Published online Nov 10, 2015. doi: 10.5317/wjog.v4.i4.77
Peer-review started: June 3, 2015
First decision: August 4, 2015
Revised: September 3, 2015
Accepted: October 1, 2015
Article in press: October 8, 2015
Published online: November 10, 2015
Processing time: 164 Days and 23 Hours
Preeclampsia (PE) is a pregnancy-specific syndrome, complicating 2%-8% of pregnancies. PE is a major cause of maternal mortality throughout the world with 60000 maternal deaths attributed to hypertensive disorders of pregnancy. PE also results in fetal morbidity due to prematurity and fetal growth restriction. The precise aetiology of PE remains an enigma with multiple theories including a combination of environmental, immunological and genetic factors. The conventional and leading hypotheses for the initial insult in PE is inadequate trophoblast invasion which is thought to result in incomplete remodelling of uterine spiral arteries leading to placental ischaemia, hypoxia and thus oxidative stress. The significant heterogeneity observed in pre-eclampsia cannot be solely explained by the placental model alone. Herein we critically evaluate the clinical (risk factors, placental blood flow and biomarkers) and pathological (genetic, molecular, histological) correlates for PE. Furthermore, we discuss the role played by the (dysfunctional) maternal cardiovascular system in the aetiology of PE. We review the evidence that demonstrates a role for both the placenta and the cardiovascular system in early- and late-onset PE and highlight some of the key differences between these two distinct disease entities.
Core tip: The conventional paradigm is that preeclampsia (PE) is solely due to placental dysfunction. However not all cases of placental dysfunction result in the syndrome of PE. Equally, placental dysfunction - as evidenced by impaired uterine artery Doppler indices, low birth weight and abnormal histology - is not always present in PE. Therefore, the heterogeneity observed in PE cannot be solely explained by placental dysfunction. There is now strong evidence supporting the role of the maternal cardiovascular system in PE. In this mini-review, we evaluate the evidence supporting a dual aetiology of PE, involving both the placenta and the maternal cardiovascular system.
- Citation: Vinayagam D, Leslie K, Khalil A, Thilaganathan B. Preeclampsia - What is to blame? The placenta, maternal cardiovascular system or both? World J Obstet Gynecol 2015; 4(4): 77-85
- URL: https://www.wjgnet.com/2218-6220/full/v4/i4/77.htm
- DOI: https://dx.doi.org/10.5317/wjog.v4.i4.77
Preeclampsia (PE) is a pregnancy-specific syndrome, complicating 2%-8% of pregnancies[1]. PE is a major cause of maternal mortality throughout the world. Over half a million women die during or after childbirth every year, and it is estimated that 60000 of these maternal deaths worldwide are attributable to hypertensive disorders of pregnancy[1,2]. In the United Kingdom, PE/eclampsia was the fourth leading cause of direct maternal deaths between 2009-2011[3]. In the triennium 2009-2011, 10 deaths in the United Kingdom were attributable to PE, giving a mortality rate of 0.42 per 100000[3], considerably lower than the mortality figures worldwide. The management of hypertension in conjunction with the use of national and local guidelines are credited with the reduction in morbidity and mortality observed in the developed world.
PE can also result in morbidity to the fetus, including fetal growth restriction, placental abruption and stillbirth. In addition, there is a considerable financial burden as a result of increased antenatal surveillance, investigations and hospital admissions along with an increased risk of operative intervention and peripartum monitoring. Following delivery, prematurity and the associated care costs, both in the short and long term, further contribute to the financial burdens associated with PE. At present, the only recognised cure for PE is delivery of the placenta. As a result, PE is the leading cause for iatrogenic preterm birth[4], which is associated with greater neonatal morbidity than delivery at term.
The importance and magnitude of PE on maternal health and neonatal morbidity is well accepted. Despite this being such an important disease, our knowledge of its aetiology is still incomplete. Forming a better understanding will aid us in making an accurate diagnosis, perform better screening and improve our ability to triage disease severity, offer targeted preventative and therapeutic measures and formulate appropriate short- and long-term postpartum management plans.
PE is a heterogenous disease exhibiting a diverse range of both fetal and maternal disease presentations. At present, we aim to identify and diagnose PE based on organ dysfunction both in the mother and in the fetus. The timing of onset of PE is as diverse as its organ involvement and can occur from the late second trimester, through to term and can even present in the postnatal period.
