Published online Nov 10, 2013. doi: 10.5317/wjog.v2.i4.108
Revised: January 5, 2012
Accepted: January 11, 2013
Published online: November 10, 2013
Processing time: 339 Days and 8.9 Hours
Ovarian cancer has as its predominant pattern of dissemination metastases to the peritoneal surfaces and disease spread within the abdomen and pelvis that most commonly causes the patients demise. To combat peritoneal metastases, cytoreductive surgery with peritoneal and visceral resections is combined with intraperitoneal and systemic chemotherapy. Chemotherapy given in the operating room after the complete visible removal of ovarian cancer is hyperthermic intraperitoneal chemotherapy. The results of the combined treatment are determined by the extent of prior surgery, the extent of disease as established by the peritoneal cancer index, and the quality of the cytoreduction as measured by the completeness of cytoreduction score. Recent clinical information on patients with recurrent ovarian cancer suggest a median overall survival of up to 60 mo. These data are greatly improved over the one year survival observed in the past.
- Citation: Frigerio L, Ansaloni L, Poiasina E, Coccolini F, Sugarbaker PH. Comprehensive management of epithelial ovarian cancer with peritoneal metastases. World J Obstet Gynecol 2013; 2(4): 108-115
- URL: https://www.wjgnet.com/2218-6220/full/v2/i4/108.htm
- DOI: https://dx.doi.org/10.5317/wjog.v2.i4.108
Epithelial ovarian cancer (EOC) is a global healthcare problem. It affects over 200000 women and causes 125000 deaths annually[1]. Within the United States, EOC is the ninth most common female cancer (21000 cases annually) and the fifth most common cause of cancer death (14600) in women[2]. Improved strategies for EOC are needed because currently less than 50% of women with EOC will survive 5 years[3,4]. In the general population the lifetime risk of ovarian cancer is 1 in 70 but there are women with much higher risk especially those with germ line mutations of BRCA1 and BRCA2 tumor suppressor genes and certain mismatch repair genes[5].
The surgical intervention in EOC with peritoneal metastases may occur with initial treatment (frontline), at interval debulking surgery following neoadjuvant chemotherapy or with recurrence. The major treatment modalities are cytoreductive surgery (CRS), perioperative chemotherapy including hyperthermic intraperitoneal chemotherapy (HIPEC), and long-term combined intravenous and intraperitoneal chemotherapy. Second look surgery may be indicated in selected patients. It has been established that improved outcome is associated with small-volume residual disease following CRS. With current thinking a highest goal of treatment must be CRS to remove all visible evidence of disease[6,7]. Or if that is not possible, surgery should leave the least amount of residual disease[8,9]. In the past, CRS that left residual cancerous lesions up to 2 cm in greatest dimension was considered “optimal”. However, the precise definition of optimal cytoreduction has been open to wide differences of opinion which have changed considerably over time. It is now accepted that leaving no visible disease should always be considered optimal CRS at all time-points for EOC surgery except for palliation[10-12].
Several divergent types of surgical procedures are necessary to achieve complete cytoreduction because EOC is often widespread within the abdominal and pelvic cavity. In the past, disease involving upper abdominal structures such as the undersurface of the diaphragm, liver surface or parenchyma, porta hepatis, pancreas, and spleen were considered obstacles to the achievement of optimal CRS. It has been shown that the resection of tumor from these sites does improve survival whether this is achieved by the gynecologic oncologists or surgical oncologists[13]. The actual sites of intra-abdominal disease not amenable to safe resection at a particular institution vary according to surgical expertise and practice. Some of the sites most likely to result in an incomplete resection include parenchymal liver metastases, extensive disease in the porta hepatis, retroperitoneal adenopathy behind and superior to the pancreas and extensive disease involving proximal small bowel and small bowel mesentery.
Successful initiation of an optimal surgical outcome requires a knowledgeable selection of patients, a strong commitment from the surgical team, and institutional support. The initial selection of patients is based on two well defined criteria: First, the ability of the patient to survive an extensive surgical procedure with acceptable morbidity and mortality and second, no evidence of intraoperative findings that would result in a futile surgical effort. Patients of advanced age, poor performance status, malnourished or with medical conditions that would decrease the likelihood of postoperative survival should be excluded. Also, patients with systemic metastases, multiple sites of bowel obstruction, common bile duct obstruction or ureteral obstruction should rarely be considered for complete CRS.
Complete CRS requires dedication from a surgeon who must have broad surgical knowledge, unusual technical skills and the stamina to endure long procedures. Realizing that these interventions are extensive and thereby costly, institutional backing is important. An effort to educate other physicians involved in this treatment, as well as nurses and ancillary personnel, should be undertaken. The steep learning curve that characterizes this treatment strategy makes it essential to design a careful plan and to regularly critically evaluate all adverse events.
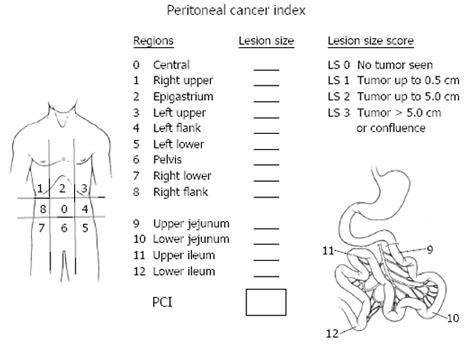
The three assessments helpful for patient selection in order to treat patients most likely to benefit are the prior surgical score (PSS), the peritoneal cancer index (PCI) and the completeness of cytoreduction score (CC). These scores are determined from 13 abdominopelvic regions. Two transverse planes and two sagittal planes are used to divide the abdomen into 9 abdominopelvic regions (0-8). The upper transverse plane is located at the lowest aspect of the costal margin. Regions 9 and 10 define the upper and lower portions of the jejunum, and regions 11 and 12 define the upper and lower portions of the ileum[14].
Surgical trauma promotes the implantation and progression of cancer nodules on peritoneal surfaces[15]. Prior surgeries may modify the natural history of ovarian cancer by inducing cancer growth at crucial anatomic sites located deep to the peritoneal layer[16]. Patients with no prior abdominopelvic surgery or biopsy only receive a PSS of 0, those with up to one abdominopelvic region dissected receive a PSS of 1, those with two to five abdominopelvic regions receive a PSS of 2 and those with six or more regions dissected receive a PSS of 3.
The PCI is a quantitative prognostic indicator determined after abdominal exploration and complete separation of intestinal adhesions (Figure 1). This index adds a lesion size parameter to the abdominopelvic regions so that a numerical score estimating the extent of carcinomatosis is available. The PCI is an accurate prognostic indicator for ovarian cancer[17].
CC is a quantitative prognostic indicator determined once the surgical resection has been completed. A patient receives a CC-0 score when no visible peritoneal carcinomatosis remains after cytoreduction, CC-1 is recorded when tumor nodules persist after cytoreduction but they measure less than 0.25 cm, CC-2 when remaining tumor measures between 0.25 to 2.5 cm. When tumor nodules are greater than 2.5 cm or there is confluence of unresectable tumor, a CC-3 score is given to the patient. Many prior studies in ovarian cancer have shown that the extent of disease remaining after cytoreduction is directly related to the survival[7-17].
The goal of CRS is to reduce the tumor burden within the abdomen and pelvis to its absolute minimal volume. The best result is a patient who is visibly free of disease at the close of the procedure. The surgery combines a series of peritonectomy procedures and visceral resections. The peritonectomy procedures include anterior parietal peritonectomy, stripping of right and left hemidiaphragm, pelvic peritonectomy, and omental bursa peritonectomy. Visceral resections include hysterectomy and oophorectomy, greater and lesser omentectomy, splenectomy, right colectomy and rectosigmoid colectomy.
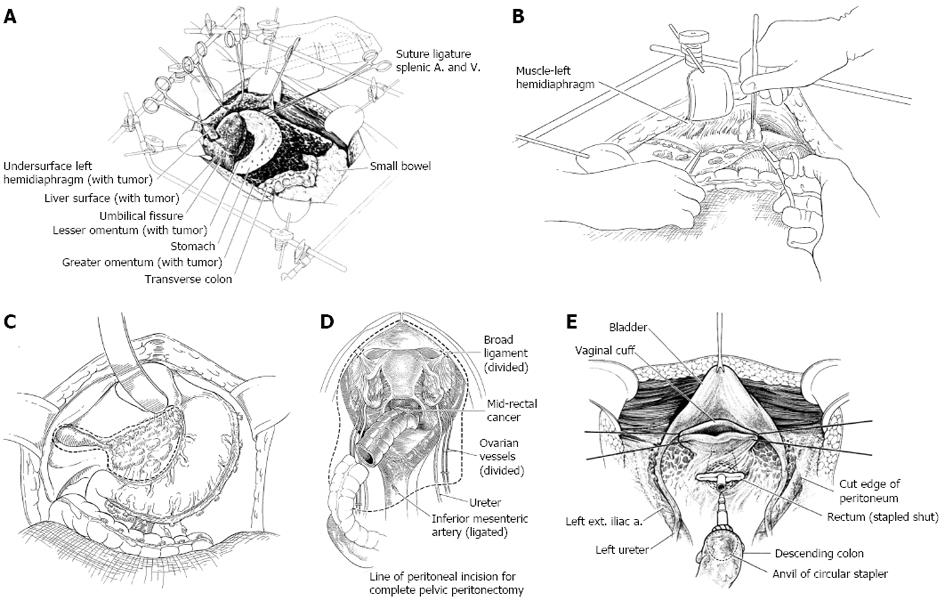
A fixed retractor that provides a rigid frame around the whole abdomen (Thompson Surgical Instruments, Traverse City, Michigan) is positioned so that continuous retraction of all parts of the abdominal incision occurs (Figure 2A). The retraction system must be securely anchored to the operating table to provide for continuous unencumbered visualization of a large operative field. An incision starting above the xiphisternal junction and continuing down to the pubis through the midline is constructed. An ellipse is created around the umbilicus to allow for the peritoneal plane to be clearly exposed throughout the extent of the abdominal incision. The fascia is divided through the linea alba from xiphoid bone to pubic bone. If there has been prior midline abdominal incision, it is widely excised. Routinely, the xiphoid is completely resected at the xiphisternal junction as part of the specimen. With the fascia divided the parietal peritoneum remains intact.
A single entry into the peritoneal cavity in the middle portion of the incision (peritoneal window) allows the surgeon to digitally and visibly assess the parietal peritoneum and the small bowel surfaces. If cancer nodules are palpated on the parietal peritoneum larger than those involving the small bowel and its mesentery, a decision for a complete dissection is made. Except for the small defect in the peritoneum required for this peritoneal exploration, the remainder of the peritoneum is kept intact to facilitate the peritonectomy.
After dissecting generously the peritoneum on both sides of the bladder, the apex of the bladder (preferably the urachus) is localized and placed on strong traction using a Babcock clamp. The peritoneum with the underlying fatty tissues are stripped away from the muscular surface of the bladder. Broad traction on the entire anterior parietal peritoneal surface and frequent room temperature saline irrigation reveals the point for tissue transection that is precisely located between the bladder musculature and its adherent fatty tissue. This dissection is continued inferiorly down to the cervix.
The self-retaining retraction system is steadily advanced more deeply into the abdominal cavity. Firm broad traction on the peritoneum at the point of dissection facilitates accurate progress. The peritoneum strips readily from the undersurface of the hemidiaphragm. The dissection connects the right and left subphrenic peritonectomy superiorly and the complete pelvic peritonectomy inferiorly. As the dissection proceeds beyond the peritoneum overlying the paracolic sulcus (line of Toldt) the dissection becomes more rapid because of the loose connections of the peritoneum to the underlying fatty tissue at this anatomic site.
