Published online Aug 12, 2014. doi: 10.5318/wjo.v4.i3.47
Revised: April 30, 2014
Accepted: July 12, 2014
Published online: August 12, 2014
Processing time: 204 Days and 14.2 Hours
Glaucoma refers to a group of diseases characterized by optic neuropathies that are commonly associated with degeneration of the retinal ganglion cells. Although intraocular pressure (IOP) is the only proven treatable factor, several studies indicate that other factors are involved in the pathogenesis of glaucoma. Since normal tension glaucoma (NTG) is the most common glaucoma at least in Japan and South Korea, development of new therapeutic strategies for glaucoma, besides reduction of IOP, is crucial. The clinical characteristics and mechanisms underlying neuronal degeneration in Alzheimer’s disease, a progressive neurodegenerative disease, are similar to those of glaucoma. Impaired cerebral blood flow (CBF) is common to both these diseases; therefore, improving CBF may be considered a new treatment for glaucoma, especially for NTG. In addition, targeting the formation and aggravation pathway for amyloid-β and administration of apolipoprotein E-containing lipoproteins may be potential strategies for glaucoma treatment.
Core tip: This review summarizes studies describing the similarities between glaucoma and Alzheimer’s disease, thereby suggesting new probable therapeutic strategies for glaucoma.
- Citation: Sugiyama T. Glaucoma and Alzheimer's disease: Their clinical similarity and future therapeutic strategies for glaucoma. World J Ophthalmol 2014; 4(3): 47-51
- URL: https://www.wjgnet.com/2218-6239/full/v4/i3/47.htm
- DOI: https://dx.doi.org/10.5318/wjo.v4.i3.47
Glaucoma refers to a group of diseases characterized by optic neuropathies that are commonly associated with degeneration of the retinal ganglion cells (RGCs)[1,2], which results in a characteristic optic nerve head (ONH) appearance and corresponding visual field defects. Global surveys indicate that glaucoma is the second leading cause of visual impairment, next to cataract[3]. Normal tension glaucoma (NTG) is the most common type of glaucoma at least in Japan and South Korea[4,5]. Currently, although intraocular pressure (IOP) is the only proven treatable factor for glaucoma, neuroprotection is increasingly being considered as a treatment strategy for glaucoma[6-8].
Alzheimer’s disease (AD), a representative neurodegenerative disease, is one of the most common causes of dementia. Hallmarks of AD include extracellular amyloid-β plaques and intracellular neurofibrillary tangles comprising abnormally phosphorylated tau protein[9,10]. The ε4 allele of apolipoprotein E (APOE) has been found to be a major genetic risk factor for AD[11].
In this review, the association of glaucoma with AD is summarized; then, based on their common pathophysiology, probable therapies for glaucoma are presented briefly.
Several reports have documented the clinical association of glaucoma with AD. Bayer et al[12] showed that patients with AD may have a significantly increased incidence of glaucoma and that ocular hypertension with normal visual fields and normal ONHs was not found in patients with AD, suggesting that the optic nerve seems to be less resistant to elevated IOP levels in AD patients[12]. Tamura et al. also found that the prevalence of open-angle glaucoma was significantly higher in AD patients than in controls[13]. Parisi reported a similar correlation between morphological and functional retinal impairment in patients with glaucoma and those with AD[14].
In addition, a decrease in amyloid-β (1-42) and an increase in tau were found in the vitreous fluid from patients with glaucoma, similar to the findings in the cerebrospinal fluid from patients with AD[15]. Others also reported the involvement of amyloid-β in animal models of glaucoma[16-19]. For example, in a rat model of chronic ocular hypertension, the RGCs demonstrated caspase activation and abnormal processing of amyloid precursor protein (APP), which includes production of amyloid-β[16]. Furthermore, APP and amyloid-β were increased in the RGC layer of DBA/2J glaucomatous mouse eyes[17]. APP and amyloid-β were also found to be highly expressed in the RGC layer of ocular hypertensive C57BL/6 mouse eyes[18]. Moreover, upregulation of amyloid-β was induced in the retina and ONH of a monkey model of chronic ocular hypertension[19].
Several reports implicate APOE in the pathogenesis of glaucoma, specifically NTG. A genetic study indicated that inheritance of the APOE Ε4 allele is associated with elevated risks for glaucomatous changes that are not related to increased IOP[20]. Other genetic studies indicated that APOE-promoter single-nucleotide polymorphisms affect the phenotype of primary open-angle glaucoma and may be associated with a risk of glaucoma occurrence[21,22]. A recent report also revealed that patients with open-angle glaucoma had higher aqueous levels of multiple biomarkers of AD, including APOE, than did cataract patients[23].
Studies using single-photon emission computed tomography (SPECT) have indicated that CBF reductions were most common in the temporoparietal regions in AD patients[24]. Disturbed CBF has been reported not only in AD patients but also in glaucoma patients. Compared to controls, glaucoma patients were found to have a lower blood velocity in the middle cerebral artery (MCA) and an absence of vasoreactivity to hypoxia[25]. The MCA supplies blood to the anterior temporal lobes where blood flow is reduced in AD patients. In addition, the same group found a significant correlation between blood velocity in the MCA and central visual function measured by foveal cone electroretinograms and the visual field[26]. This finding suggests that diminished central visual function may be a manifestation of widespread cerebrovascular insufficiency in certain patients with glaucoma. Another group also reported enhanced transmission of oscillations in the mean arterial pressure onto CBF in patients with glaucoma including NTG[27]. They suggested that impaired cerebral autoregulation might contribute to an increased risk of cerebrovascular disorders in glaucoma patients.
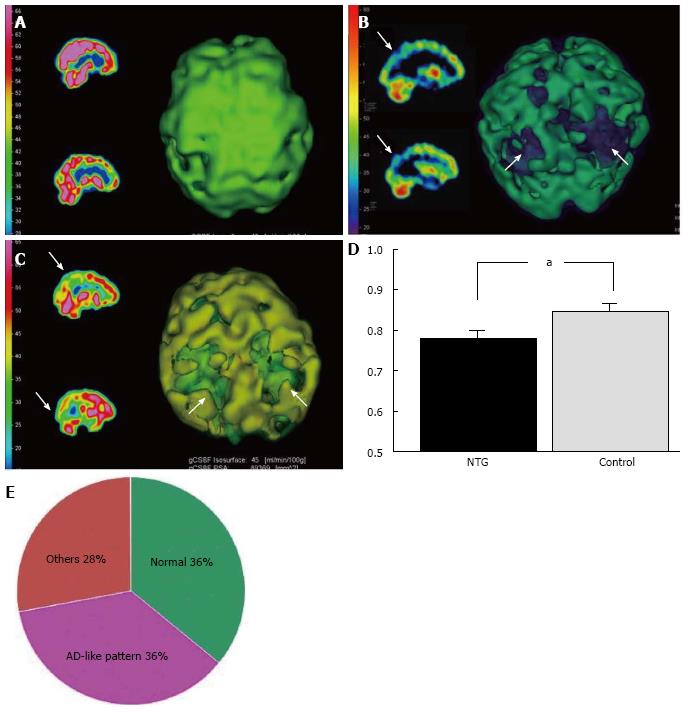
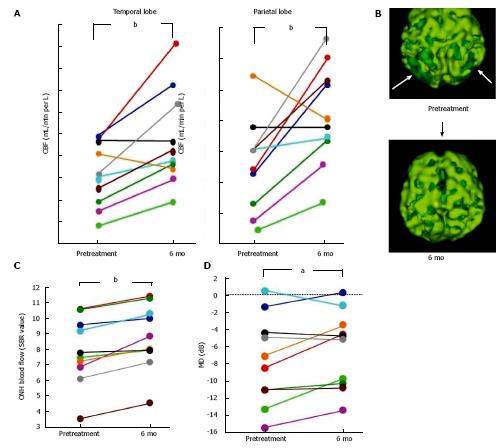
AD patients usually have a characteristic cerebral perfusion pattern, a decrease in CBF ranging from the parietal lobe to the temporal lobe, as shown in Figure 1B. In our SPECT study, we classified cerebral perfusion pattern into normal, AD-like (Figure 1A and C) and other patterns. We found that 22.6% of NTG patients exhibited an AD-like cerebral perfusion pattern[28]. Relative CBF in the parietal lobe was lower in NTG patients than in controls (Figure 1D)[28]. In a subsequent study, we increased the number of subjects and found that 36% of the NTG patients showed an AD-like pattern (Figure 1E)[29]. We also obtained a preliminary result regarding the effects of donepezil, an anti-AD drug, on NTG patients. Visual field, ONH blood flow, and CBF in the temporal and parietal lobes were improved after 6 mo of oral administration of donepezil although the IOP remained unchanged (Figure 2)[29]. This result implies that an AD-like cerebral perfusion abnormality might be involved in the pathogenesis of NTG in certain patients, and improving CBF can be a therapeutic strategy for glaucoma.
Several types of recent or new therapeutic strategies for glaucoma have been suggested on the basis that similar pathological mechanisms underlie neuronal death in both AD and glaucoma. One such strategy is α-2 adrenergic receptor activation: compensating for common loss of noradrenergic innervation of RGCs and central neurons in glaucoma and AD[30]. A recent randomized, double-masked, multicenter clinical trial showed that NTG patients treated with an α-2 adrenergic receptor agonist brimonidine were less likely to show progression in visual field defects than were patients treated with timolol[31].
On the other hand, another report suggested that targeting different components of the formation and aggravation pathway for amyloid-β could be effective in reducing glaucomatous RGC apoptosis in vivo[32]. Several studies have reported the protective effects of an anti-AD drug donepezil (an acetylcholinesterase inhibitor) on RGC death in vitro and in vivo[33,34]. One of them suggested that not only the activation of acetylcholine receptors but also a mechanism unrelated to acetylcholinesterase inhibition might contribute to the protective effect of donepezil[34].
Recently, it was reported that administration of APOE-containing lipoproteins protected RGCs from glutamate-induced apoptosis in vitro and partially protected RGCs from neurodegeneration in glutamate aspartate transporter-deficient mice, which exhibit many features similar to human NTG[35].
In conclusion, eliciting the relevance of AD when considering the pathogenesis of glaucomatous optic neuropathy may provide novel therapeutic strategies for protecting the optic nerve in glaucoma.
I would like to express my appreciation to Professor Tsunehiko Ikeda for giving me the opportunity to study this topic and to continue investigating it at the Department of Ophthalmology at Osaka Medical College until March 2013, and Dr. Keita Utsunomiya for collaborating with me during the SPECT study.
P- Reviewer: Hong YJ, Razeghinejad MR S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Schwartz M, Belkin M, Yoles E, Solomon A. Potential treatment modalities for glaucomatous neuropathy: neuroprotection and neuroregeneration. J Glaucoma. 1996;5:427-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Naskar R, Wissing M, Thanos S. Detection of early neuron degeneration and accompanying microglial responses in the retina of a rat model of glaucoma. Invest Ophthalmol Vis Sci. 2002;43:2962-2968. [PubMed] |
| 3. | Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844-851. [PubMed] |
| 4. | Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G, Inoue Y, Kitazawa Y; Tajimi Study Group, Japan Glaucoma Society. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641-1648. [PubMed] |
| 5. | Kim JH, Kang SY, Kim NR, Lee ES, Hong S, Seong GJ, Hong YJ, Kim CY. Prevalence and characteristics of glaucoma among Korean adults. Korean J Ophthalmol. 2011;25:110-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Tătaru CP, Purcărea VL. Antiglaucoma pharmacotherapy. J Med Life. 2012;5:247-251. [PubMed] |
| 7. | Chen SD, Wang L, Zhang XL. Neuroprotection in glaucoma: present and future. Chin Med J (Engl). 2013;126:1567-1577. [PubMed] |
| 8. | Johnson TV, Martin KR. Cell transplantation approaches to retinal ganglion cell neuroprotection in glaucoma. Curr Opin Pharmacol. 2013;13:78-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245-4249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2928] [Cited by in RCA: 3098] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 10. | Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913-4917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2356] [Cited by in RCA: 2567] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 11. | Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 876] [Cited by in RCA: 815] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 12. | Bayer AU, Ferrari F, Erb C. High occurrence rate of glaucoma among patients with Alzheimer’s disease. Eur Neurol. 2002;47:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 197] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Tamura H, Kawakami H, Kanamoto T, Kato T, Yokoyama T, Sasaki K, Izumi Y, Matsumoto M, Mishima HK. High frequency of open-angle glaucoma in Japanese patients with Alzheimer’s disease. J Neurol Sci. 2006;246:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 14. | Parisi V. Correlation between morphological and functional retinal impairment in patients affected by ocular hypertension, glaucoma, demyelinating optic neuritis and Alzheimer’s disease. Semin Ophthalmol. 2003;18:50-57. [PubMed] |
| 15. | Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005;49:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | McKinnon SJ, Lehman DM, Kerrigan-Baumrind LA, Merges CA, Pease ME, Kerrigan DF, Ransom NL, Tahzib NG, Reitsamer HA, Levkovitch-Verbin H. Caspase activation and amyloid precursor protein cleavage in rat ocular hypertension. Invest Ophthalmol Vis Sci. 2002;43:1077-1087. [PubMed] |
| 17. | Goldblum D, Kipfer-Kauer A, Sarra GM, Wolf S, Frueh BE. Distribution of amyloid precursor protein and amyloid-beta immunoreactivity in DBA/2J glaucomatous mouse retinas. Invest Ophthalmol Vis Sci. 2007;48:5085-5090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kipfer-Kauer A, McKinnon SJ, Frueh BE, Goldblum D. Distribution of amyloid precursor protein and amyloid-beta in ocular hypertensive C57BL/6 mouse eyes. Curr Eye Res. 2010;35:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Ito Y, Shimazawa M, Tsuruma K, Mayama C, Ishii K, Onoe H, Aihara M, Araie M, Hara H. Induction of amyloid-β(1-42) in the retina and optic nerve head of chronic ocular hypertensive monkeys. Mol Vis. 2012;18:2647-2657. [PubMed] |
| 20. | Vickers JC, Craig JE, Stankovich J, McCormack GH, West AK, Dickinson JL, McCartney PJ, Coote MA, Healey DL, Mackey DA. The apolipoprotein Ε4 gene is associated with elevated risk of normal tension glaucoma. Mol Vis. 2002;8:389–393. [PubMed] |
| 21. | Copin B, Brézin AP, Valtot F, Dascotte JC, Béchetoille A, Garchon HJ. Apolipoprotein E-promoter single-nucleotide polymorphisms affect the phenotype of primary open-angle glaucoma and demonstrate interaction with the myocilin gene. Am J Hum Genet. 2002;70:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Nowak A, Przybylowska-Sygut K, Gacek M, Kaminska A, Szaflik JP, Szaflik J, Majsterek I. Neurodegenerative Genes Polymorphisms of the -491A/T APOE, the -877T/C APP and the Risk of Primary Open-angle Glaucoma in the Polish Population. Ophthalmic Genet. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Inoue T, Kawaji T, Tanihara H. Elevated levels of multiple biomarkers of Alzheimer’s disease in the aqueous humor of eyes with open-angle glaucoma. Invest Ophthalmol Vis Sci. 2013;54:5353-5358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Holman BL. Perfusion and receptor SPECT in the dementias--George Taplin memorial lecture. J Nucl Med. 1986;27:855-860. [PubMed] |
| 25. | Harris A, Zarfati D, Zalish M, Biller J, Sheets CW, Rechtman E, Migliardi R, Garzozi HJ. Reduced cerebrovascular blood flow velocities and vasoreactivity in open-angle glaucoma. Am J Ophthalmol. 2003;135:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Harris A, Siesky B, Zarfati D, Haine CL, Catoira Y, Sines DT, McCranor L, Garzozi HJ. Relationship of cerebral blood flow and central visual function in primary open-angle glaucoma. J Glaucoma. 2007;16:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Tutaj M, Brown CM, Brys M, Marthol H, Hecht MJ, Dutsch M, Michelson G, Hilz MJ. Dynamic cerebral autoregulation is impaired in glaucoma. J Neurol Sci. 2004;220:49-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Sugiyama T, Utsunomiya K, Ota H, Ogura Y, Narabayashi I, Ikeda T. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am J Ophthalmol. 2006;141:394-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Yoshida Y, Sugiyama T, Utsunomiya K, Ogura Y, Ikeda T. A pilot study for the effects of donepezil therapy on cerebral and optic nerve head blood flow, visual field defect in normal-tension glaucoma. J Ocul Pharmacol Ther. 2010;26:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Tatton W, Chen D, Chalmers-Redman R, Wheeler L, Nixon R, Tatton N. Hypothesis for a common basis for neuroprotection in glaucoma and Alzheimer’s disease: anti-apoptosis by alpha-2-adrenergic receptor activation. Surv Ophthalmol. 2003;48:S25-S37. [PubMed] |
| 31. | Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: results from the Low-Pressure Glaucoma Treatment Study. Am J Ophthalmol. 2011;151:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 32. | Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci USA. 2007;104:13444-13449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 33. | Miki A, Otori Y, Morimoto T, Okada M, Tano Y. Protective effect of donepezil on retinal ganglion cells in vitro and in vivo. Curr Eye Res. 2006;31:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Sakamoto K, Ohki K, Saito M, Nakahara T, Ishii K. Histological protection by donepezil against neurodegeneration induced by ischemia-reperfusion in the rat retina. J Pharmacol Sci. 2010;112:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Hayashi H, Eguchi Y, Fukuchi-Nakaishi Y, Takeya M, Nakagata N, Tanaka K, Vance JE, Tanihara H. A potential neuroprotective role of apolipoprotein E-containing lipoproteins through low density lipoprotein receptor-related protein 1 in normal tension glaucoma. J Biol Chem. 2012;287:25395-25406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |