Revised: September 17, 2012
Accepted: October 10, 2012
Published online: November 16, 2012
AIM: To explore the optimal interval of intraocular pressure (IOP) measurement for screening glaucoma in healthy people.
METHODS: From January to December 2005, we consecutively enrolled all participants (> 20 years old) attending the Center for Preventive Medicine at St. Luke’s International Hospital in Tokyo, Japan, for the annual health check program. The program promoted the early detection of chronic diseases and their risk factors. We excluded people who had glaucoma or a high IOP (≥ 22 mmHg) at baseline. The annual health check-ups collected all demographic information and medical history with an initial evaluation, including IOP measurement. IOP was measured in both eyes with a full auto-tonometer TX-F (Canon, Tokyo, Japan). Participants with an IOP ≥ 22 mmHg in either eye were considered to require additional evaluation for glaucoma. We divided the participants into two groups based on age: under 65 years old and over 65 years old. The United States Department of Health and Human Services Centers for Medicare and Medicaid Services guideline was used as a reference.
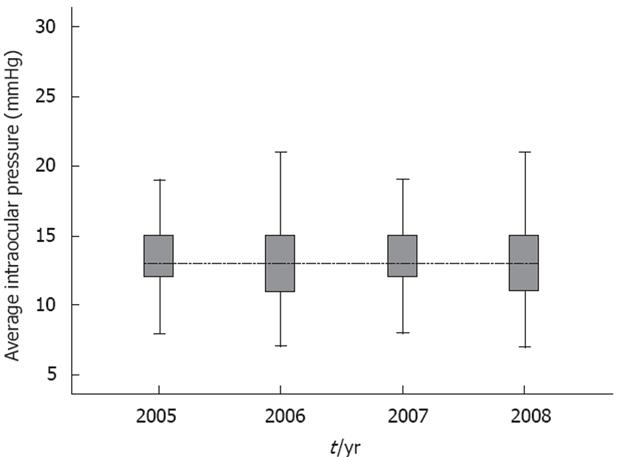
RESULTS: From January 2005 to July 2008, 12 385 participants underwent check-ups each year. The mean ± SD IOP in the higher eye at baseline was 13.4 (2.6) in 2005, 13.2 (2.7) in 2006, 13.3 (2.6), and 12.8 (2.6) in 2008. In addition, we analyzed the differences with an analysis of variance (ANOVA), and additional analysis was performed with Bonferroni’s correction. The difference between the 4 years was significant (P < 0.01) with ANOVA. Bonferroni analysis revealed significant differences between 2005 and 2006 (P < 0.01), 2005 and 2008 (P < 0.01), 2006 and 2007 (P < 0.01), 2006 and 2008 (P < 0.01), and 2007 and 2008 (P < 0.01). Only the difference between 2005 and 2007 was not significant (P = 0.1). Logistic regression suggested that only age (P < 0.01) and baseline IOP (P < 0.01) were associated with high IOP; the presence of diabetes, HgbA1c level, gender, systolic blood pressure, diastolic blood pressure, low-density lipoprotein and family history were non-significant.
CONCLUSION: Annual IOP check-ups may be recommended for participants aged ≥ 65 years with baseline IOPs of 17-21 mmHg. A check-up every 3 years or more may be recommended for patients with IOPs < 17 mmHg.
- Citation: Kobayashi D, Takahashi O, Glasziou PP, Fukui T. Optimal screening interval for intraocular pressure measurement for Asian glaucoma patients. World J Ophthalmol 2012; 2(1): 1-5
- URL: https://www.wjgnet.com/2218-6239/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5318/wjo.v2.i1.1
Glaucoma is a significant public health problem: approximately 45 million patients globally have open-angle glaucoma, and approximately 8.4 million patients become blind because of glaucoma[1-3]. Although it has been reported that the prevalence of glaucoma for Asians is less than for Caucasian and African races, approximately 4 million patients suffer from glaucoma in Japan[4-6]. Therefore, it is necessary to treat glaucoma and prevent blindness[7-10].
Intraocular pressure (IOP) measurement is one method to evaluate glaucoma[3,11], and it is sufficient as a screening procedure[12]. An IOP of 22 mmHg is considered a screening cut-off level[13,14], and IOP is measured with a contact or non-contact IOP tonometer. A non-contact tonometer is as reliable as Goldmann applanation[15]. Patients with IOP values greater than 22 mmHg require additional work-up for glaucoma treatments.
There are various recommendations for glaucoma screening[16-18]. The United States Preventive Services Task Force found insufficient evidence to recommend either for or against screening adults for glaucoma[19]. Routine population-based mass screening for glaucoma may not be cost-effective[20]. However, some guidelines suggest that screening high-risk participants, for example, the elderly, African-Americans, those who have a family history or those who have high IOP at baseline, may be effective[21] and cost-effective[20]. Therefore, we aimed to evaluate the optimal screening interval for glaucoma, especially among high-risk groups in Japan.
From January to December 2005, we consecutively enrolled all participants (> 20 years old) attending the Center for Preventive Medicine at St. Luke’s International Hospital in Tokyo, Japan, for the annual health check program. The program promoted the early detection of chronic diseases and their risk factors. The data collected contained current and past medical history, including diabetes[22], and family history, including glaucoma[23-26]. We excluded people who had glaucoma or a high IOP (≥ 22 mmHg) at baseline.
The annual health check-ups collected all demographic information and medical history with an initial evaluation, including IOP measurement. IOP was measured in both eyes with a full auto-tonometer TX-F (Canon, Tokyo, Japan). Participants with an intraocular pressure ≥ 22 mmHg in either eye were considered to require additional evaluation for glaucoma. The diagnosis of glaucoma was reported by individual participants. We divided participants into two groups based on age: under 65 years old and over 65 years old. The United States Department of Health and Human Services Centers for Medicare and Medicaid Services guideline was used as a reference. A total of 12 385 participants were enrolled in our study. The number of participants under 65 years old was 10 600, and the number of participants aged 65 years or older was 1785. Among those who were under 65 years old, 1331 had an IOP of 17-21 mmHg at baseline, 6781 had an IOP of 12-16 mmHg, and 1219 had an IOP of 11 mmHg or lower. Among those who were aged 65 or older, 184 had an IOP of 17-21 mmHg at baseline, 1048 had an IOP of 12-16 mmHg, and 553 had an IOP of 11 mmHg or lower.
All data analyses were performed with an exact binominal using SPSS software 15.0J (IBM Japan, Tokyo, Japan) and Stata version 10 (STATA Corp, College Station, TX).
From January 2005 to July 2008, 12 385 participants underwent check-ups every year. The mean ± SD age of participants was 50 (12) years, and 7617 (53%) were male. A total of 1852 (15%) were current smokers, and 436 (3.7%) had diabetes. A total of 133 (1.1%) had a family history of glaucoma. The mean ± SD body mass index was 22.5 (3.2) kg/m2, the height was 163.7 (8.7) cm, the systolic blood pressure (SBP) was 119.3 (17.7) mmHg, the diastolic blood pressure (DBP) was 74.2 (11.3) mmHg, the fasting plasma glucose was 100.4 (15.6) mg/dL, and the hemoglobin A1c (HbA1c) at baseline was 5.1 (0.6%). The total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels at baseline were 206.1 (33.5) mg/dL, 117.9 (29.6) mg/dL, 62.2 (15.7) mg/dL, and 102.4 (75.5) mg/dL, respectively (Table 1).
| Characteristics | mean ± SD |
| Age (yr) | 50 ± 12 |
| Male | 7617 (53) |
| Current smoker | 1852 (15.0) |
| Height (cm) | 163.7 ± 8.7 |
| Body mass index (kg/m2) | 22.5 ± 3.2 |
| Diabetes | 463 (3.7) |
| Family history of glaucoma | 133 (1.1) |
| Higher intraocular pressure in each eye (mmHg) | 13.3 ± 2.6 |
| Systolic blood pressure (mmHg) | 119.3 ± 17.7 |
| Diastolic blood pressure (mmHg) | 74.2 ± 11.3 |
| Glucose (mg/dL) | 100.4 ± 15.6 |
| Hemoglobin A1c (%) | 5.1 ± 0.6 |
| Total-cholesterol (mg/dL) | 206.1 ± 33.5 |
| Low-density lipoprotein-cholesterol (mg/dL) | 117.9 ± 29.6 |
| High-density lipoprotein-cholesterol (mg/dL) | 62.2 ± 15.7 |
| Triglyceride (mg/dL) | 102.4 ± 75.5 |
The mean ± SD IOP in the higher eye at baseline was 13.4 (2.6) in 2005, 13.2 (2.7) in 2006, 13.3 (2.6) in 2007, and 12.8 (2.6) in 2008. In addition, we analyzed the differences with an analysis of variance (ANOVA), and additional analysis was performed with Bonferroni’s correction. The difference between the 4 years was significant (P < 0.01) with the ANOVA. Bonferroni analysis revealed significant differences between 2005 and 2006 (P < 0.01), 2005 and 2008 (P < 0.01), 2006 and 2007 (P < 0.01), 2006 and 2008 (P < 0.01), and 2007 and 2008 (P < 0.01). Only the difference between 2005 and 2007 was not significant (P = 0.1) (Figure 1).
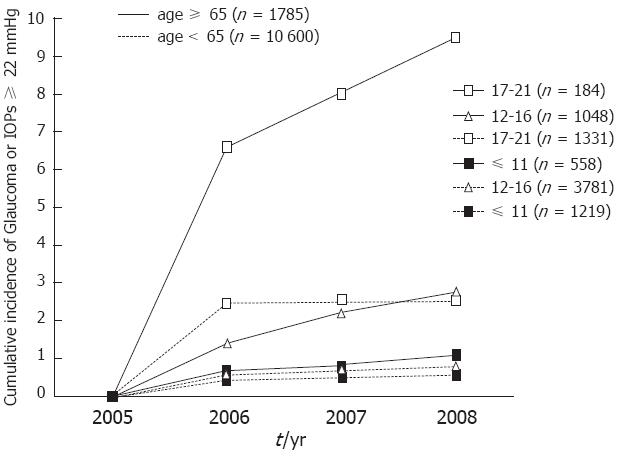
After three years, for participants with an IOP of 11 or less than 11 mmHg, 12-16 mmHg and 17-21 mmHg at baseline, the cumulative incidence [95% confidence interval (CI) was 1.1% (0.4%-2.4%), 2.8% (1.9%-4.1%) and 9.5% (5.5%-15.0%)], respectively, in the group over 65 years old and 0.5% (0.3%-0.9%), 0.8% (0.6%-1.0%) and 2.6% (1.8%-3.6%), respectively, in the group under 65 years old (Figure 2). We analyzed these data with a log-rank test. The result revealed that the group over 65 years old with a baseline IOP of 17-21 mmHg had significant differences compared to all of the other groups (P < 0.01) (Figure 2).
Logistic regression suggested that only age (P < 0.01) and baseline IOP (P < 0.01) were associated with high IOP; the presence of diabetes, HgbA1c level, gender, SBP, DBP, LDL and family history were non-significant.
Our study shows that the likelihood of IOP increasing to approximately 22 mmHg is strongly predicted by baseline IOP level and age. The screening interval for glaucoma by IOP measurement may be determined from a participant’s age and baseline IOP.
For participants over 65 years old, an IOP of 17 mmHg at baseline may be considerable. In the group with IOPs of 17-21 mmHg at baseline, the cumulative incidence of glaucoma or high IOP was approximately 7% in the following year. In contrast, in the groups with IOPs ≤ 11 mmHg and 12-16 mmHg at baseline, the cumulative incidence was below 3% in 3 years. Therefore, annual IOP check-ups may be appropriate for individuals with IOPs of 17-21 mmHg at baseline, and check-ups every 3 years or more may be appropriate for individuals with IOPs below 17 mmHg.
The cumulative incidence of glaucoma or high IOP was low for participants less than 65 years old. In groups whose IOP was ≤ 11 mmHg and 12-16 mmHg at baseline, the cumulative incidence was below 1% in 3 years. In the group that had IOPs of 17-21 mmHg at baseline, the cumulative incidence was below 3% in 3 years. Therefore, check-ups every 3 years or more may be appropriate for people under 65 years old in the Asian population.
The American Optometric Association recommends annual eye examinations for people at risk for glaucoma. Our results demonstrated that elderly patients with high baseline IOP meet the criteria of being at high risk for glaucoma.
In our study, participants whose baseline IOPs were 17-22 mmHg had a high incidence of high IOPs in the first year. In our opinion, this result was due to measurement error of IOPs[27]. Because IOP measurement using the non-contact method is prone to error, participants who had borderline IOPs at baseline and a wide range of measurement error tended to have high IOPs the following year. Because we analyzed cumulative incidence, the incidence of high IOPs tended to be high in the first year for those with IOPs of 17-22 mmHg.
There are some limitations to our study. First, our data lack possible risk factors for glaucoma, such as cataracts[28,29], steroid use[30] and myopia[31-33]. More frequent examination may be recommended for individuals who have these risk factors. Second, there are some missing data in our study because not all participants returned every year. Although 34 234 participants came to the health check-up in 2005, only 12 385 (36.2%) continued to come for 4 years. Because our health check-up was not mandatory, some participants did not return. Third, our data did not have the results of other optic nerve measurements to diagnose glaucoma[34-36]. Because Asians are more likely to have normal tension glaucoma compared to other races, further evaluations are required in a future study. Finally, our data lack evaluation for glaucoma. Although we could identify participants with high IOP using non-contact measurements from our data, we could not identify glaucoma patients. Additional studies that include glaucoma patient information are necessary to decide the optimal screening interval for glaucoma.
In conclusion, for the high-risk group (age ≥ 65 years and baseline IOP 17-21 mmHg), careful IOP check-up might be recommended. For all others, check-ups every 3 years or more appear to be reasonable.
Glaucoma is one of the most serious causes of blindness. Early detection and treatment are required. However, the screening interval for glaucoma is still controversial. This study aims to evaluate the optimal screening interval for glaucoma with non-contact intraocular pressure measurement.
The United States Preventive Services Task Force and National Institute for Health and Clinical Excellence address this type of screening guideline.
This study is innovative because of the evaluation of a screening interval for glaucoma, which is still controversial.
This study may be useful for glaucoma screening with non-contact intraocular pressure measurement. However, additional evaluations are needed to evaluate high-risk populations.
In this study, a large population undergoing general medical screening had their intraocular pressure evaluated at enrolment and annually thereafter. The authors attempt to define the optimal interval between intraocular pressure measurements.
Peer reviewers: Thomas Yorio, PhD, Professor, Department of Pharmacology and Neuroscience, University of North Texas Health Science Center, Fort Worth, TX 76107, United States; Colin Ian Clement, BSc, MBBS, PhD, FRANZC, Glaucoma Unit, Sydney Eye Hospital, 8 Macquarie Street, Sydney, NSW 2000, Australia; Naj Sharif, PhD, Director, Adjunct Professor, Molecular Pharmacology (R6-19), Alcon Research, Ltd, 6201 S Freeway, Fort Worth, TX 76134-2099, United States; Young Jae Hong, MD, PhD, Emeritus Professor, Glaucoma Center, Nune Eye Hospital, 907-16 Daechi-Dong, Gangnam-Ku, Seoul 135-280, South Korea
S- Editor Song XX L- Editor A E- Editor Lu YJ
| 1. | Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4855] [Cited by in RCA: 4914] [Article Influence: 258.6] [Reference Citation Analysis (0)] |
| 2. | Cedrone C, Mancino R, Cerulli A, Cesareo M, Nucci C. Epidemiology of primary glaucoma: prevalence, incidence, and blinding effects. Prog Brain Res. 2008;173:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Cook C, Foster P. Epidemiology of glaucoma: what's new? Can J Ophthalmol. 2012;47:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 4. | Iwase A, Suzuki Y, Araie M, Yamamoto T, Abe H, Shirato S, Kuwayama Y, Mishima HK, Shimizu H, Tomita G. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 341] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 5. | Stein JD, Kim DS, Niziol LM, Talwar N, Nan B, Musch DC, Richards JE. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011;118:1031-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Ang LP, Ang LP. Current understanding of the treatment and outcome of acute primary angle-closure glaucoma: an Asian perspective. Ann Acad Med Singapore. 2008;37:210-215. [PubMed] |
| 7. | Tarongoy P, Ho CL, Walton DS. Angle-closure glaucoma: the role of the lens in the pathogenesis, prevention, and treatment. Surv Ophthalmol. 2009;54:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Lee DA, Higginbotham EJ. Glaucoma and its treatment: a review. Am J Health Syst Pharm. 2005;62:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Glen FC, Crabb DP, Garway-Heath DF. The direction of research into visual disability and quality of life in glaucoma. BMC Ophthalmol. 2011;11:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Garudadri C, Senthil S, Rao HL. Evidence-based approach to glaucoma management. Indian J Ophthalmol. 2011;59 Suppl:S5-S10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Burr JM, Botello-Pinzon P, Takwoingi Y, Hernández R, Vazquez-Montes M, Elders A, Asaoka R, Banister K, van der Schoot J, Fraser C. Surveillance for ocular hypertension: an evidence synthesis and economic evaluation. Health Technol Assess. 2012;16:1-271, iii-iv. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Rouhiainen H, Teräsvirta M. Incidence of open-angle glaucoma and screening of the intraocular pressure with a non-contact tonometer. Acta Ophthalmol (Copenh). 1990;68:344-346. [PubMed] |
| 13. | Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994;101:1851-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:1661-1669. [PubMed] |
| 15. | Babalola OE, Kehinde AV, Iloegbunam AC, Akinbinu T, Moghalu C, Onuoha I. A comparison of the Goldmann applanation and non-contact (Keeler Pulsair EasyEye) tonometers and the effect of central corneal thickness in indigenous African eyes. Ophthalmic Physiol Opt. 2009;29:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | [The Japan Glaucoma Society Guidelines for Glaucoma (3rd Edition)]. Nihon Ganka Gakkai Zasshi. 2012;116:3-46. [PubMed] [DOI] [Full Text] |
| 17. | National Collaborating Centre for Acute Care (UK). Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension. London: National Collaborating Centre for Acute Care (UK) 2009; . [PubMed] |
| 18. | Shah S, Murdoch IE. NICE - impact on glaucoma case detection. Ophthalmic Physiol Opt. 2011;31:339-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | United States Preventive Services Task Force. Screening for Glaucoma. Cited 2011-04-21. Available from: http: //www.uspreventiveservicestaskforce.org/uspstf05/glaucoma/glaucrs. |
| 20. | Tielsch JM, Katz J, Singh K, Quigley HA, Gottsch JD, Javitt J, Sommer A. A population-based evaluation of glaucoma screening: the Baltimore Eye Survey. Am J Epidemiol. 1991;134:1102-1110. [PubMed] |
| 21. | American Academy of Ophthalmology. Primary Open-Angle Glaucoma PPP. Cited 2010-11-14. Available from: http: //one.aao.org/CE/PracticeGuidelines/PPP_Content.aspx?cid=93019a87-4649-4130-8f94-b6a9b19144d2. |
| 22. | Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Zegers RH, Reinders EF, de Smet MD. Primary open-angle glaucoma: the importance of family history and role of intraocular pressure. Med J Aust. 2008;188:312-313. [PubMed] [DOI] [Full Text] |
| 25. | Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch Ophthalmol. 1994;112:69-73. [PubMed] |
| 26. | Ekström C. Risk factors for incident open-angle glaucoma: a population-based 20-year follow-up study. Acta Ophthalmol. 2012;90:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Michaelides M, Bunce C, Adams GG. Glaucoma following congenital cataract surgery--the role of early surgery and posterior capsulotomy. BMC Ophthalmol. 2007;7:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Wong IB, Sukthankar VD, Cortina-Borja M, Nischal KK. Incidence of early-onset glaucoma after infant cataract extraction with and without intraocular lens implantation. Br J Ophthalmol. 2009;93:1200-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Chadha V, Cruickshank I, Swingler R, Sanders R. Advanced glaucomatous visual loss and oral steroids. BMJ. 2008;337:a670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Marcus MW, de Vries MM, Junoy Montolio FG, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology. 2011;118:1989-1994.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 32. | Xu L, Wang Y, Wang S, Wang Y, Jonas JB. High myopia and glaucoma susceptibility the Beijing Eye Study. Ophthalmology. 2007;114:216-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010-2015. [PubMed] |
| 34. | Johnson CA, Keltner JL, Cello KE, Edwards M, Kass MA, Gordon MO, Budenz DL, Gaasterland DE, Werner E. Baseline visual field characteristics in the ocular hypertension treatment study. Ophthalmology. 2002;109:432-437. [PubMed] [DOI] [Full Text] |
| 35. | Ocular Hypertension Treatment Study Group and the European Glaucoma Prevention Study Group. The accuracy and clinical application of predictive models for primary open-angle glaucoma in ocular hypertensive individuals. Ophthalmology. 2008;115:2030-2036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Güerri N, Polo V, Larrosa JM, Ferreras A, Fuertes I, Pablo LE. Performance of imaging devices versus optic disc and fiber layer photography in a clinical practice guideline for glaucoma diagnosis. Eur J Ophthalmol. 2012;22:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |