Copyright
©2014 Baishideng Publishing Group Inc.
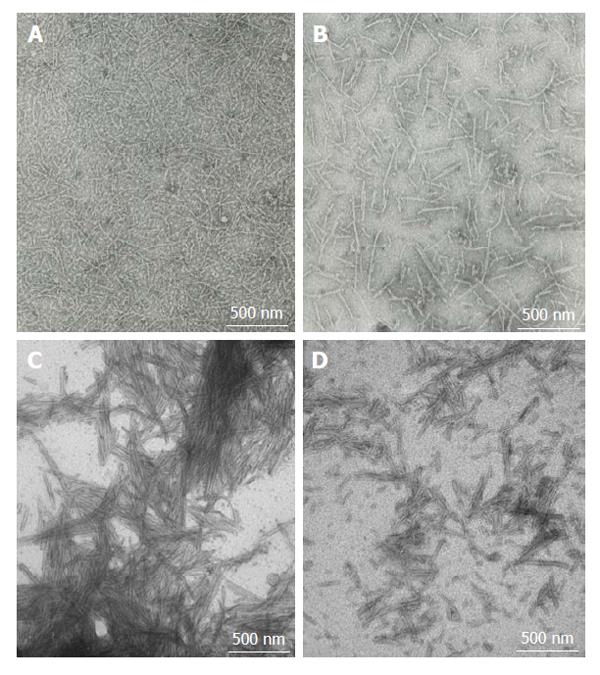
Figure 1 Fibrillar amyloid-beta peptide and alpha-synuclein.
A and B: Amyloid-beta (10 mg/mL) was aggregated at room temperature for 72 h with shaking; C and D: α-synuclein (2 mg/mL) was aggregated by incubation at 37 °C for 144 h with shaking. Aggregation leads to formation of fibrils with varying structures. Fibrils were negatively stained with 2% phosphotungstic acid and their formation was confirmed using Transmission Electron Microscopy at 75 kV. Images display 40000 × magnification.
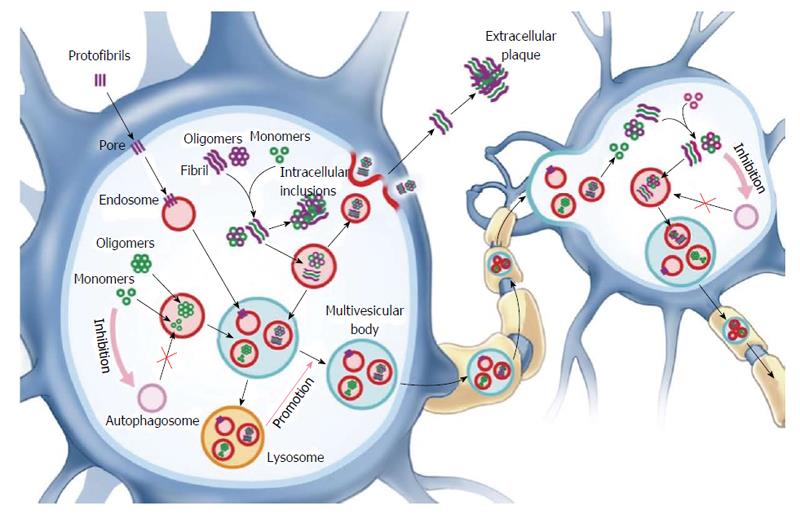
Figure 2 Several mechanisms are proposed to underlie the extracellular release of amyloidogenic proteins from neurons.
Lysosomal dysfunction due to uptake and accumulation of proteopathic species that are not degraded promotes the release of misfolded species by exosomes and exocytosis. Uptake by nearby neurons likely occurs via an endocytic mechanism and allows proteopathic species (oligomers and fibrils) to seed the templating of endogenous protein into a misfolded conformation. Pore formation by insertion of protofibrillar amyloid-beta and alpha-synuclein prompts a cellular response to undergo endocytosis and degradation. Inhibition of autophagosome formation may contribute to amyloid deposition as well as transfer of amyloidogenic species.
- Citation: Ibrahim T, McLaurin J. Protein seeding in Alzheimer’s disease and Parkinson’s disease: Similarities and differences. World J Neurol 2014; 4(4): 23-35
- URL: https://www.wjgnet.com/2218-6212/full/v4/i4/23.htm
- DOI: https://dx.doi.org/10.5316/wjn.v4.i4.23