Although often classified as mild, moderate and severe, a newer and perhaps more relevant method of clinical classification would be based upon timing of onset. Early onset PE is thus defined as PE occurring prior to and requiring delivery before 34 wk gestation and late onset PE developing and requiring delivery after this gestational age. There are distinct differences between these two types of PE, in terms of their pathophysiological basis, effects on mother and fetus as well as their long term implications. Late PE accounts for approximately 75% of cases, whilst the remaining 25% are early onset PE[5,6].
Ten percent to fifteen percent of pregnant women will develop some form of hypertensive disease that requires further assessment and follow up. Currently, United Kingdom guidelines on screening for identification of those at high risk of developing PE is performed in the first trimester and is based on maternal demographics and obstetric/medical risk factors[7]. The NICE model recommends commencing preventative measures (e.g., low dose aspirin) and formulation of an appropriate antenatal management plan in high risk women. However the NICE model categorises more than 60% of women as high risk, but predicts less than 30% of those destined to develop PE[8].
The precise pathophysiological basis of PE remains an enigma with multiple, plausible aetiological theories involving a combination of environmental, immunological and genetic factors.
The conventional, leading hypotheses, which has stood the test of time, for the initial pathophysiological insult in PE is inadequate trophoblast invasion and thus incomplete remodelling of uterine spiral arteries This leads to placental ischaemia, hypoxia and thus oxidative stress[9,10]. The placental hypoxaemia sets off a biochemical cascade of angiogenic/antiangionic factors which leads to subsequent endothelial cell dysfunction with its observed maternal signs and symptoms of PE. Whilst placental hypoxaemia and the subsequent oxidative stress is a significant contributing factor, we acknowledge that a definitive link between PE and placental hypoxia is not confirmed and further research is required.
However not every pregnancy characterised by abnormal placentation results in PE - fetal growth restriction is one such example in where there is suboptimal placentation with detrimental fetal effects but minimal maternal effects. Others will go on to develop the syndrome of PE which is characterised by hypertension and proteinuria and multiple organ involvement.
One possible reason for the development of PE is that there is a concurrent maladaption of the maternal cardiovascular system along with abnormal placentation, and this predisposes an individual to developing PE. This would also explain why not all women with poor placentation with its sequalae (such as fetal growth restriction) develop PE; their cardiovascular system adapts appropriately to the ongoing pregnancy and associated haemodynamic changes.
The development of PE does not necessarily require a uterus or indeed a fetus, as the condition has been reported in abdominal[11] and molar pregnancies[12] respectively. However, a placenta is essential for the disease to occur, and is therefore central in the pathogenesis. Furthermore, it is well documented that the cure for PE is delivery of the placenta, further supporting the crucial role played by the placenta in the development of PE.
The paradigm is that the “placenta causes PE”. Whilst we firmly believe that the placenta is a pre-requisite and therefore crucial to the development of PE, herein we critically review the evidence that the placenta is indeed the only organ of causality in PE.
The majority of the evidence for the placental origin hypothesis of PE is based on the clinical features of the disorder. Broadly speaking, they constitute clinical risk factors, placental blood flow evaluation, fetal growth restriction and placental biomarkers.
Both PE and fetal growth restriction (FGR) are thought to share clinical risk factors such as increased maternal age, ethnic origin, increased body mass index (BMI), diabetes and other co-morbidities. These associations are conventionally advocated by national guidelines[7,13] as a method to screen routine populations for their risk of developing PE. However, analysis of data from 40000 pregnancies collected for the World Health Organization Antenatal care trial[14] showed that surprisingly, PE and fetal growth restriction had different risk factor profiles. Mothers with PE compared with those with fetal growth restriction were more likely to have a history of diabetes, renal or cardiac disease, chronic hypertension, previous PE, increased BMI and extremes of maternal age. Conversely, fetal growth restriction was associated with higher risk of low birth weight in previous pregnancies, but not with previous PE. The same analysis demonstrated that PE and gestational hypertension shared many risk factors - conventionally associated with cardiovascular disease in the non-pregnant population. These data infer that the aetiology of PE is not necessarily as a result of placental dysfunction.
The pathological hallmark of placental insufficiency is incomplete spiral artery remodelling, and this is seen in both fetal growth restriction as well as in some, but not all cases of PE. Uterine artery Doppler waveform studies have been shown to aid in the prediction of pregnancies that will be complicated by fetal growth restriction or PE[8,15-17]. Increased uterine artery resistance indices are related to incomplete trophoblast invasion of maternal spiral arteries, which results in a high-resistance placental circulation and therefore an underperfused fetoplacental unit. This placental hypoperfusion is a feature seen in both PE and fetal growth restriction.
The association with increased uterine artery Doppler resistance indices and a higher propensity to develop PE and FGR is widely reported and used in current clinical practice in the management of high risk pregnancies. Velauthar et al[17] carried out a meta-analysis of over 55000 patients looking at the predictive value of performing uterine artery Doppler measurements in the first trimester. The authors reported a sensitivity and specificity of 47.8% and 92.1% respectively for the detection of early onset PE. The sensitivities and specificities for detection of late onset PE were 21.5% and 90.3%; for PE at any gestation these were 26.4% and 93.4%. Although first trimester uterine artery Doppler screening is a highly specific means of screening for early PE, it is less so for late PE.
Verlohren et al[18] have corroborated this finding in their published data of second trimester uterine artery Doppler screening. The investigators looked at outcomes in over 27000 cases that had uterine artery measurements in the second trimester. They reported that the prevalence of resistance indices above the 90th centile were present in 63.6% of early PE, 15.5% of late PE and 8.8% in the control group. This significant finding shows that increased uterine artery Doppler indices, are a poor predictor of late onset PE.
It is well recognised that increased uterine artery dopplers are an index for inadequate placentation, and thereby a surrogate marker for increased risk of PE and FGR. Data from these large-scale, high quality studies indicate that inadequate placentation is a key feature of early onset PE and FGR. The reduced prevalence of increased uterine artery indices observed in late-onset PE lends further support to the argument that the heterogeneity observed in PE, is due to early onset PE being related to a dysfunctional placenta, whilst late onset PE may not be associated with placental insufficiency.
The fetal effects of a dysfunctional placenta include fetal growth restriction, and this is seen in many cases of early-onset PE, but less so in cases of late PE. The majority of neonates in late PE are of normal size[18]. This observation is consistent with the notion that whilst early PE due to placental insufficiency is also closely correlated to FGR, there is an additional aetiological factor to placental insufficiency in late onset PE.
Verlohren et al[18] reported on the distribution of small-for-gestational-age (SGA) neonates in their large cohort. They found the incidence of SGA to be 66.2% in the early PE group, 16.7% in the late PE group and 10.8% in the control group.
The distribution of large-for-gestational age neonates did not observe a similar pattern; the prevalence being greatest in the late PE group as compared to all other groups. Interestingly, both SGA and large-for-gestational-age (LGA) births were more prevalent in the late PE group, demonstrating a bimodal skewed birth weight distribution in late PE. Whilst the association between PE and LGA babies has been observed previously, the bimodal distribution is certainly a novel finding.
The increased incidence of SGA births in early-onset PE, associated with increased uterine artery impedances, is an expected finding given the underlying placental insufficiency, unlike the reported increased prevalence of both SGA and LGA in late-onset PE. The authors postulate that this finding implies that whilst the SGA form of late-onset PE is due to placental insufficiency, the LGA form (with its associated normal uterine artery impedance measurements) is secondary to the inability of the maternal heart to meet the demands of the (oversized) placenta. The subsequent placental hypoperfusion as a result of the inadequate pump action, leads to placental hypoxia and the biochemical cascade that leads to endothelial cell dysfunction observed in PE.
The oxidative stress in the placenta leads to an imbalance in angiogenic and antiangiogenic factors. An imbalance in these factors can adversely affect vascular homeostasis, and therefore contribute to the array of symptoms displayed in PE. The angiogenic factors that are believed to play a role include vascular endothelial growth factor (VEGF) and placental growth factor (PlGF)[10,19,20], the latter being used as a potential diagnostic biomarker for PE[19,20]. PlGF is produced by trophoblasts and interacts with cell surface receptors such as Flt-1. The soluble form of this, soluble fms-like tyrosine kinase-1 (sFlt-1) is an antiangiogenic factor which has also emerged as a key factor and potential biomarker in PE. sFlt-1 has been shown to block PlGF and therefore play a causative role in the development of PE[10,21]. Maynard et al[10] demonstrated a rise in arterial pressures and proteinuria with exogenous administration of sFlt-1. Furthermore, uteroplacental ischaemia has been shown to increase sFlt-1 levels in experimental models, as well as a decrease in VEGF/PlGF[22,23].
Another biological factor that has been studied and may have a role in PE is Heme Oxygenase-1 (HO-1). HO-1 and its metabolites carbon monoxide (CO) and bilirubin exert protective effects against oxidative stimuli, and in vitro studies have reported that HO-1 downregulates sFlt-1[24]. HO-1 has been shown to inhibit sFlt-1 release[24,25] and it is possible that a loss of HO-1 plays a part in the pathophysiology of PE. There are no published studies looking at the differences in the expression of HO-1 in early-onset vs late-onset PE.
Poon et al[26] conducted a first trimester screening study using uterine artery Doppler indices, serum pregnancy associated plasma protein A (PAPP-A) and placental growth factor measurements in a large cohort of over 57000 cases. They found that in the pre-term SGA group, serum PAPP-A and PlGF were reduced, indicating an underlying pathology of impaired placentation. The authors reported that for a false-positive rate of 5%, the detection of early-onset PE (< 34 wk gestation) using PlGF was 59.3%, however in all cases of PE below 42 wk gestation, the detection rate was significantly lower at 29.1% - this figure included all of the early-onset along with late-onset cases of PE. The evidence suggests that placental biomarkers have a good performance for screening for early-onset PE, but a poor performance when screening for late-onset PE.
The biomarkers discussed above have been used in clinical trials as a potential screening tool for the detection of PE. Elevated sFlt-1/PlGF ratios have been observed in early-onset PE[19,20]. Chappell et al[19] published data from a multicentre trial using a PlGF assay (Triage, Alere, California, United States). PlGF concentrations below the 5th centile had a sensitivity of 96% and a negative predictive value of 98% for PE requiring delivery within the next fourteen days, in gestational ages below 35 wk. Beyond 35 wk, the test was not as good at excluding PE. This further strengthens the argument for a role of placental insufficiency in early cases of PE, with a weaker association between PlGF and late-onset PE.
Many scientific evaluations of the pathology associated with PE have been used to justify the placental origins hypothesis of PE. These may be genetic, molecular or histological in nature.
There is an unquestionable link between the development of PE and subsequent cardiovascular disease. Despite the incomplete understanding of the aetiology of PE, familial clustering has been observed[27,28] and reported in PE and therefore supports a genetic link. Many susceptibility genes for PE have been reported in the literature - the function of the majority of these loci remain unknown. The very few PE genetic loci with known associations have previously been implicated in adult cardiovascular disease. Despite further work in this field being required, it does seem entirely plausible that due to the similarities between PE and cardiovascular disease with clinical risk factors and postpartum cardiovascular legacy, there is indeed a shared genetic link between PE and cardiovascular disease.
Recently published data on the molecular biology, also provide insightful evidence. Yung et al[29] investigated whether there was molecular evidence of a difference in placental stress response between cases of early and late PE. Investigating multiple stress-signalling pathways, the investigators demonstrated that activation of stress-signalling pathways was negatively correlated with gestational age, with a clear inflection for placentae beyond 34 wk gestation. Activation of these pathways was significantly higher in early PE than in late PE or controls. The authors reported no difference between placentas in late PE and normotensive controls. It appears that this group of investigators have provided the first molecular evidence of placental stress response in early PE with a lack of such a response in cases of late PE. This supports a placental cause for early PE, but further suggests that placental dysfunction may not necessarily be implicated in late onset PE.
Histopathological studies of placentae have also reported a difference between early and late PE[30-32]. Whilst in the majority of cases of PE the placentae are histologically normal, it is also found that histopathological lesions are mainly seen in preterm PE[22]. Pathak et al[33] carried out a blinded analysis and reported that placental hypoperfusion in the form of massive perivillous fibrin deposition was much more frequent in those with PET (OR 20.2) and SGA (8.9). However we acknowledge that this was in a small sample of pathological cases. Ogge et al[32] demonstrated the prevalence of placental hypoperfusion lesions were greatest in cases of early-onset PE (58%), as compared to 33% in late onset PE and 16% in term controls.
An interesting, recent study investigated placental perfusion using magnetic resonance imaging in early and late PE[34]. The study reports that women with early PE did indeed show smaller placental perfusion fractions when compared to controls. Interestingly, women with late onset PE had a larger placental perfusion fraction when compared to matched controls for gestational age. The increased placental perfusion fraction seen in late PE further supports the argument that late-onset PE is associated with larger placentae and babies, and therefore is unlikely to be due to placental insufficiency.
The maternal cardiovascular system undergoes a series of changes in pregnancy, all of which have a role in ensuring adequate uterine perfusion, oxygen delivery and provision of nutrients to meet the demands of the growing fetus. These adaptations enable the pregnancy and the growth of the fetus to continue unimpaired. They include a rise in cardiac output, heart rate and plasma volume and a concomitant fall in total vascular resistance (TVR).
Despite a significant volume of literature regarding placental dysfunction in PE, data regarding the cardiac changes associated with PE are more scant, and also more controversial. The conventional belief was that early-onset PE is associated with reduced cardiac output and increased total vascular resistance, with maternal cardiac function succumbing early in the disease process. With regard to late-onset PE, the original data implied that this was a condition of raised cardiac output and reduced total vascular resistance, however this model has not been reported consistently[35,36]. The discrepancies reported in the cardiovascular profiles in women who present with PE can be attributed to certain confounding factors - antihypertensive medication, co-morbidities, different gestational ages and stages of labour.
Another significant reason for the discrepancies reported in the literature with regards to haemodynamics in PE is the use of cardiac output instead of the corrected index for body surface area, cardiac index. Cardiac index (CI) represents the cardiac output per square meter of body surface area. We believe that using this corrected index is superior to using cardiac output, which does not factor in height or weight of the subject. As human beings come in different shapes and sizes, with varied metabolic demands, we feel that comparison of cardiac output without correcting for body surface area is both inadequate and inaccurate.
Prior to the onset of symptoms of early-onset PE, there is a shift towards a reduced cardiac index in conjunction with a raised total vascular resistance, increased mean arterial pressure, contracted intravascular volume and reduced venous reserve capacity[37-42]. These findings suggest that the high resistance/low volume haemodynamic profile observed in women destined to develop early-onset PE is observed in the latent phase of the disease at mid-gestation[39,40].
In a study by Melchiorre et al[39] women who subsequently developed late-onset PE presented with raised total vascular resistance, but importantly, there was no difference in cardiac index between late-onset PE and control groups. Whilst these findings have not been reported consistently in the literature, it is worth noting that in studies that did not corroborate the findings observed by Melchiorre et al[41], corrected cardiac indices were not employed.
In preeclamptics medicated with anti-hypertensives, the CI normalises and is comparable to CI in normotensive controls. The observed reduction in arterial compliance, has also been shown to be corrected following antihypertensive treatment in patients with PE[42].
Although some of the initial work into cardiac changes in PE showed conflicting results, more recent work has demonstrated a more consistent collection of findings. This is in part due to improved and newer techniques such as colour tissue Doppler and 2-dimensional speckle tracking derived strain and strain rate imaging, which are able to detect early and subtle changes in myocardial function in an objective manner, when used in conjunction with validated diagnostic algorithms.
Prior to the onset of clinical symptoms in women who develop early onset PE, there is an abnormal remodelling of the left ventricle which consists of concentric remodelling and hypertrophy[39,41]. Moreover in this group of women, there is evidence of mild diastolic dysfunction as well as impaired myocardial relaxation[39,41]. This impaired diastolic function is thought to be associated with the increased cardiac afterload (increased TVR) and abnormal left ventricular remodelling[39,41]. The abnormal pattern of remodelling observed in PE is similar to that observed in non-pregnant individuals with essential hypertension and is consistent with an impairment that is afterload-induced[39,41,43].
Studies assessing early myocardial changes, have reported that mild-moderate isolated left ventricular diastolic dysfunction is seen in approximately half of women with early onset PE[39,41,43] with one in five women having biventricular systolic dysfunction with associated left ventricular hypertrophy[41,43]. Melchiorre et al[39,41] have also demonstrated impairment in myocardial contractility in early onset PE using colour tissue Doppler and 2-D speckle tracking. Left atrial remodelling in PE has also been reported. These findings suggest that the pregnant heart in PE is working at its maximum potential capacity, and any additional stress could result in a deterioration of cardiovascular function.
Other authors have suggested raised intra-abdominal pressure (IAP) as a major aetiological factor to the development of PE[44,45]. They hypothesize that women with raised IAP will have compromised venous return to the heart, which will result in decreased blood flow in the vascular beds of the placenta, uterus, kidneys and liver. The result of this impaired blood flow and venous congestion would include placental ischaemia, oedema of the lower extremities, glomerulopathy associated with hypertension and proteinuria and hepatic dysfunction[44,46]. This hypothesis is in concordance with the cardiovascular origin of PE proposed in this review. The myocardial dysfunction of PE noted in many studies will also compound the compromised venous return to the heart. These two theories are therefore not mutually exclusive.
Although we have classically believed that treatment of PE is by delivery of the placenta in order to avoid impending maternal harm, the subtle, subclinical cardiac changes associated with a diagnosis of PE are not cured by birth of the infant or delivery of the placenta. Melchiorre et al[47] showed that at 1 year postpartum 56% of women with early onset PE had asymptomatic left ventricular dysfunction compared to 14% of women with late onset PE and 8% of healthy controls. The same authors also reported a 40% incidence of essential hypertension at 2 years post-delivery.
Indeed a diagnosis of PE is considered a risk factor for long term cardiovascular disease[48,49]. Following a diagnosis of PE, in particular early onset PE, it therefore seems prudent that these patients are adequately risk assessed in the years following pregnancy, in order to address and mitigate future cardiovascular risks.
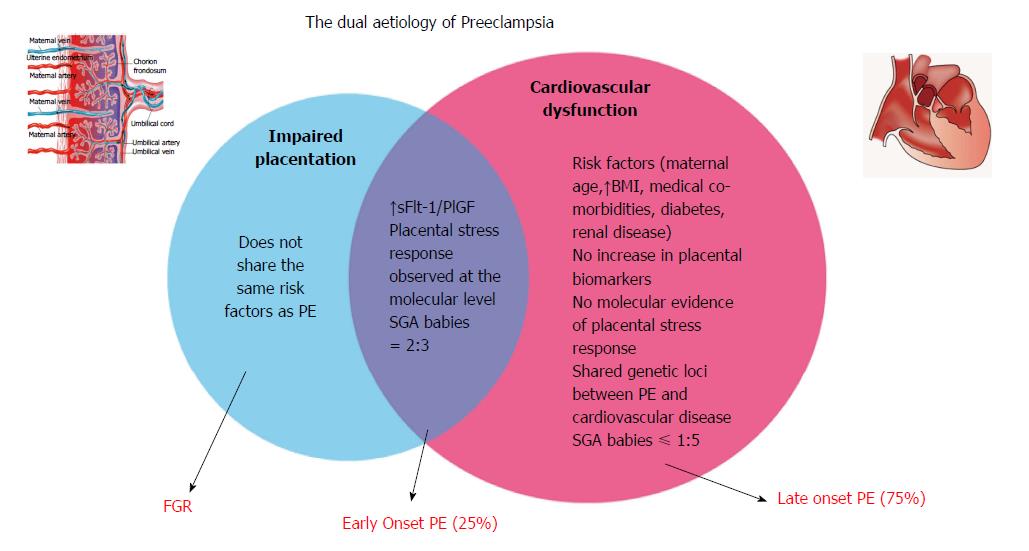
Herein we argue that in order for PE to manifest in an expectant mother, there is an element of both a dysfunctional placenta as well as an abnormal cardiac response. Figure 1 depicts the common and specific key components of impaired placentation and cardiovascular dysfunction. We cannot ignore the role of the maternal cardiovascular system in the development of PE, and thus PE must be recognised and managed, both in the acute and in the long term phases, as a cardiovascular-placental syndrome.
PE is a heterogenous disorder. There is an abundance of evidence supporting the role of the placenta in PE. The evidence indicates that placental dysfunction is prevalent in early-onset PE, however this is not as clear in cases of late-onset PE, as evidenced by uterine artery Doppler indices, birthweight distribution, placental biomarkers, histological and pathological studies. Table 1 summarises the main differences in placental and cardiovascular parameters observed in early-onset vs late-onset pre-eclampsia
| Early onset PE | Late onset PE | |
| Fetal growth restriction | ↑↑↑ | ↑ |
| Hormones - PlGF | ↓ | ↔ |
| Hormones - sFlt-1 | ↑ | ↔ |
| Hormones - PAPP-A | ↓ | ↔ |
| Uterine artery Doppler resistance | ↑↑↑ | ↑/↔ |
| Molecular stress response | ↑ | ↔ |
| Histological evidence of hypoperfusion | ↑↑ | ↑/↔ |
| Haemodynamics - Cardiac index | ↓ | ↔ |
| Haemodynamics - TVR index | ↑↑ | ↑ |
| Left Ventricular geometry – | ↑↑ | ↑↑ |
| concentric remodelling | ||
| Left Ventricular geometry – | ↑ | ↓ |
| concentric hypertrophy | ||
| Chamber diastolic function | ↓↓ | ↓ |
| Chamber systolic function | ↓ | ↔ |
The majority of PE is late-onset, and has classically been described as “maternal” or “heterogenous” PE. By and large, maternal PE remains unexplained. Several theories involving an exaggerated maternal systemic inflammatory response and increased systemic levels of oxidative stress have been published in the past[50]. Whilst this is supported by the observed increase in PE in women with certain pro-inflammatory systemic conditions (autoimmune disease, renal disease, etc.)[51], another, and we believe stronger, argument can be made for the role of the maternal cardiovascular system in the development of PE.
PE is a complex disorder which is no doubt closely related to placental insufficiency. However we strongly believe that the placenta is not the only culprit; the inability of the maternal heart to adapt to placental dysfunction also forms a significant, however as yet incompletely understood, part of the enigma.
We propose that whilst intrinsic placental dysfunction and the mal-adaptation of the maternal cardiovascular system leads to early-onset PE, late PE is associated with an acquired placental dysfunction as a result of the maternal heart not being able to meet the demands of the placenta. Both the intrinsic and acquired placental dysfunction results in placental hypoxia which sets off a cascade of events result in the multisystem disorder of PE. In view of the evidence, we propose a paradigm shift of our current understanding of pre-eclampsia, as a disease entity not solely due to the placenta, but as a cardiovascular-placental syndrome. In answer to our original question which forms the title of this review, the answer is both - as an element of dysfunction in both the cardiovascular system and the placenta are required in order for PE to develop.
P- Reviewer: Wong RJ, Xu XH S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. 2009;33:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1775] [Article Influence: 110.9] [Reference Citation Analysis (0)] |
| 2. | Bhutta ZA, Chopra M, Axelson H, Berman P, Boerma T, Bryce J, Bustreo F, Cavagnero E, Cometto G, Daelmans B. Countdown to 2015 decade report (2000-10): taking stock of maternal, newborn, and child survival. Lancet. 2010;375:2032-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 475] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 3. | Knight M, Kenyon S, Brocklehurst P, Neilson J, Shakespeare J, Kurinczuk JJ, editors . on behalf of MBRRACE-UK. Saving lives, Improving Mother’s Care - Lessons learned to inform future maternity care from the UK and Ireland Confidential Enquiries into Maternal deaths and morbidity 2009-2012. Oxford: National Perinatal Epidemiology Unit, University of Oxford 2014; . |
| 4. | Meis PJ, Goldenberg RL, Mercer BM, Iams JD, Moawad AH, Miodovnik M, Menard MK, Caritis SN, Thurnau GR, Bottoms SF. The preterm prediction study: risk factors for indicated preterm births. Maternal-Fetal Medicine Units Network of the National Institute of Child Health and Human Development. Am J Obstet Gynecol. 1998;178:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 230] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX-XIV. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1129] [Cited by in RCA: 1126] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 6. | Wright D, Akolekar R, Syngelaki A, Poon LC, Nicolaides KH. A competing risks model in early screening for preeclampsia. Fetal Diagn Ther. 2012;32:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 7. | National Collaborating Centre for Women’s and Children’s Health (UK). Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. National Institute for Health and Clinical Excellence: Guidance. London: RCOG Press 2010; . |
| 8. | Papageorghiou AT, Yu CK, Erasmus IE, Cuckle HS, Nicolaides KH. Assessment of risk for the development of pre-eclampsia by maternal characteristics and uterine artery Doppler. BJOG. 2005;112:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376:631-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2100] [Cited by in RCA: 2257] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 10. | Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2783] [Cited by in RCA: 2951] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 11. | Seki H, Kuromaki K, Takeda S, Kinoshita K. Ovarian pregnancy diagnosed in the third trimester: a case report. J Obstet Gynaecol Res. 1997;23:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Brittain PC, Bayliss P. Partial hydatidiform molar pregnancy presenting with severe preeclampsia prior to twenty weeks gestation: a case report and review of the literature. Mil Med. 1995;160:42-44. [PubMed] |
| 13. | American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1452] [Cited by in RCA: 2186] [Article Influence: 198.7] [Reference Citation Analysis (0)] |
| 14. | Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, Farnot U, Bergsjø P, Bakketeig L, Lumbiganon P. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 339] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Kleinrouweler CE, Bossuyt PM, Thilaganathan B, Vollebregt KC, Arenas Ramírez J, Ohkuchi A, Deurloo KL, Macleod M, Diab AE, Wolf H. Value of adding second-trimester uterine artery Doppler to patient characteristics in identification of nulliparous women at increased risk for pre-eclampsia: an individual patient data meta-analysis. Ultrasound Obstet Gynecol. 2013;42:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Spencer K, Yu CK, Savvidou M, Papageorghiou AT, Nicolaides KH. Prediction of pre-eclampsia by uterine artery Doppler ultrasonography and maternal serum pregnancy-associated plasma protein-A, free beta-human chorionic gonadotropin, activin A and inhibin A at 22 + 0 to 24 + 6 weeks’ gestation. Ultrasound Obstet Gynecol. 2006;27:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Velauthar L, Plana MN, Kalidindi M, Zamora J, Thilaganathan B, Illanes SE, Khan KS, Aquilina J, Thangaratinam S. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol. 2014;43:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Verlohren S, Melchiorre K, Khalil A, Thilaganathan B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet Gynecol. 2014;44:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Chappell LC, Duckworth S, Seed PT, Griffin M, Myers J, Mackillop L, Simpson N, Waugh J, Anumba D, Kenny LC. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation. 2013;128:2121-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 20. | Chappell LC, Bramham K, Shennan AH. Short-term prediction of preeclampsia: how close are we? Biomark Med. 2014;8:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2659] [Cited by in RCA: 2728] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 22. | Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, Waugh R, McKenzie P, Kirwan P, Hennessy A. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 23. | Gilbert JS, Babcock SA, Granger JP. Hypertension produced by reduced uterine perfusion in pregnant rats is associated with increased soluble fms-like tyrosine kinase-1 expression. Hypertension. 2007;50:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 24. | Cao J, Inoue K, Li X, Drummond G, Abraham NG. Physiological significance of heme oxygenase in hypertension. Int J Biochem Cell Biol. 2009;41:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | George EM, Cockrell K, Aranay M, Csongradi E, Stec DE, Granger JP. Induction of heme oxygenase 1 attenuates placental ischemia-induced hypertension. Hypertension. 2011;57:941-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Poon LC, Syngelaki A, Akolekar R, Lai J, Nicolaides KH. Combined screening for preeclampsia and small for gestational age at 11-13 weeks. Fetal Diagn Ther. 2013;33:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 27. | Arngrímsson R, Sigurõardóttir S, Frigge ML, Bjarnadóttir RI, Jónsson T, Stefánsson H, Baldursdóttir A, Einarsdóttir AS, Palsson B, Snorradóttir S. A genome-wide scan reveals a maternal susceptibility locus for pre-eclampsia on chromosome 2p13. Hum Mol Genet. 1999;8:1799-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 135] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | van Dijk M, Mulders J, Poutsma A, Könst AA, Lachmeijer AM, Dekker GA, Blankenstein MA, Oudejans CB. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Yung HW, Atkinson D, Campion-Smith T, Olovsson M, Charnock-Jones DS, Burton GJ. Differential activation of placental unfolded protein response pathways implies heterogeneity in causation of early- and late-onset pre-eclampsia. J Pathol. 2014;234:262-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol. 2014;210:66.e1-66.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 31. | van der Merwe JL, Hall DR, Wright C, Schubert P, Grové D. Are early and late preeclampsia distinct subclasses of the disease--what does the placenta reveal? Hypertens Pregnancy. 2010;29:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS. Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia. J Perinat Med. 2011;39:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Pathak S, Lees CC, Hackett G, Jessop F, Sebire NJ. Frequency and clinical significance of placental histological lesions in an unselected population at or near term. Virchows Arch. 2011;459:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Sohlberg S, Mulic-Lutvica A, Lindgren P, Ortiz-Nieto F, Wikström AK, Wikström J. Placental perfusion in normal pregnancy and early and late preeclampsia: a magnetic resonance imaging study. Placenta. 2014;35:202-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Bosio PM, McKenna PJ, Conroy R, O’Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94:978-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 156] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstet Gynecol. 1990;76:1061-1069. [PubMed] |
| 37. | Aardenburg R, Spaanderman ME, Courtar DA, van Eijndhoven HW, de Leeuw PW, Peeters LL. A subnormal plasma volume in formerly preeclamptic women is associated with a low venous capacitance. J Soc Gynecol Investig. 2005;12:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 428] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 39. | Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid-gestational maternal cardiovascular profile in preterm and term pre-eclampsia: a prospective study. BJOG. 2013;120:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Jia RZ, Liu XM, Wang X, Wu HQ. Relationship between cardiovascular function and fetal growth restriction in women with pre-eclampsia. Int J Gynaecol Obstet. 2010;110:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130:703-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 212] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 42. | Khalil A, Jauniaux E, Harrington K. Antihypertensive therapy and central hemodynamics in women with hypertensive disorders in pregnancy. Obstet Gynecol. 2009;113:646-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 43. | Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertens Pregnancy. 2012;31:454-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Sugerman HJ. Hypothesis: preeclampsia is a venous disease secondary to an increased intra-abdominal pressure. Med Hypotheses. 2011;77:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997-1004; discussion 1004-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Gyselaers W, Mullens W, Tomsin K, Mesens T, Peeters L. Role of dysfunctional maternal venous hemodynamics in the pathophysiology of pre-eclampsia: a review. Ultrasound Obstet Gynecol. 2011;38:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 48. | Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123:1243-1262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1288] [Cited by in RCA: 1269] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 49. | Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, Lawlor DA. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 330] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 50. | Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1152] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 51. | Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1133] [Cited by in RCA: 1097] [Article Influence: 54.9] [Reference Citation Analysis (0)] |