When the anterior parietal peritonectomy has been completed, removal of this large peritoneal layer eradicates cancer implants from the posterior aspect of the anterior abdominal wall. Complete exploration of the abdomen and pelvis proceeds.
To begin peritonectomy of the left upper quadrant, the peritoneum is progressively stripped off the posterior rectus sheath (Figure 2B). Broad traction must be exerted on the tumor specimen throughout the left upper quadrant. Strong traction combined with ball-tip electrosurgical dissection allows separation of the peritoneum with tumor from all normal tissue in the left upper quadrant including the diaphragm muscle, the left adrenal gland, and the superior aspect of perirenal fat. The splenic flexure of the colon is divided from the peritoneum of the left abdominal gutter and moved medially.
To free the mid-abdomen of tumor, the greater omentectomy-splenectomy is performed. The greater omentectomy is elevated and then separated from the transverse colon using electrosurgery. The omental tissue on the anterior aspect of the transverse mesocolon is also resected. The gastroepiploic vessels on the greater curvature of the stomach are ligated and divided. Also, the short gastric vessels are transected. This freely exposes the splenic artery and vein at the tail of the pancreas. These vessels are ligated in continuity and proximally suture ligated taking care not to traumatize pancreas parenchyma. This allows the greater curvature of the stomach to be reflected to the right from the pylorus to the gastroesophageal junction.
Peritoneum is stripped away from the right posterior rectus sheath to begin the peritonectomy in the right upper quadrant of the abdomen. Strong traction on the peritoneum infiltrated by tumor is used to elevate the rolled muscular edge of the hemidiaphragm into the operative field. Again, ball-tipped electrosurgery on pure cut is used to dissect at the interface of tumor and normal tissue. Coagulation current is used to divide the blood vessels as they are encountered and before they bleed.
The gallbladder is removed in a routine fashion from its fundus toward the cystic artery and cystic duct (Figure 2C). Blunt dissection of the cystic artery and cystic duct away from the common duct and right hepatic artery distinguishes these structures from the surrounding tumor and fatty tissue. These structures are ligated and divided.
To strip the peritoneum from the anterior aspect of the hepatoduodenal ligament, its peritoneal reflection to the liver surface is divided. Special care is taken not to injure the left hepatic artery, which is usually the most superficial of the portal structures. The peritoneum, often layered by tumor nodules, is firmly grasped using a Russian forceps and peeled away from the common bile duct and hepatic artery. The underside of the hepatoduodenal ligament must be visualized and peritoneum stripped if tumor is present.
The left lateral segment of the liver is retracted left to right to expose the hepatogastric ligament and lesser omentum in its entirety. A circumferential release of this ligament from the fissure between the left lateral portion of the liver and segment 1 occurs first. The arcade of right gastric artery to left gastric artery along the lesser curvature of the stomach must be skeletonized. After electrosurgically dividing the peritoneum on the lesser curvature of the stomach, digital dissection with extreme pressure from the surgeon’s thumb and index finger separates lesser omental fat and tumor from the vascular arcade. As much of the anterior vagus nerve is spared as is possible with patience and persistence. The tumor and fatty tissue surrounding the right and left gastric arteries are split away from the vascular arcade. In this manner the specimen is centralized over the major branches of the left gastric artery. With strong traction on the specimen, the lesser omentum is released from the left gastric artery and vein.
With the left lateral segment of the liver retracted to the right, further exposure of the peritoneal floor of the omental bursa is achieved through elevation of the left side of the caudate lobe of the liver. The peritoneal reflection between caudate and vena cava is electrosurgically divided. Also, the peritoneal reflection towards the left hepatic vein is divided. Then, blunt stripping of the peritoneum covering the crus of the right hemidiaphragm is completed. This peritoneal stripping continues over the lymph nodes along the common hepatic artery and up into the tissues of the lesser omentum. Care is taken to avoid the origin and branches of the left gastric artery. Care is also taken to eliminate tumor nodules from the shelf created by the caudate lobe beneath the posterior aspect of the hepatoduodenal ligament.
To being the rectosigmoid colon resection a linear stapler is used to divide the sigmoid colon just above the limits of the pelvic peritoneal metastases; this is usually at the junction of sigmoid and descending colon. The vascular supply of the distal portion of the bowel is traced back to its origin on the aorta. The inferior mesenteric artery and vein are ligated, suture-ligated, and divided. This allows one to pack all the viscera, including the proximal descending colon into the upper abdomen.
Ball-tipped electrosurgery is used to dissect at the limits of the pelvic peritonectomy (Figure 2D). The surgeon works in a centripetal fashion. Extra-peritoneal ligation of the uterine arteries is performed just above the ureter and close to the base of the bladder. In women, the bladder is moved gently off the cervix and the vagina is entered. The vaginal cuff anterior and posterior to the cervix is transected using ball-tipped electrosurgery, and the rectovaginal septum is entered. Ball-tipped electrosurgery is used to divide the perirectal fat beneath the peritoneal reflection. This ensures that all tumors that occupy the peritoneum within the cul-de-sac are removed intact with the specimen. The anterior rectal musculature is skeletonized using ball-tipped electrosurgery. Preservation of the lower half of the rectum will allow for a larger stool reservoir and diminish frequent bowel movements. A stapler is used to close off the rectal stump and the rectum is sharply divided above the stapler.
Additional sutures are placed to close the apex of the vagina (Figure 2E). These sutures are left long so that they may be used to elevate the vaginal cuff and clearly expose the stump of the rectum.
Once peritonectomy and visceral resections and after para-rectal and para-vesical spaces have been observed, systematic pelvic and aortic lymphadenectomy are performed.
Pelvic lymphadenectomy dissection begins at the origin of the external iliac vessels and continues caudally around them along the medial border of the psoas muscle. The aponeurotic fascia is kept intact and the branches of the genito-femoral nerve are carefully spared to limit the risk of postoperative neurological sequelae. The dissection proceeds through the areolar plane between the adventitia of the artery and the lymphatic tissue. The lower limit of the external iliac lymphadenectomy is represented by the deep inferior epigastric vessels. Lymph nodes along the external iliac vessels are removed en bloc with those adjacent to the common iliac vessels. The psoas fascia, superficially, and the fascia covering the internal obturator and levator ani muscles, deeply, constitute the lateral boundaries of lymphadenectomy, while the medial margin is represented by an ideal plane between the umbilical artery, anteriorly, and the rectum, posteriorly. After obturator nerve identification, lymphadenectomy of the obturator fossa is performed with the mobilization of the superficial obturator nodes which are removed en bloc with the perilymphatic fatty tissue around the internal iliac vessels at the origin of the internal pudendal vessels. Lymphadenectomy is completed by removing deep obturator and gluteal nodes.
Aortic lymphadenectomy begins at the aortic bifurcation up to the renal vessels. After having exteriorized the transverse colon and the small bowel, the superficial intercavo-aortic, precaval, and preaortic nodal groups are removed. Lymph nodes located lateral to the vena cava (i.e., paracaval nodal group) are separated from it and removed en bloc. Removal of the lymph nodes lateral to the aorta is carried out up to the level of the left renal vein, after entering the plane between the Toldt’s and Gerota’s fasciae, mobilizing the descending colon from the renal capsule, the psoas muscle, and the ovarian pedicle and displacing the ureter laterally. Lastly, lymph nodes behind the vena cava (i.e., retrocaval nodal group) and the lumbar vessels (i.e., deep intercavo-aortic nodal group) are removed if enlarged, by dissecting from the pre-vertebral fascia after displacing the vena cava and the aorta laterally and medially (Figure 3).
After the completion of cytoreduction when no visible cancer remains, it is invariably true that invisible to the naked eye, an immense number of cancer cells will remain within the peritoneal cavity. Tumor manipulation, transected lymphatic ducts leaking tumor cells throughout the procedure, and small tumor nodules remaining on the abdominal and pelvic surfaces of organs not amenable to peritonectomy procedures, namely small bowel, make necessary the implementation of some method that will eradicate residual tumor cells. Another well known site for persistent disease is the suture lines that are an ideal site for cancer cell implants. Tumor cell entrapment occurs on these raw surfaces with fibrin accumulating and tissues compressed together by stitches or staples. Suture lines are at high risk for recurrence if constructed before the HIPEC.
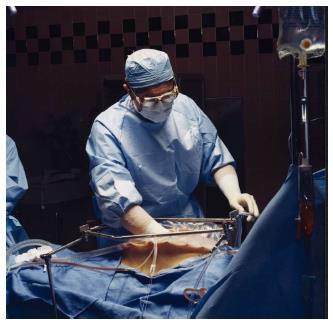
An abdominopelvic reservoir is constructed by tenting up the skin edges on a fixed retractor that allows hand distribution of the chemotherapy agent and total containment. The gloved hand guarantees that the perfusate reaches all surfaces within the peritoneal cavity, such as the space between the bowel loops, the space behind the liver, and the pelvic cavity (Figure 4).
In order to keep the temperature at a constant 42 °C, a hyperthermia pump forces the solution through a heat exchanger. Then it proceeds into the abdominopelvic cavity through an inflow catheter. The hyperthermic chemotherapy fluid is drained from the abdomen through four closed drains going back to the heat exchanger, and closing the circuit. The inflow catheter and the closed suction drains are secured watertight with purse-string sutures on the skin of the abdomen to avoid leaks and spillage. The chemotherapy solution circulates for 90 min at 42 °C.
After the 90 min of HIPEC with manual distribution, the surgeon may assume that fibrin and tissue debris and the microscopic residual disease they contain have been eradicated. At this time, all the anastomosis and any additional reconstruction can occur. Closed-suction drains and an inflow catheter are properly positioned for subsequent early postoperative intraperitoneal chemotherapy with paclitaxel. Standardized orders for bidirectional intraperitoneal chemotherapy are given in Table 1. Cisplatin and doxorubicin are given by intraperitoneal administration and ifosfamide by intravenous continuous infusion (55-56).
| Add cisplatin (50 mg/m2) ________ mg to 2 L of 1.5% dextrose peritoneal dialysis solution |
| Add doxorubicin (15 mg/m2) ________ mg to same 2 L of 1.5% dextrose peritoneal dialysis solution |
| Add ifosfamide (1300 mg/m2) ________ mg to 1 L normal saline |
| Begin continuous iv infusion over 90 min simultaneous with ip chemotherapy |
| Add mesna disulfide (260 mg/m2) ________ mg in 100 mL 0.9% sodium chloride to be given iv as a bolus 15 min prior to ifosfamide infusion |
| Add mesna disulfide (260 mg/m2) ________ mg in 100 mL 0.9% sodium chloride to be given iv as a bolus 4 h after ifosfamide infusion |
| Add mesna disulfide (260 mg/m2) ________ mg in 100 mL 0.9% sodium chloride to be given iv as a bolus 8 h after ifosfamide infusion |
| Send all the above to operating room No. _______ at _______ o’clock on ___________ (date) for a 90-min treatment |
In the first five postoperative days the patient receives normothermic intraperitoneal paclitaxel (20-40 mg/m2 per day), with the goal of consolidating the intraperitoneal chemotherapy treatments. The extremely favorable area under the curve ratio and the remarkable drug penetration of up to 80 cell layers deserve mention. Standardized orders for early postoperative intraperitoneal chemotherapy with paclitaxel are given in Table 2.
| Paclitaxel ________ mg (20-40 mg/m2× _______ m2) (maximum dose: 80 mg) in_______mL 6% Hespan® (Braun B, Irvine, CA) via the Tenckhoff catheter or IP port daily |
| Start date: ________________ Stop date: __________________ (For daily doses > 500 mL total volume, pharmacy will split dose equally into two bags) |
| Instill as rapidly as possible via the Tenckhoff catheter or ip port |
| Dwell for 23 h |
| Drain from Jackson-Pratt drains for one hour prior to the next instillation |
| Continue to drain the abdominal cavity by Jackson-Pratt drains after the last dose of ip chemotherapy |
| During the initial 6 h after chemotherapy instillation, patient’s bed should be kept flat |
| The patient should be on the right side during instillation |
| Turn ½ hour post instillation onto the left side and continue to change sides at ½ hour intervals for 6 h |
| Monitor with a pulse oximeter during the first 6 h of each intraperitoneal chemotherapy |
In patients who have failed the standard treatments of primary ovarian cancer the survival is short with an estimated median survival of 9 mo. The median survival of 28 patients with advanced primary and recurrent EOC who had an attempt at complete cytoreduction combined with perioperative chemotherapy at the Washington Cancer Institute was 45.8 mo[16]. Further analysis by Look et al[16] of the clinical features that affected survival showed that extent of prior surgery and completeness of cytoreduction were independent factors significantly affecting survival. Those patients with extensive prior surgery, that is with three or more abdominopelvic regions subjected to surgical dissection, were less likely to receive a complete cytoreduction and their survival was significantly shorter. Patients with a low PSS who had less than three abdominopelvic regions previously dissected had a median survival of 6.5 years, compared to 1.5 years for those patients with a higher PSS (P < 0.001). Patients with an adequate cytoreduction had a median survival of 55.9 mo; suboptimal cytoreduction showed an 8 mo survival (P = 0.037).
Tentes and colleagues reported on the PCI as a quantitative prognostic indicator in 60 women with ovarian cancer[17]. Those patients with a PCI lower than 10 had a median survival of 80 mo and a 5-year survival of 65%, while those patients with a PCI greater than 10 had a median survival of 38 mo and a 5-year survival rate of 29% (P = 0.0253).
Recently, Bijelic et al[18] published a systematic review analyzing 14 studies that reported on cytoreduction and HIPEC. Ten studies reported a positive impact of CRS and HIPEC on survival and in 4, survival was not analyzed. Morbidity ranged from 5% to 36% and the median mortality was 3%.
This comprehensive management plan has been reported in approximately 35 manuscripts over the last two decades. As experience in patient selection and refinements in surgical technology have occurred, a gradual improvement in survival benefits and a decrease in morbidity and mortality have occurred. Clinical information from 7 recent reports are presented in Table 3.
| Ref. | n | Median follow-up (mo) | Median disease-free survival (mo) | Median overall survival (mo) | Overall 5-year survival (mo) | Median length of hospital stay (d) | Mortality (%) | Morbidity Grade 3 (%) | Morbidity Grade 4 (%) |
| Bereder et al[20] | 246 | NR | 13 | 49 | 35 | 17 | 0.4 | 12 | |
| Pavlov et al[21] | 56 | 60 | 26 | 38 | NR | 14 | 2 | 0 | 2 |
| Fagotti et al[22] | 25 | 18 | 10 | NR | NR | 13 | 0 | 8 | 8 |
| Guardiola et al[23] | 47 | 23 | 14 | NR | NR | 18 | 0 | NR | 13 |
| Di Giorgio et al[24] | 47 | NR | 20 | 24 | 17 | 221 | 4 | 9 | 13 |
| Bae et al[25] | 67 | NR | NR | NR | 66 | NR | 0 | 0 | 0 |
| Cotte et al[26] | 81 | 47 | 19 | 28 | NR | 172 | 3 | 5 | 2 |
In conclusion, this highly specialized treatment needs to be performed by qualified surgeons who are knowledgeable about peritonectomy procedures. Accepting the fact that there might be a selection bias, the results with this comprehensive treatment are encouraging. Multi-institutional studies are in progress to further validate the benefits of complete CRS in patients with ovarian cancer. The strong rationale, the initial favorable results by competent groups, and the demonstrated safety justify the current use of complete CRS.
P- Reviewer: Dursun P S- Editor: Gou SX L- Editor: A E- Editor: Zheng XM
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 2. | American Cancer Society. Cancer Facts and Figures 2010. Available from: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010. Accessed October 7, 2010. |
| 3. | Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A. SEER Cancer Statistics Review, 1975-2006. Bethesda, MD: National Cancer Institute. Available from: http://seer.cancer.gov/csr/1975_2006/. Based on November 2008 SEER data submission, posted to the SEER web site, 2009. Accessed October 7, 2010. |
| 4. | Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S161-S192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 758] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 5. | Tavassoli FA, Devilee P. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARC Press 2003; . |
| 6. | Munnell EW. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961. Am J Obstet Gynecol. 1968;100:790-805. [PubMed] |
| 7. | Griffiths CT, Craig JM, Kistner RW, Rothman KJ, Steiner GJ, Tomic M. Effect of castration, estrogen, and timed progestins on induced endometrial carcinoma in the rabbit. Gynecol Oncol. 1975;3:259-275. [PubMed] |
| 8. | Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 1992;47:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 359] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 702] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 10. | Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, Levine DA, Hensley M, Barakat RR. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 11. | Eisenkop SM, Spirtos NM. What are the current surgical objectives, strategies, and technical capabilities of gynecologic oncologists treating advanced epithelial ovarian cancer? Gynecol Oncol. 2001;82:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 137] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998;69:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 384] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 13. | Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Levine DA, Poynor EA, Aghajanian C, Jarnagin WR, DeMatteo RP, D’Angelica MI, Barakat RR. The addition of extensive upper abdominal surgery to achieve optimal cytoreduction improves survival in patients with stages IIIC-IV epithelial ovarian cancer. Gynecol Oncol. 2006;103:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 229] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15:49-58. |
| 15. | Sugarbaker PH. Peritoneum as the first-line of defense in carcinomatosis. J Surg Oncol. 2007;95:93-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Look M, Chang D, Sugarbaker PH. Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int J Gynecol Cancer. 2004;14:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Tentes AA, Tripsiannis G, Markakidis SK, Karanikiotis CN, Tzegas G, Georgiadis G, Avgidou K. Peritoneal cancer index: a prognostic indicator of survival in advanced ovarian cancer. Eur J Surg Oncol. 2003;29:69-73. [PubMed] |
| 18. | Bijelic L, Jonson A, Sugarbaker PH. Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol. 2007;18:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am. 2003;12:703-727, xiii. [PubMed] |
| 20. | Bereder J, Glehen O, Habre J, Desantis M, Cotte E, Mounier N, Ray-Cocquard I, Karimdjee B, Bakrin N, Bernard J. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from ovarian cancer: A multiinstitutional study of 246 patients. J Clin Oncol. 2009;27:abstr 5542. |
| 21. | Pavlov MJ, Kovacevic PA, Ceranic MS, Stamenkovic AB, Ivanovic AM, Kecmanovic DM. Cytoreductive surgery and modified heated intraoperative intraperitoneal chemotherapy (HIPEC) for advanced and recurrent ovarian cancer -- 12-year single center experience. Eur J Surg Oncol. 2009;35:1186-1191. [PubMed] |
| 22. | Fagotti A, Paris I, Grimolizzi F, Fanfani F, Vizzielli G, Naldini A, Scambia G. Secondary cytoreduction plus oxaliplatin-based HIPEC in platinum-sensitive recurrent ovarian cancer patients: a pilot study. Gynecol Oncol. 2009;113:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Guardiola E, Delroeux D, Heyd B, Combe M, Lorgis V, Demarchi M, Stein U, Royer B, Chauffert B, Pivot X. Intra-operative intra-peritoneal chemotherapy with cisplatin in patients with peritoneal carcinomatosis of ovarian cancer. World J Surg Oncol. 2009;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Di Giorgio A, Naticchioni E, Biacchi D, Sibio S, Accarpio F, Rocco M, Tarquini S, Di Seri M, Ciardi A, Montruccoli D. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer. 2008;113:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, Ahn WS, Namkoong SE. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Cotte E, Glehen O, Mohamed F, Lamy F, Falandry C, Golfier F, Gilly FN. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg. 2007;31:1813-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |