Published online Aug 10, 2017. doi: 10.5317/wjog.v6.i3.16
Peer-review started: May 10, 2017
First decision: May 25, 2017
Revised: June 25, 2017
Accepted: July 10, 2017
Article in press: July 11, 2017
Published online: August 10, 2017
To analyze serum levels of nitric oxide (NO), an indicator of cardiovascular health, in post-menopausal females with and without hypothyroidism.
NO was analyzed colorimetrically in 30 newly diagnosed hypothyroid postmenopausal females and 30 postmenopausal females with normal thyroid profile. Results were compared and subjected to appropriate statistical analysis.
The levels of serum NO were found to be significantly decreased in postmenopausal females with hypothyroidism as compared to the levels in those with normal thyroid profile (P value < 0.05). A negative correlation of NO was observed with thyroid stimulating hormone whereas a positive correlation of NO was observed with free T3 (FT3), free T4 (FT4), T3 and T4 though it was statistically significant only for FT4 among postmenopausal females with hypothyroidism.
Postmenopausal hypothyroid females may be at a risk of compromised cardiovascular health as indicated by low NO levels. Regular monitoring and risk assessment is essential for timely intervention.
Core tip: It is already established that post-menopausal women on account of low levels of reproductive hormones are at a greater risk of cardiovascular accidents. Hypothyroidism, itself, is also a risk factor for poor cardiovascular health. In this study, significantly low levels of nitric oxide (NO) were observed in postmenopausal hypothyroid females as compared to those in postmenopausal euthyroid females, thereby, indicating the significant role played by NO in cardioprotection as well as a need for regular monitoring of NO levels and thyroid profile in postmenopausal phase of life.
- Citation: Dahiya K, Dalal D, Malhotra V, Aggarwal S, Malik AK, Ghalaut VS, Dahiya P. Is nitric oxide level affected in postmenopausal women with hypothyroidism? World J Obstet Gynecol 2017; 6(3): 16-20
- URL: https://www.wjgnet.com/2218-6220/full/v6/i3/16.htm
- DOI: https://dx.doi.org/10.5317/wjog.v6.i3.16
Menopause is a physiological process characterized by loss of reproductive function, depletion of ovarian follicles and estrogen production resulting in appearance of a variety of symptoms including irregular menstruation, vasomotor instability (hot flashes and night sweats), genitourinary tissue atrophy, breast tenderness, vaginal dryness, mood variations and increased propensity for developing osteoporosis and heart disease[1,2].
Estrogen is found to be protective against cardiovascular disease by a variety of mechanisms including favorable effects on lipoprotein, glucose and insulin homeostasis, changes in extracellular matrix composition, atherosclerotic plaque destabilization and the facilitation of collateral vessel formation[2]. Postmenopausal estrogen deficiency is associated with higher blood levels of free fatty acids which contribute to the pathogenesis of the metabolic syndrome and insulin resistance, symptoms of which might, sometimes, overlap with those of hypothyroidism[3].
Hypothyroidism is a condition resulting from inadequate production of thyroid hormones and is characterized by increased levels of thyroid stimulating hormone (TSH) and low levels of T3 and T4. Hypothyroidism is of two types, primary and secondary, depending on the organ of defect[4]. The prevalence of primary hypothyroidism is 10/1000 in general population[3]. It is more common in women than men. The ratio of female to male is approximately 6:1[5]. Studies have shown a relationship between thyroid hormones and nitric oxide (NO). According to a report, thyroid hormone can induce endothelium independent relaxation[6,7]. One of these studies showed that expression of both endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) was modulated in thyroid disorders[7].
NO is recognized as a cellular signaling molecule which plays many important roles in the body. Important biological functions of NO include maintenance of blood pressure, facilitating neuro-transmission, regulating platelet functions and modulation of the immune system[8]. It is synthesized from amino acid L-Arginine which gets oxidized to NO by the action of the NOS enzymes[9]. Pathophysiological basis of cardiovascular changes is reported to be endothelial dysfunction which occurs mainly via a decrease in endothelium-dependent vasodilatation mediated by NO[10].
Some studies have suggested that estrogen associated cardio-protection is mediated by estrogen induced increase in the release of NO from the vascular endothelium[11,12]. This finding was recently confirmed in post-menopausal women, in whom estradiol was observed to be acutely attenuated and they showed abnormal coronary vasomotor responses to acetylcholine[11]. With all this background information in mind, this study was planned to estimate NO levels in postmenopausal hypothyroid and euthyroid females.
Thirty postmenopausal women with newly diagnosed primary hypothyroidism (group A, diagnosed by increased TSH and clinical examination) and thirty euthyroid controls (group B) presenting as outdoor patients in Department of Obstetrics and Gynecology/Department of Endocrinology were included in the study. All the subjects were enrolled after obtaining their informed consent and taking care of all ethical issues including approval from Postgraduate Board of Studies of the Institute. The sample size was calculated keeping power of the test at 9.0[13].
Subjects with any other chronic disease or on any type of treatment were excluded from the study.
Venous blood samples were collected aseptically from all recruited subjects and serum was separated. Serum samples of all the subjects were analyzed for complete thyroid profile including total T3 (TT3), total T4 (TT4), free T3 (FT3), free T4 (FT4), TSH and NO on the same day of collection. TT3, TT4 were estimated by standard radioimmunoassay, TSH by immunoradiometric assay and FT3 and FT4 were analyzed using a chemiluminescence technique (Advia Centaur CP, Siemens). Newly diagnosed primary subclinical and overt hypothyroidism (TSH > 4.25 μIU/mL, free T3 < 2.4 pg/mL, free T4 < 0.7 ng/dL) in postmenopausal women (> 1 year of menopause but < 2 years of menopause) assessed on the basis of history, clinical examination and thyroid function tests were included in the study group A and postmenopausal women (> 1 year of menopause but < 2 years of menopause) assessed on the basis of history, clinical examination and normal thyroid function test were included in the study group B.
NO was measured colorimetrically using Griess reaction which measures nitrite formed from NO which is a stable and nonvolatile end product of NO which, itself, has a short half-life of 6-10 s. In this method, nitrite reacts under acidic conditions with sulfanilic acid to form a diazonium cation which subsequently couples to the aromatic amine 1-naphthylamine to produce a purple coloured complex whose absorbance is read at 546 nm[14] body mass index (BMI) was also calculated and recorded for these patients.
Data of both the groups was compared using student’s t-test while the correlation was calculated using Pearson’s coefficient of correlation.
The mean age of postmenopausal females in group A was 53.2 ± 1.57 years with a range of 51-57 years and in group B 52.8 ± 2.38 years with range being 46-57 years (P = 0.492). Hypothyroid patients were found to have greater BMI as compared to euthyroid subjects but it was statistically non-significant (P = 0.993). The levels of thyroid profile and NO in both the groups are shown in Table 1. Serum NO levels were observed to be significantly decreased in patients (group A) as compared to those in controls (group B) (P value < 0.05).
| Parameters | Group A | Group B | P value |
| Body mass index (kg/m2) | 30.37 ± 3.26 | 29.03 ± 3.56 | 0.993 |
| TSH (μIU/mL) | 9.596 ± 2.349 (5.9-15.5) | 3.663 ± 1.193 (0.94-4.4) | < 0.001 |
| S. T4 (ng/mL) | 1.262 ± 0.707 (0.93-2.1) | 6.405 ± 1.727 (3.2-9.2) | < 0.001 |
| S. T3 (ng/mL) | 56.266 ± 15.72 (22-77) | 98.433 ± 9.640 (85-111) | < 0.001 |
| FT3 (pg/dL) | 0.493 ± 0.180 (0.09-0.83) | 1.230 ± 0.213 (2.1-4.1) | < 0.001 |
| FT4 (ng/mL) | 1.206 ± 0.352 (0.93-2.1) | 2.835 ± 0.507 (0.93-1.8)) | < 0.001 |
| NO (μmol/L) | 25.832 ± 5.286 (16.5-38) | 33.671 ± 5.173 (23.16-47) | < 0.001 |
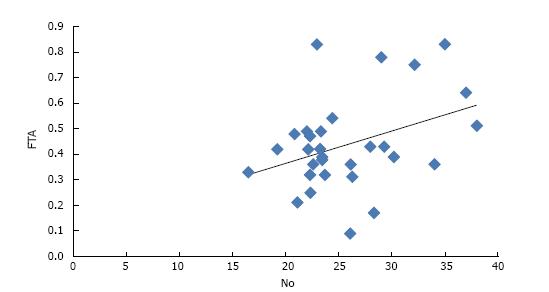
In group A, a negative correlation of NO was observed with TSH whereas a positive correlation of NO was found with FT3, FT4, T3 and T4 (Table 2). Amongst all, only FT4 was found to have a statistically significant correlation with NO (P value = 0.04) (Figure 1). In group B, a positive correlation of NO was observed with T3, T4, FT3 and FT4 though it was not significant statistically (Table 3).
| Parameter | r value | P value |
| Estrogen | 0.21 | 0.24 |
| Progesterone | -0.14 | 0.43 |
| TSH | -0.1 | 0.58 |
| T4 | 0.36 | 0.50 |
| T3 | 0.16 | 0.38 |
| Free T3 | 0.22 | 0.22 |
| Free T4 | 0.37 | 0.04 |
| Parameter | r value | P value |
| Estrogen | 0.13 | 0.47 |
| Progesterone | -0.06 | 0.72 |
| TSH | 0.00 | 0.97 |
| T4 | 0.17 | 0.36 |
| T3 | 0.17 | 0.35 |
| Free T3 | 0.26 | 0.15 |
| Free T4 | 0.31 | 0.09 |
The natural menopause is defined as 12 consecutive months of amenorrhea according to some major studies[15-17]. The present study included recently menopausal women with duration of menopause of more than one year but less than 2 years, to minimize the age related effects. There could be age-related fall in circulating T4 concentrations which could result in increased TSH secretion. Alternatively, with aging there is reduction in TSH bioactivity, the responsiveness of the thyroid to TSH or some occult thyroid disease, leading to increased TSH levels[18]. With aging, there is progressive decline of glutathione concentration in cells of liver, kidney and retina etc. Thus, the functioning of various organs is affected due to increased reactive oxygen species, mitochondrial damage and cellular dysfunction. The first year of menopause is considered as menopause transition phase, during which there are ongoing changes in hormonal levels. Studies have demonstrated that, the potential for hormone secretion by residual follicles in older women is variably diminished[19,20].
In this study, BMI of postmenopausal hypothyroid females was observed to be 30.37 ± 3.26 kg/m2, which was slightly higher than the BMI of postmenopausal euthyroid females, which was 29.03 ± 3.56 kg/m2. This increase in BMI in postmenopausal hypothyroid females is due to various factors because of decreased estrogen and thyroid hormone levels in these females. Some studies have also proposed that postmenopausal women have higher BMI than premenopausal women because of action of estrogen[21,22].
In the present study, NO levels in postmenopausal hypothyroid females were observed to be significantly lower than those in postmenopausal euthyroid females (P = 0.00). In a study, NO levels were observed to be higher (75 ± 5 μmol/L) in postmenopausal healthy females[23]. In another study, NO levels were increased significantly in hypothyroid females (57.61 ± 15.8 μmol/L and 36.24 ± 7.61 μmol/L in postmenopausal hypothyroid and euthyroid females respectively). Above study observed a positive correlation between TSH and NO.
In the present study, a negative correlation was observed between TSH and NO but it was not statistically significant (P = 0.58). The correlation of NO with free T4 was found to be statistically significant in postmenopausal hypothyroid females with a “r” value of 0.37 and P value of 0.04. Thyroid hormones directly increase NO production in vascular smooth muscle cells through PI3K/Akt signaling pathway and produce rapid relaxation of vascular smooth muscle cells. T3 is reported to significantly induce the expression of three NOS isoforms in smooth muscle cells[23].
Various studies have shown relationship between thyroid hormone and NO and that thyroid hormone can also induce endothelium-independent relaxation[24,25] but data from some studies suggest that smooth muscle cells rather than the endothelium, are the primary target of thyroid hormones[19]. One study in rats showed that the hyperthyroid state was associated with increased formation of NO, but there was a reduced capacity for responding to NO when compared to the hypothyroid state[26]. The same study showed that both eNOS and nNOS expression were modulated in hypothyroidism and hyperthyroidism. Another study on rats showed that thyroid hormones affect the level and activity of nNOS and therefore NO levels.
Thus, it may be concluded that cardiovascular health is compromised in postmenopausal women which is further deteriorated by hypothyroidism, indicated by lower NO levels in postmenopausal hypothyroid women as compared to postmenopausal euthyroid women. This indicates that normal level of thyroid hormone along with reproductive hormones is essential for cardiac health. Further, regular monitoring and risk assessment regarding cardiovascular disease is essential in postmenopausal hypothyroid females and timely interventions may help to avoid further complications.
Hypothyroidism and menopause, both pose a risk to the cardiac health of women.
As suggested by literature that estrogen induced cardio protection might be mediated by release of nitric oxide (NO), this study was conducted to estimate and compare serum NO levels in postmenopausal women with and without hypothyroidism.
NO was analyzed as nitrite colorimetrically in 30 postmenopausal hypothyroid and euthyroid females each.
A statistically significant decrease was observed in NO levels in postmenopausal hypothyroid females as compared to their euthyroid counterparts (P < 0.05).
It may be concluded that cardiovascular health is compromised in postmenopausal women because of low estrogen, which is further deteriorated by hypothyroidism, indicated by lower NO.
This research directs towards future perspectives in analyzing other endothelial function markers along with actual estimation of NO and its association with reproductive hormone levels in larger study groups.
Manuscript source: Invited manuscript
Specialty type: Obstetrics and gynecology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Khajehei M, Schulten HJ, Zhang XQ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci. 2005;28:364-375. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Pansini F, Mollica G, Bergamini CM. Management of the menopausal disturbances and oxidative stress. Curr Pharm Des. 2005;11:2063-2073. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Naziroğlu M, Simşek M, Simşek H, Aydilek N, Ozcan Z, Atilgan R. The effects of hormone replacement therapy combined with vitamins C and E on antioxidants levels and lipid profiles in postmenopausal women with Type 2 diabetes. Clin Chim Acta. 2004;344:63-71. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Kratzsch J, Pulzer F. Thyroid gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:57-75. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525-531. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Jiang CW, Sarrel PM, Lindsay DC, Poole-Wilson PA, Collins P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br J Pharmacol. 1991;104:1033-1037. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Sudhir K, Chou TM, Mullen WL, Hausmann D, Collins P, Yock PG, Chatterjee K. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26:807-814. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Tuteja N, Chandra M, Tuteja R, Misra MK. Nitric Oxide as a Unique Bioactive Signaling Messenger in Physiology and Pathophysiology. J Biomed Biotechnol. 2004;2004:227-237. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Schmidt HH, Nau H, Wittfoht W, Gerlach J, Prescher KE, Klein MM, Niroomand F, Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988;154:213-216. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046-1051. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365-F388. [PubMed] [Cited in This Article: ] |
| 12. | Wang M, Wang Y, Weil B, Abarbanell A, Herrmann J, Tan J, Kelly M, Meldrum DR. Estrogen receptor beta mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R972-R978. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ghalaut VS, Sangwan L, Dahiya K, Ghalaut PS, Dhankhar R, Saharan R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J Oncol Pharm Pract. 2012;18:186-190. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | McKinlay SM. The normal menopause transition: an overview. Maturitas. 1996;23:137-145. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Kaufert P, Lock M, McKinlay S, Beyenne Y, Coope J, Davis D, Eliasson M, Gognalons-Nicolet M, Goodman M, Holte A. Menopause research: the Korpilampi workshop. Soc Sci Med. 1986;22:1285-1289. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350-356. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Schindler AE. Thyroid function and postmenopause. Gynecol Endocrinol. 2003;17:79-85. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Longcope C. Metabolic clearance and blood production rates of estrogens in postmenopausal women. Am J Obstet Gynecol. 1971;111:778-781. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Korenman SG, Perrin LE, McCallum TP. A radio-ligand binding assay system for estradiol measurement in human plasma. J Clin Endocrinol Metab. 1969;29:879-883. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Signorelli SS, Neri S, Sciacchitano S, Di Pino L, Costa MP, Pennisi G, Ierna D, Caschetto S. Duration of menopause and behavior of malondialdehyde, lipids, lipoproteins and carotid wall artery intima-media thickness. Maturitas. 2001;39:39-42. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Gavaler JS, Deal SR, Van Thiel DH, Arria A, Allan MJ. Alcohol and estrogen levels in postmenopausal women: the spectrum of effect. Alcohol Clin Exp Res. 1993;17:786-790. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Pueyo ME, Arnal JF, Rami J, Michel JB. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. Am J Physiol. 1998;274:C214-C220. [PubMed] [Cited in This Article: ] |
| 23. | McAllister RM, Grossenburg VD, Delp MD, Laughlin MH. Effects of hyperthyroidism on vascular contractile and relaxation responses. Am J Physiol. 1998;274:E946-E953. [PubMed] [Cited in This Article: ] |
| 24. | McAllister RM, Luther KL, Pfeifer PC. Thyroid status and response to endothelin-1 in rat arterial vessels. Am J Physiol Endocrinol Metab. 2000;279:E252-E258. [PubMed] [Cited in This Article: ] |
| 25. | Nakaki T, Nakayama M, Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990;189:347-353. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Gonzales RJ, Walker BR, Kanagy NL. 17beta-estradiol increases nitric oxide-dependent dilation in rat pulmonary arteries and thoracic aorta. Am J Physiol Lung Cell Mol Physiol. 2001;280:L555-L564. [PubMed] [Cited in This Article: ] |