Published online Aug 12, 2014. doi: 10.5318/wjo.v4.i3.82
Revised: July 4, 2014
Accepted: August 10, 2014
Published online: August 12, 2014
Processing time: 253 Days and 9.7 Hours
AIM: To estimate the cumulative probability of Nd:YAG capsulotomy at a teaching institution and evaluate secondary risk factors.
METHODS: The records of all patients who underwent phacoemulsification with intraocular lens (IOL) placement between 2005-2010 were retrospectively reviewed. The cumulative probability of Nd:YAG capsulotomy (capsulotomy) was calculated using Kaplan-Meier survival analysis and secondary risk factors were evaluated using the Cox proportional hazards regression model.
RESULTS: One thousand three hundred and fifty four charts were reviewed. A total of 70 capsulotomies were performed. The mean follow-up was 19.4 mo (standard deviation 17 mo). The cumulative probability of capsulotomy was 4% at 1 year, 5% at 2 years, and 9% at 3 years. Multivariate analysis demonstrated an increased risk with younger age (HR = 1.03, CI 1.01-1.05, P = 0.007), placement of sulcus IOL (HR = 2.57, CI 1.32-4.99, P = 0.005), ocular trauma (HR = 2.34, CI 1.13-4.83, P = 0.02), and phacoemulsification by a more experienced surgeon (HR = 4.32, CI 1.89-9.87, P = 0.001).
CONCLUSION: Cumulative probability of capsulotomy was lower than previously reported. Posterior capsule opacification was strongly associated with younger age and factors associated with high-risk cataract surgery. Surgeon awareness to the risk factors that correlate with posterior capsulotomy may allow for more thorough pre-operative disclosure and enhance patient satisfaction.
Core tip: Posterior capsule opacification (PCO) is a known late sequelae of cataract surgery. Our study uncovers risk of PCO in teaching institutions is associated with surgeon experience in that YAG capsulotomy rates are higher in patients whose cataract surgery was performed by a more experienced surgeon. Capsulotomy rates overall were lower than previously reported.
- Citation: Junk AK, Dunn EN, Galor A, Wellik SR, Pelletier J, Gregori N, Feuer W. Cumulative probability and risk analysis for Nd:YAG laser capsulotomy. World J Ophthalmol 2014; 4(3): 82-86
- URL: https://www.wjgnet.com/2218-6239/full/v4/i3/82.htm
- DOI: https://dx.doi.org/10.5318/wjo.v4.i3.82
Posterior capsule opacification (PCO) is the most common late sequelae after cataract surgery. Its definitive treatment, Nd:YAG laser posterior capsulotomy poses a financial burden on health care systems worldwide and causes rare, but serious patient morbidity[1,2]. Identifying the incidence and risk factors for PCO is important, not only for prevention, but also to provide patients with better pre-operative disclosure of the late changes possible after cataract surgery.
The cumulative probability of capsulotomy after phacoemulsification ranges between 1.95% and 11.8% at one year[3-5] and increases to 18.5% to 20.7% at three year. Independent and well established risk factors for capsulotomy include younger age at the time of cataract extraction and intraocular lens (IOL) properties[6,7]. A metaanalysis evaluating 66 prospective randomized controlled clinical trials comparing poly-methylmethacrylate (PMMA), hydrogel, hydrophobic acrylic and silicone IOLs did not detect a significant influence of IOL material on PCO incidence. In contrast, sharp edged IOL optics had a significantly lower incidence of PCO compared to round edged IOL optics. Haptic design, whether one-piece or three-piece IOLs did not bear influence on PCO incidence. Johansson et al[8] reported a cumulative incidence of capsulotomy five year after cataract extraction with IOL placement in the capsular bag of 17% with sharp-edged hydrophobic acrylic IOLs, 24% with round-edged hydrophobic acrylic IOLs, and 30% with sharp-edged hydrophilic acrylic IOLs. Additionally, they observed that patients surviving the five year study were more likely to have had capsulotomy. Along with modern IOL design and evolution in surgical technique, from extracapsular cataract extraction to small incision phacoemulsification, capsulotomy rates have declined since. While it is encouraging that the incidence of capsulotomy appears to be decreasing the reported rates in the literature cannot be extrapolated to a patient population at a teaching institution.
This study sets out to evaluate the influence of surgeon experience on PCO incidence. Prior studies evaluating the influence of surgical experience on PCO did not further differentiate data by year of training[6]. While patients may be stratified at the time of preoperative evaluation to match eyes more prone to intraoperative challenges with more experienced surgeons at a teaching institution prior trauma is commonplace among veterans as most were experienced some trauma during training and service. Likewise, intraoperative floppy iris syndrome and the ubiquitous intake of alpha adrenergic for benign prostate hyperplasia in the veteran population cannot be avoided by a novice surgeon in this population. Given the uniform patient population and identical surgical technique this study is uniquely positioned to evaluate the cumulative incidence of capsulotomy and associated risk factors including any influence of year of training.
After receiving institutional review board approval, the medical records of 1354 patients who underwent cataract extraction at the Veteran Affairs Hospital in Miami, Florida between 2005 and 2010 were retrospectively reviewed. Patients who underwent phacoemulsification with IOL placement in the capsular bag, ciliary sulcus, or anterior chamber were included in the review. Patients with anterior capsule tear, posterior capsule tear, anterior or posterior vitrectomy were included. Patients with concomitant unrelated surgery (corneal transplant, trabeculectomy, glaucoma drainage device, epiretinal membrane peel, endolaser) were included. Eyes that underwent intraoperative conversion to manual extracapsular cataract extraction were excluded. For patients who underwent sequential cataract extraction, only the first eye was included in the analysis.
The following information for each patient was recorded: age at surgery; date of surgery; date of capsulotomy; date of last visit; gender; ethnicity; history of diabetes, glaucoma, ocular trauma, and uveitis; intraoperative anterior vitrectomy, pars plana vitrectomy, concomitant glaucoma surgery, posterior capsule rupture; experience level of primary surgeon; and IOL model (SN60WF (hydrophobic acrylic, sharp-edged, 1-piece), MA60AC (hydrophobic acrylic, round-edged, 3-piece), SN60T/SN6AT (hydrophobic acrylic, sharp-edged, 1-piece), and MTA (PMMA, convexoplano, 1-piece).
Surgeries were performed by an ophthalmology team, which included an attending surgeon and a second year ophthalmology resident [post-graduate year (PGY) 3], or a third year ophthalmology resident (PGY4), or by an attending surgeon (PGY5 or greater) and assistant surgeon in training. Patients were preoperatively stratified and patients with a phacodonesis due to ocular trauma, prior eye surgery, monocular patients, pseudoexfoliation of the lens, pupil dilation less than 6 mm, corneal opacity, and endothelial guttatae were operated by a PGY4 or higher level surgeon. After routine postoperative follow-up at one day, one week, and one month after surgery with their primary surgeon, patients were followed every six to twelve months. The indications for capsulotomy were driven by patients’ symptoms (decreased visual acuity, decreased contrast sensitivity, or significant glare) with objective evidence of PCO. The final decision for capsulotomy was made by an attending physician.
The cumulative probability of capsulotomy was calculated by Kaplan-Meier survival analysis. The Cox proportional hazards regression model was used to evaluate potential risk factors including: age; gender; ethnicity; diabetes; glaucoma; ocular trauma, uveitis, anterior vitrectomy, pars plana vitrectomy, concomitant glaucoma surgery; posterior capsular rupture; IOL type, IOL placement; and surgeon experience.
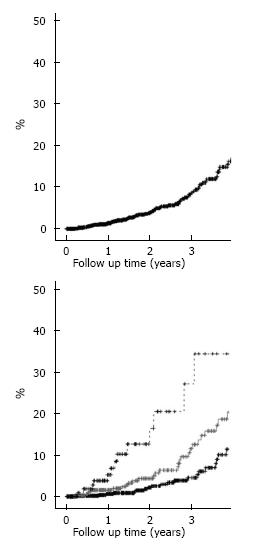
Table 1 displays the patient’s demographic and clinical information. 1354 cataract extractions with phacoemulsification and IOL placement were included in the study with a total of 70 capsulotomies performed after a mean follow-up of 19.4 mo (SD 17 mo). The cumulative probability of capsulotomy in our population was 4% at 1 year, 5% at 2 year, and 9% at 3 year (Figure 1A).
| Number eyes (patients) | 1354 (1354) |
| Age at time of cataract surgery, mean ± SD (n) | 69 ± 10 (1354) range 38-94 |
| Months follow-up, mean ± SD | 19.4 ± 17 mo |
| Gender, % male (n) | 97 (1318) |
| Ethnicity | |
| % White, non-Hispanic (n) | 63 (315) |
| % Black, non-Hispanic | 23 (113) |
| % White, Hispanic | 14 (68) |
| Diabetes, % yes (n) | 35 (477) |
| Glaucoma, % yes (n) | 33 (452) |
| Ocular trauma, % yes (n) | 5 (62) |
| Uveitis, % yes (n) | 4 (57) |
| Selected lens type, % (n) | |
| Monofocal, 3-piece (MA60) | 29 (389) |
| Monofocal and toric, 1-piece (SN60, SN6A) | 69 (937) |
| Anterior chamber lens (MTA) | 1.6 (22) |
| Surgeon level, % (n) | |
| PGY2 surgeon | 56 (757) |
| PGY3 surgeon | 33 (437) |
| PGY5 or higher (Experienced surgeon) | 11 (148) |
| Sulcus lens placed, % (n) | 6.6 (89) |
| Anterior vitrectomy performed, % (n) | 4 (54) |
| Planned Pars Plana Vitrectomy performed, % (n) | 4 (51) |
| Unplanned Pars Plana Vitrectomy performed, % (n) | 1 (10) |
| Concomitant glaucoma surgery | 2.7 (36) |
| Posterior capsular rupture | 2.9 (39) |
Table 2 displays the variables found to significantly increase the likelihood of capsolotomy. Younger individuals were at increased risk for capsulotomy compared to older individuals. Sulcus IOL placement portended an approximate 2.5 fold increased risk for subsequent capsulotomy. Clinical signs of ocular trauma increased the risk of capsulotomy 2.3 fold. Increasing surgeon experience increased the risk of capsulotomy with a 2.39 fold increased risk for PGY4 surgeons and a 4.32 fold increased risk for PGY 5 and above surgeons. The three year capsulotomy rate for PGY3/attending teams was 4%, for PGY4/attending teams was 13%, and for PGY5/attending teams was 24% (Figure 1B). Factors not found to affect the risk of capsulotomy included patients’, ethnicity, and comorbidities such as diabetes, glaucoma, concomitant glaucoma surgery, uveitis, intraoperative anterior vitrectomy, pars plana vitrectomy, posterior capsule rupture, shape or material of the IOL.
| Variable | Uni-variable | Multi-variable | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Decade of age1 | 0.68 (0.53-0.86) | 0.003 | 0.74 (0.61-0.90) | 0.007 |
| Surgeon | ||||
| PGY3/PGY2 | 2.83 (1.63-4.9) | < 0.0005 | 2.39 (1.40-4.09) | 0.002 |
| PGY5/PGY2 | 6.03 (2.96-12.3) | < 0.0005 | 4.32 (1.89-9.87) | 0.001 |
| Sulcus lens, yes/no | 3.43 (1.9-6.16) | < 0.0005 | 2.57 (1.32-4.99) | 0.005 |
| Ocular trauma, yes/no | 2.84 (1.41-5.72) | 0.003 | 2.34 (1.13-4.83) | 0.020 |
We found a cumulative probability of capsulotomy after phacoemulsification of 4% at 1 year, 5% at 2 year, and 9% at 3 year. The highest level of evidence describing the probability of capsulotomy after cataract extraction is provided in a meta-analysis of 49 studies that reported a 20.7% cumulative probability of capsulotomy at three year[9]. While it is encouraging that the capsulotomy rate at our institution is much lower at three year, it is important to consider that the metaanalysis included patients who underwent traditional extracapsular cataract extraction, a surgical technique known to be associated with a higher risk of PCO formation. However, studies evaluating PCO incidence after phacoemulsification found similarly high rates of PCO, at 18.5% cumulative probability of capsulotomy after 3 year[3].
Differences in population demographics and lens choice may partially explain differences between published capsulotomy rates. The surgical population at the Veterans Affairs Hospital was younger with a mean age of 69 ± 10 year compared to 76.7 year and 74 year in other studies[4,8]. Our data confirms that younger age is an independent risk factor for capsulotomy. Female patients may carry a higher rate of capsulotomy[6]. Given that 97% of our patients were men our data may be skewed towards a lower incidence of capsulotomy. However, we could not confirm prior studies showing an advantage of sharped edged IOL optics over round edged optics placed into the capsular bag with regards to capsulotomy rates.
In addition to younger age, sulcus placement, clinical signs of ocular trauma and increasing surgeon experience were independent risk factors for capsulotomy. We found a 3% increase in the probability of capsulotomy with a one year difference in age. This translates into a 34% increased risk for PCO with a 10 years difference in age. The greater proliferation rate of equatorial lens epithelial cells in younger individuals is believed to be responsible for increased rate of PCO[10] .
Patients in our study who had IOL placement into the ciliary sulcus were also at higher risk of capsulotomy. Sulcus IOLs have previously been reported to increase the risk of visually significant PCO[11,12]. A post-mortem prospective study of 3493 eyes found that fixation of the IOL outside the capsular bag was a significant risk factor for capsulotomy or central PCO in the visual axis[12]. When neither haptic nor IOL optic is placed inside the capsular bag, the IOL does not form an effective barrier to lens fiber migration and PCO ensues in the visual axis[12-14].
The increased probability of capsulotomy with increasing surgeon experience level stands in contrast to Elgohary[6] were no difference in capsolotomy rates were found by surgeon experience. Our observation may be partially explained by a referral bias at our institution where more experienced surgeons are referred more complex cases including those with poor mydriasis, pseudoexfoliation, evidence of ocular trauma, prior retinal or glaucoma surgery, extreme axial length, prior intravitreal injection, and mature cataracts even though none of these factors reached statistical significance as an independent risk factor for capsulotomy. While patients with ocular comorbidities may undergo more frequent follow up beyond routine annual exams and PCO may be detected and treated earlier, frequent follow up in diabetic patients did not result in a higher incidence of PCO compared to non-diabetics (15.3% vs 21.2% at 3 year)[6]. On the other hand, our data may be biased in that an unknown number of patients may have sought evaluation and treatment for PCO outside of the VA system locally or at a different VA eye care facility.
Furthermore, the use of capsulotomy as opposed to PCO formation as a primary endpoint and the reliance on multiple observers to diagnose the PCO may limit our findings. The probability of capsulotomy has been shown to increase steadily until it levels off at five year after phacoemulsification[4,9]. Further follow up will be required to assess whether this trend holds in our population.
Despite its limitations, this study demonstrates that the cumulative incidence of capsulotomy is significantly lower than previously reported in this teaching institution. However, PCO remains a common late sequelae of modern cataract surgery. The identification of patients at greater risk of capsulotomy, such as younger patients or those at risk for sulcus-placed IOL will allow for more thorough pre-operative disclosure, appropriate follow-up care, and enhance patient satisfaction.
This study sets out to evaluate the influence of surgeon experience on posterior capsule opacification (PCO) incidence. Prior studies evaluating the influence of surgical experience on PCO did not further differentiate data by year of training. Along with modern intraocular lens (IOL) design and evolution in surgical technique, from extracapsular cataract extraction to small incision phacoemulsification, capsulotomy rates have declined. While it is encouraging that the incidence of capsulotomy appears to be decreasing the reported rates in the literature cannot be extrapolated to a patient population at a teaching institution.
The increased probability of capsulotomy with increasing surgeon experience level stands in contrast to prior observations. Patient referral based on case complexity may have introduced a selection bias.
Compared to prior studies, capsulotomy rates are in decline. This is attributable to new intraocular lens design and materials, advancements in surgical technique. In this study surgeon experience appeared to be associated with higher risk for capsulotomy. However this is likely due to selection bias, as more complex cases associated with greater risk of complications were referred to more experienced surgeons.
The identification of patients at greater risk of capsulotomy, such as younger patients or those at risk for sulcus-placed IOL will allow for more thorough pre-operative disclosure, appropriate follow-up care, and enhance patient satisfaction.
This is a nice and well presented manuscript of posterior capsular opacification cumulative risk.
P- Reviewer: Joo CK, Jhanji V, Navas A S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Steinberg EP, Javitt JC, Sharkey PD, Zuckerman A, Legro MW, Anderson GF, Bass EB, O’Day D. The content and cost of cataract surgery. Arch Ophthalmol. 1993;111:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular edema, retinal detachment, and glaucoma after Nd: YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112:373-380. [PubMed] |
| 3. | Ando H, Ando N, Oshika T. Cumulative probability of neodymium: YAG laser posterior capsulotomy after phacoemulsification. J Cataract Refract Surg. 2003;29:2148-2154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Baratz KH, Cook BE, Hodge DO. Probability of Nd: YAG laser capsulotomy after cataract surgery in Olmsted County, Minnesota. Am J Ophthalmol. 2001;131:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010;CD003738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Elgohary MA, Dowler JG. Incidence and risk factors of Nd: YAG capsulotomy after phacoemulsification in non-diabetic and diabetic patients. Clin Experiment Ophthalmol. 2006;34:526-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ninn-Pedersen K, Bauer B. Cataract patients in a defined Swedish population, 1986 to 1990. V. Postoperative retinal detachments. Arch Ophthalmol. 1996;114:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Johansson B. Clinical consequences of acrylic intraocular lens material and design: Nd: YAG-laser capsulotomy rates in 3 x 300 eyes 5 years after phacoemulsification. Br J Ophthalmol. 2010;94:450-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology. 1998;105:1213-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Wormstone IM, Liu CS, Rakic JM, Marcantonio JM, Vrensen GF, Duncan G. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997;38:396-404. [PubMed] |
| 11. | Ayed T, Rannen R, Naili K, Sokkah M, Gabsi S. [Risk factors for secondary cataract: a case-control study with multivariate analysis]. J Fr Ophtalmol. 2002;25:615-620. [PubMed] [DOI] [Full Text] |
| 12. | Ram J, Pandey SK, Apple DJ, Werner L, Brar GS, Singh R, Chaudhary KP, Gupta A. Effect of in-the-bag intraocular lens fixation on the prevention of posterior capsule opacification. J Cataract Refract Surg. 2001;27:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Peng Q, Apple DJ, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, Schoderbek R, Guindi A. Surgical prevention of posterior capsule opacification. Part 2: Enhancement of cortical cleanup by focusing on hydrodissection. J Cataract Refract Surg. 2000;26:188-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 117] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Duncan G, Wormstone IM, Davies PD. The aging human lens: structure, growth, and physiological behaviour. Br J Ophthalmol. 1997;81:818-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |