasopharyngeal cancer. Int J Radiat Oncol Biol Phys. 2009;75:711-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 112. | Feldmeier J, Carl U, Hartmann K, Sminia P. Hyperbaric oxygen: does it promote growth or recurrence of malignancy? Undersea Hyperb Med. 2003;30:1-18. [PubMed] [Cited in This Article: ] |
| 113. | Neovius EB, Lind MG, Lind FG. Hyperbaric oxygen therapy for wound complications after surgery in the irradiated head and neck: a review of the literature and a report of 15 consecutive patients. Head Neck. 1997;19:315-322. [PubMed] [Cited in This Article: ] |
| 114. | Filntisis GA, Moon RE, Kraft KL, Farmer JC, Scher RL, Piantadosi CA. Laryngeal radionecrosis and hyperbaric oxygen therapy: report of 18 cases and review of the literature. Ann Otol Rhinol Laryngol. 2000;109:554-562. [PubMed] [Cited in This Article: ] |
| 115. | Abe M, Shioyama Y, Terashima K, Matsuo M, Hara I, Uehara S. Successful hyperbaric oxygen therapy for laryngeal radionecrosis after chemoradiotherapy for mesopharyngeal cancer: case report and literature review. Jpn J Radiol. 2012;30:340-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 116. |
Narozny W, Sicko Z, Kot J, Stankiewicz C, Przewozny T, Kuczkowski J; Hyperbaric oxygen therapy in the treatment of complications of irradiation in head and neck area. |
| 117. | Dequanter D, Jacobs D, Shahla M, Paulus P, Aubert C, Lothaire P. The effect of hyperbaric oxygen therapy on treatment of wound complications after oral, pharyngeal and laryngeal salvage surgery. Undersea Hyperb Med. 2013;40:381-385. [PubMed] [Cited in This Article: ] |
| 118. | Annane D, Depondt J, Aubert P, Villart M, Géhanno P, Gajdos P, Chevret S. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893-4900. [PubMed] [Cited in This Article: ] |
| 119. | Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012;5:CD005005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 120. | Fingeret MC, Teo I, Goettsch K. Body image: a critical psychosocial issue for patients with head and neck cancer. Curr Oncol Rep. 2015;17:422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 121. | Iteld L, Yu P. Pharyngocutaneous fistula repair after radiotherapy and salvage total laryngectomy. J Reconstr Microsurg. 2007;23:339-345. [PubMed] [Cited in This Article: ] |
| 122. | Galli J, De Corso E, Volante M, Almadori G, Paludetti G. Postlaryngectomy pharyngocutaneous fistula: incidence, predisposing factors, and therapy. Otolaryngol Head Neck Surg. 2005;133:689-694. [PubMed] [Cited in This Article: ] |
| 123. | Smith TJ, Burrage KJ, Ganguly P, Kirby S, Drover C. Prevention of postlaryngectomy pharyngocutaneous fistula: the Memorial University experience. J Otolaryngol. 2003;32:222-225. [PubMed] [Cited in This Article: ] |
| 124. | Righini C, Lequeux T, Cuisnier O, Morel N, Reyt E. The pectoralis myofascial flap in pharyngolaryngeal surgery after radiotherapy. Eur Arch Otorhinolaryngol. 2005;262:357-361. [PubMed] [Cited in This Article: ] |
| 125. | Demir Z, Velidedeoğlu H, Celebioğlu S. Repair of pharyngocutaneous fistulas with the submental artery island flap. Plast Reconstr Surg. 2005;115:38-44. [PubMed] [Cited in This Article: ] |
| 126. | Rennekampff HO, Tenenhaus M. Turnover flap closure of recalcitrant tracheostomy fistula: a simplified approach. Plast Reconstr Surg. 2007;119:551-555. [PubMed] [Cited in This Article: ] |
| 127. | Sadigh PL, Wu CJ, Feng WJ, Hsieh CH, Jeng SF. New double-layer design for 1-stage repair of orocutaneous and pharyngocutaneous fistulae in patients with postoperative irradiated head and neck cancer. Head Neck. 2015;Jan 12; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |