Copyright
©2013 Baishideng.
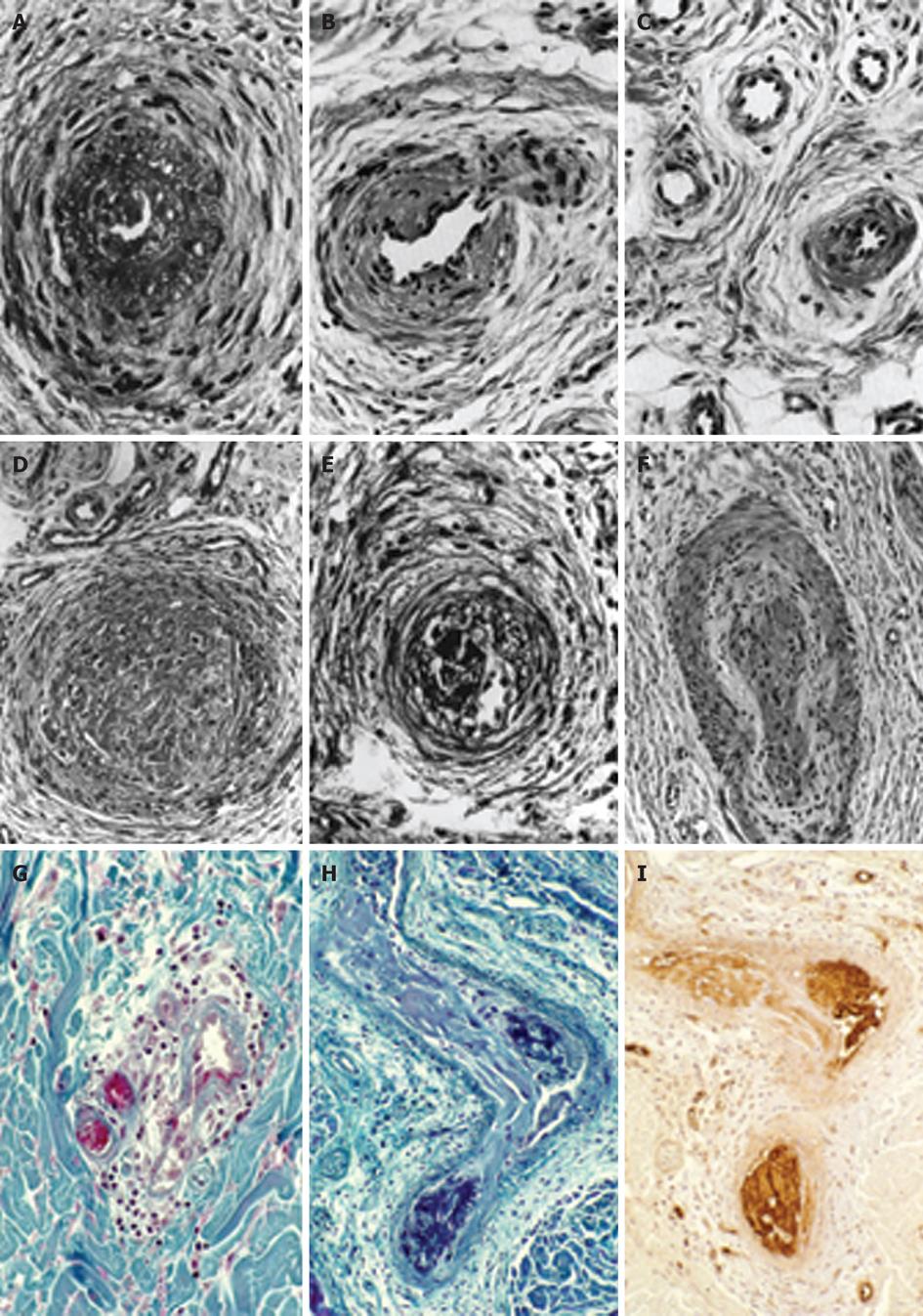
Figure 1 The histopathological appearances of a skin punch biopsy from areas of recently relapsed erythromelalgia 1 wk after aspirin discontinuation arteriolar l fibromuscular intimal proliferation, swollen endothelial cells, slight intravascular and perivascular infiltration with inflammatory cells, some perivascular fibrosis and no occlusive arteriolar thrombi.
The histopathological appearances from relapsed erythromelalgia complicated by blue ischemic spots 3 wk after aspirin discontinuation show arterioles with pronounced fibromuscular intimal proliferation without occlusive trombi (A-C) and fibromuscular intimal proliferation with occlusive thrombotic arteriolar lesions (D-F) perivascular oedema, vascular and perivascular fibrosis and infiltration by inflammatory cells (thromboangiitis obliterans). Originated from Michiels et al[20]. Biopsy from red-bluish discolored erythromelalgic skin area showing arteriolarthrombi with a weak fibrin staining (H) as compared to a positive fibrin staining from a patient with primary antipholipid syndrome and recurrent arterial skin thromboses (G). The occlusive arteriolar thrombi revealed a strong staining for von Willebrand factor (I) indicating platelet rich thrombi. This documents that thrombi from skin areas of erythromelalgia complicated by acrocyanosis are indeed platelet-rich and fibrin-poor thrombi. Originated from van Genderen et al[24].
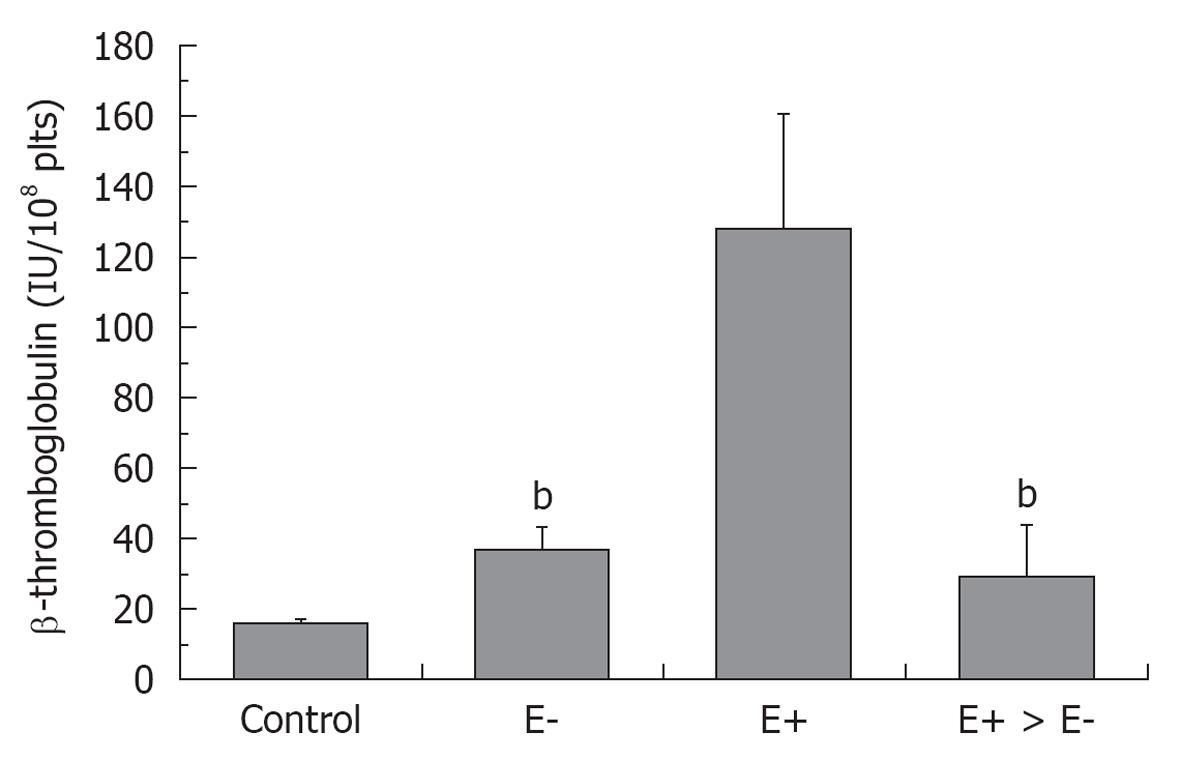
Figure 2 β-thromboglobuline levels in healthy control, and in essential thrombocythemia (E) either asymptomatic (E-), complicated by erythromelalgia (E+) and after treatment of E+ with aspirin (E+ → E-)[24].
bP < 0.01, significant differences between E- and E+ > E- as compared to E+.
Figure 3 Etiology and pathophpysiology of platelet-mediated fibromuscular intimal proliferation and platelet thrombi in erythromelalgia and its acrocyanotic complications in thrombocythemia vera (essential thrombocythemia and polycythemia vera) and etiology of major thrombosis on top of erythromelagic microvascular circulation disturbances in polycythemia vera.
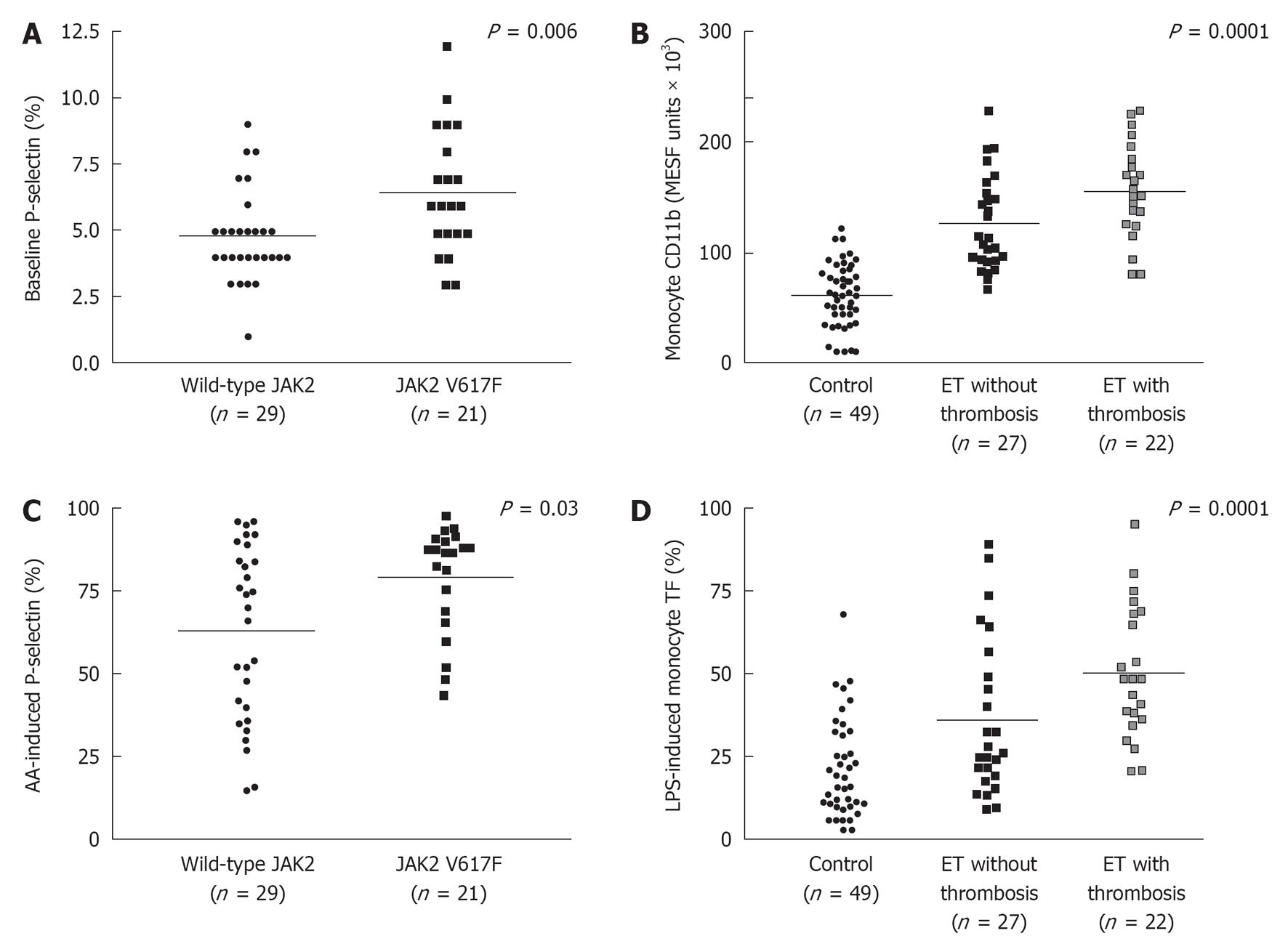
Figure 4 Baseline P-selectin levels (A), and arachidonic acid-induced P-selectin levels (C) in JAK2V617F mutated essential thrombocythemia and JAK2 wild type essential thrombocythemia; monocyte CD11b (B), and LPS-induced monocyte tissue factor expressions (D) in controls, essential thrombocythemia without thrombosis and essential thrombocythemia with thrombosis.
Majority of essential thrombocythemia (ET) patients were on myelosuppressive therapy (mainly hydroxyurea), and a minority used aspirin at time of laboratory investigations. AA: Arachidonic acid.
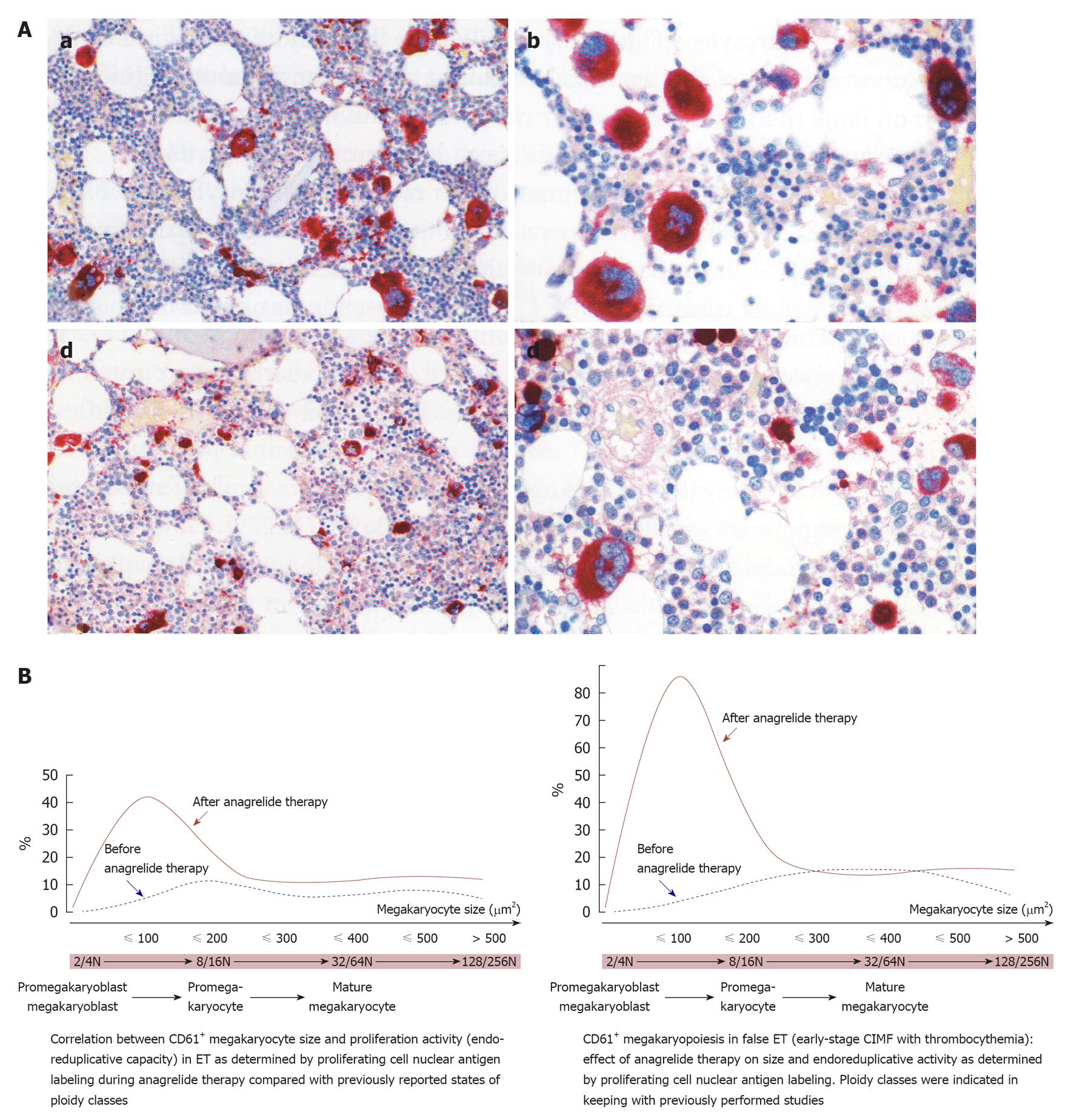
Figure 5 The effect of anagrelide treatment on CD61+ stained megakaryocyte size, proliferation and differentiation in bone marrow biopsies.
A: Change of megakaryocyte size from large before treatment (a and b) to small after treatment (c and d) with anagrelide, a selective inhibitor of megakaryocyte differentiation; B: The peculiar phenomenon of megakaryocyte change by anagrelide is generated by an arrest of endomitotic activity of the thrombocythemia-specific reversion of large to small megakaryocytes before and after anagrelide treatment in essential thrombocythemia (ET, left) and in hypercellularfalse ET (early prefibrotic stage chronic idiopathic myelofibrosis, right). Before anagrelide therapy (platelets 9.7 × 1011/L), the megakaryocytes in ET are loosely clustered with predominance of large and giant forms (a, b). After 1 year anagrelide monotherapy (platelets 4.5 × 1011/L), the megakaryocytes are small (c, d), but the number of small megakaryocytes remained increased (d). Originated from Michiels et al[58].
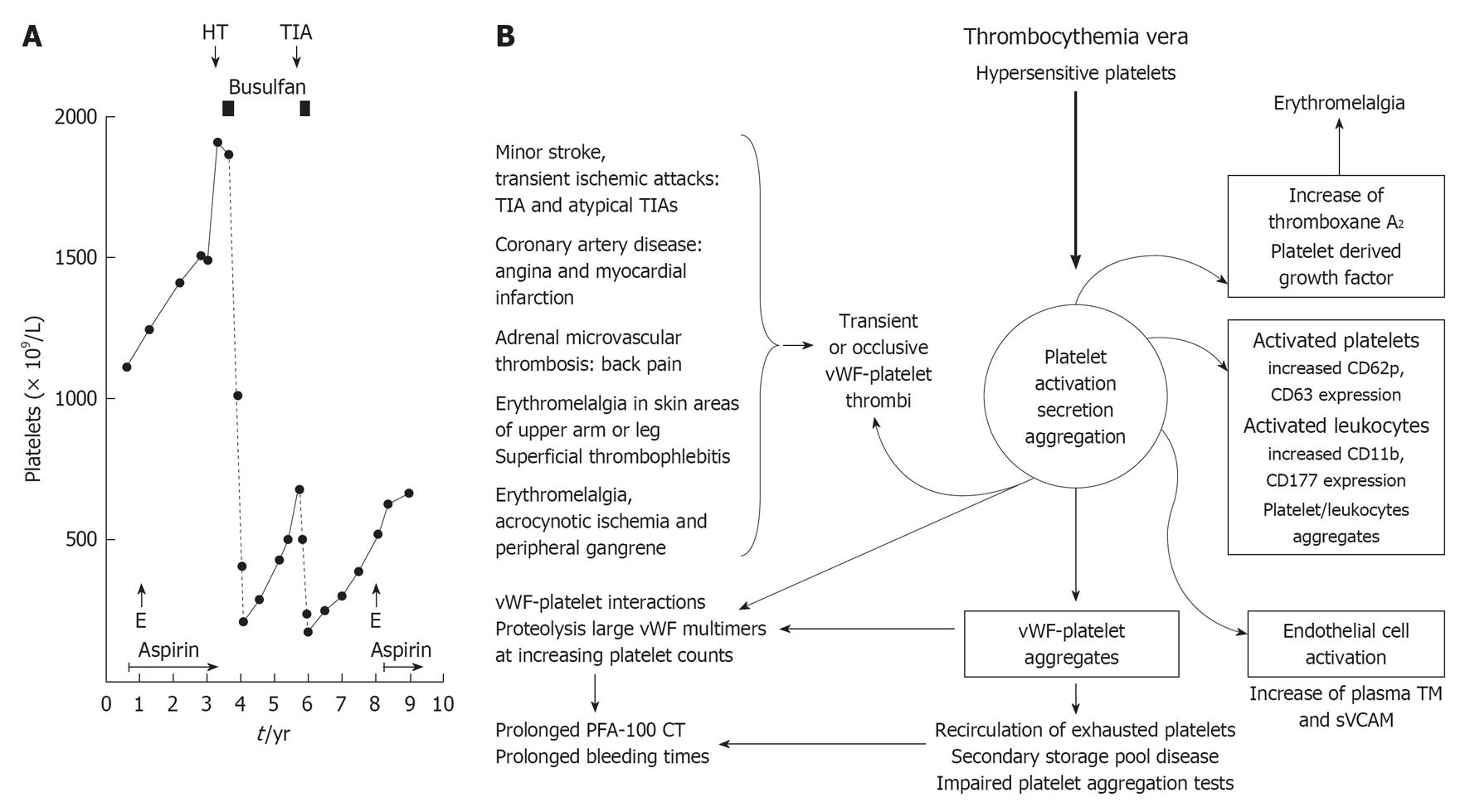
Figure 6 JAK2V617F or MPL515 constitutively activated (hypersensitive) platelets in thrombocythemia spontaneously aggregate and secrete products (platelet derived growth factor et) at high shear stress in the end-arterial cerebral, ocular, coronary and peripheral circulation.
This is associated with a broad spectrum of microvascular ischemic circulation disturbances (B) caused by transient von Willebrand factor (vWF)-rich platelet agregates or occlusive vWF-rich platelet thrombi on histological investigation (Figure 1). Circulating activated platelets express increase of platelet membrane surface markers, e.g., CD62p, and circulating platelet aggregates induce endothelial cell activation with increased thrombomoduline (TM) and soluble vascular adhesion molecule (sVCAM) plasma levels (right). Increase of platelet counts from below to far above 1000 × 109/L is associated with platelet-induced proteolysis of large vWF multimers with clinical features of hemorrhagic thrombocythemia (HT, A) due to an acquired von Willebrand disease type 2A [prolonged PFA-110 closure times (CT) and bleeding times]. TIA: Transient cerebral ischemic attack; E: Erythromelalgia.
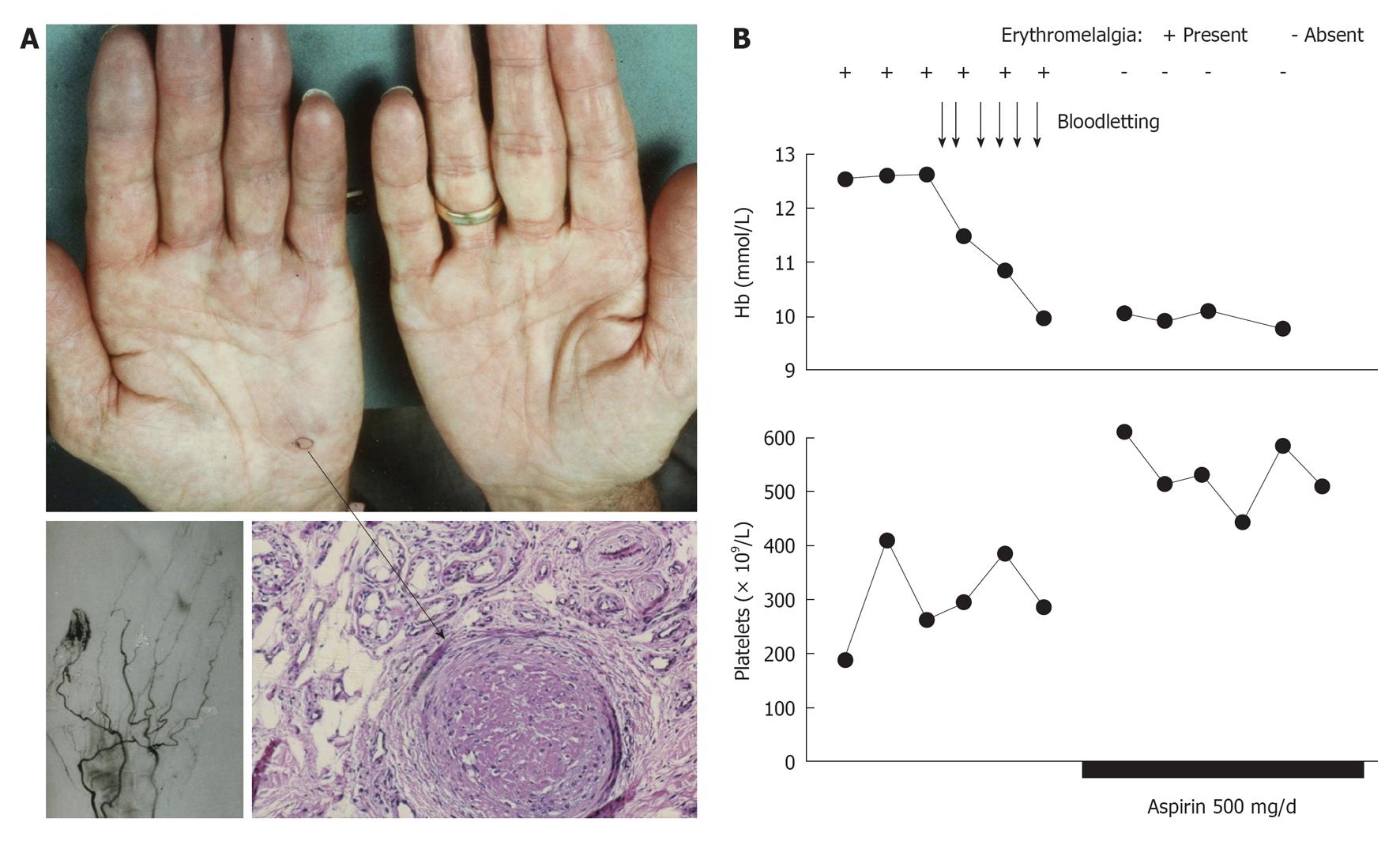
Figure 7 Acrocyanotic ischemic progression in a case of polycythemia vera 6 mo after presentation of erythromelalgia in the fingers of the right hand caused by endthrombotic occlusions of the superficial arterial arcade of the digital arteries.
A: A skin biopsy taken from the blue ischemic spot of the handpalm (arrow) showed an occluded arteriole consistent with thrombo-angiitis obliterans; B: The erythromelalgic acrocyanotic congested fingers persited after treatment of polycythemia vera with phlebotomy for 1 mo (4 wk), but treatment with aspirin during a subsequent second month (4 wk) induced complete relief of the painfull blue swelling, which was associated with a significant increase of circulating platelets counts from below to above 400 × 109/L. Four weeks of phlebomotomy treatment (horizontal axis) had no effect on acrocyanotic erythromelalgia of the fingers, and subsequent treatment with aspirin one dose daily for several weeks was associated with the disappearance of painfull acrocyantic erythromelalic sign and symptoms and a significant rise in circulating platelet counts of about 200 × 109/L. Originated from Michiels et al[83].
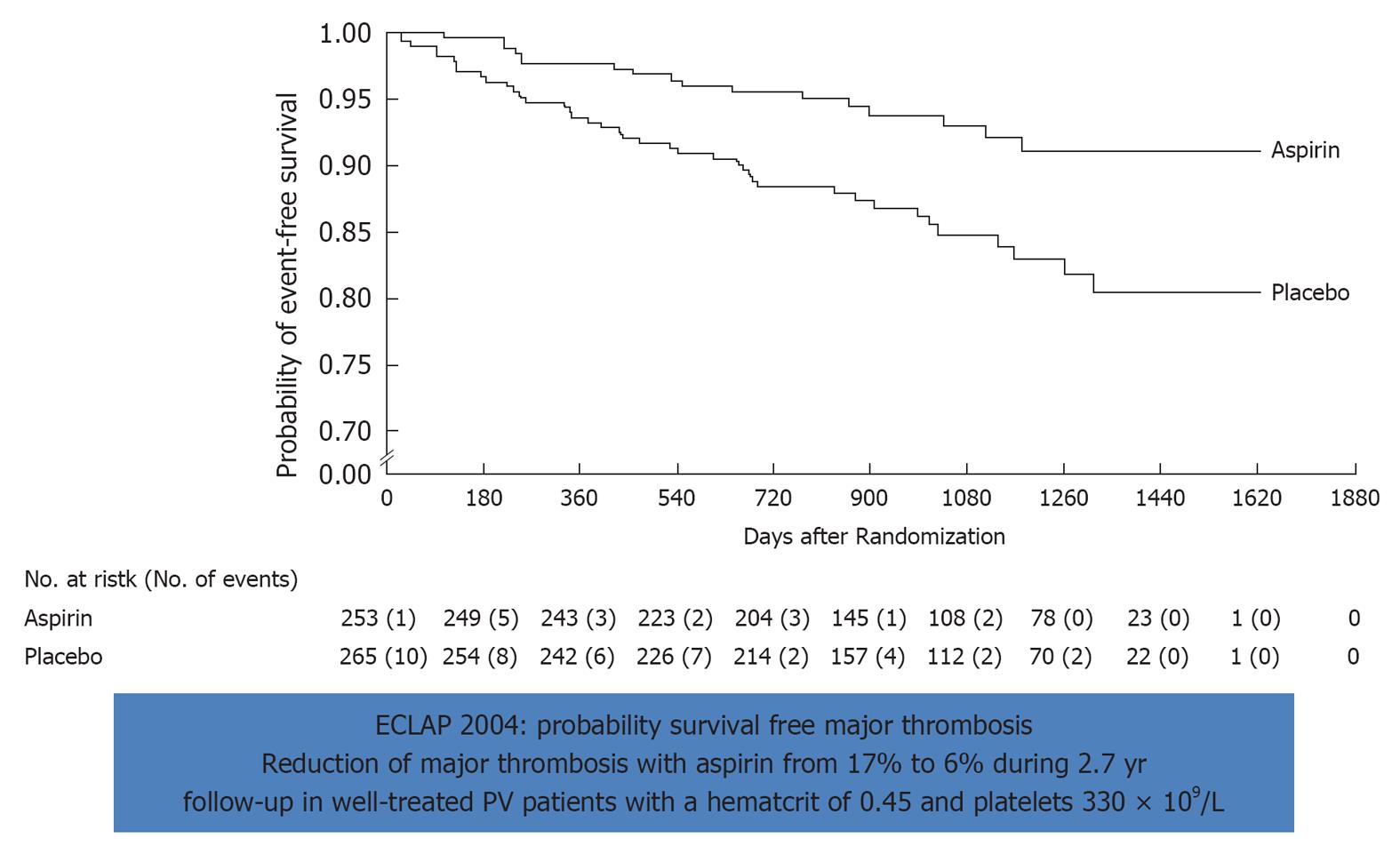
Figure 8 Effect of low dose aspirin on major thrombosis in treated polycythemia vera with bloodletting (40%) or hydrea (60%).
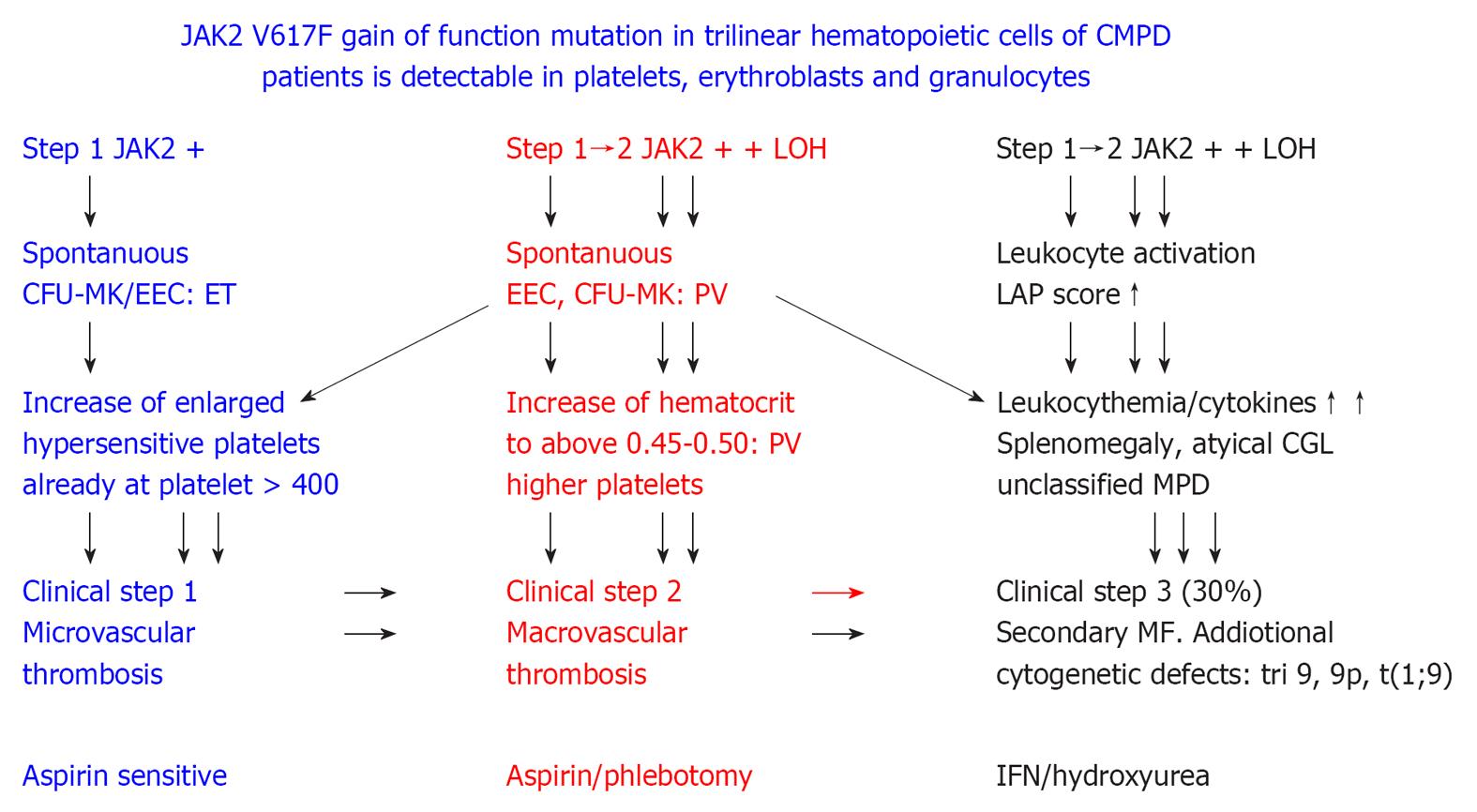
Figure 9 2005 ECMP concept on the molecular etiology of essential thrombocythemia, prodromal and overt polycythemia vera, leukocythemia, platelet-mediated microvascular thrombosis, increased blood volume and secondary myelofibrosis in JAK2 muated classical myeloproliferative disorders.
ET: Essential thrombocythemia; PV: Polycythemia vera; LAP: Leukocyte alkaline phosphatase; MPD: Myeloproliferative disorders; IFN: Interferon. Originated from Michiels et al[106,107].
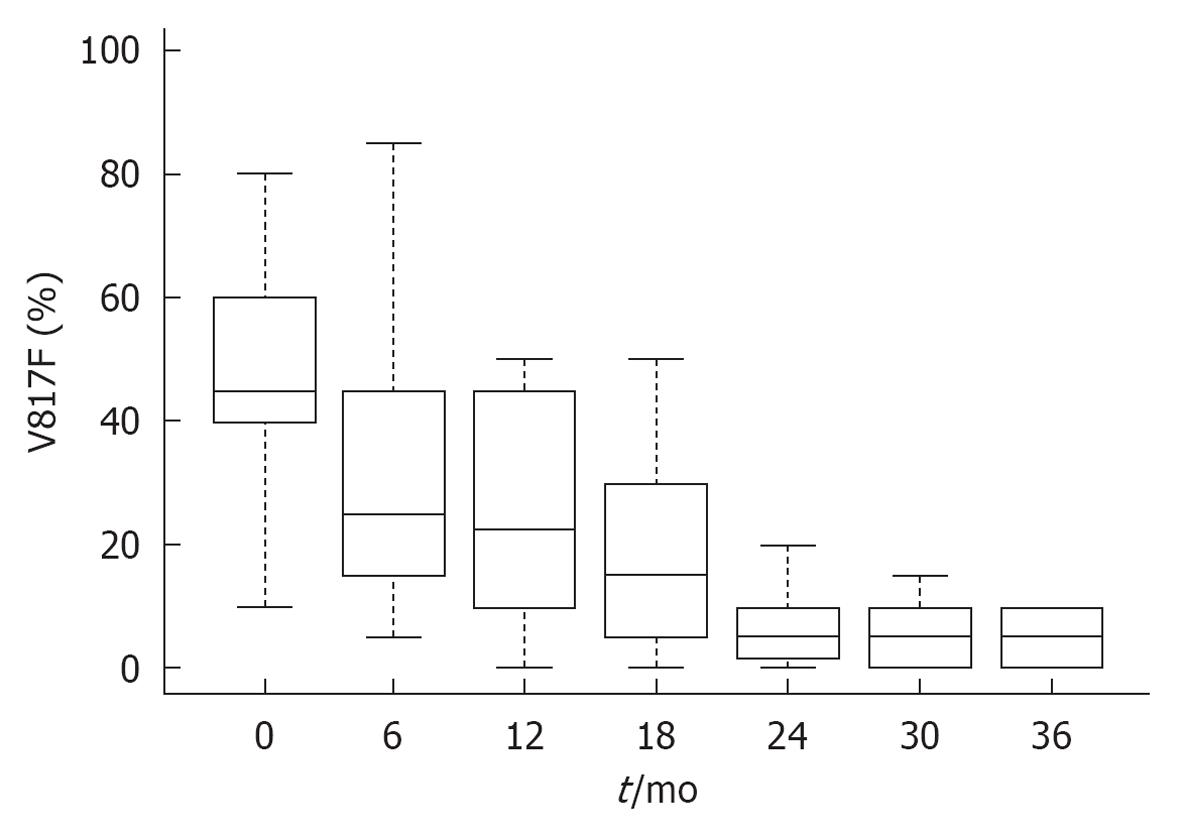
Figure 10 JAK2V617F molecular responses in 38 patients with polycythemia vera during the first three years of treatment with interferon α-2a (Pegasys R) Courtesy of Jean Jacques Kiladjian, France.
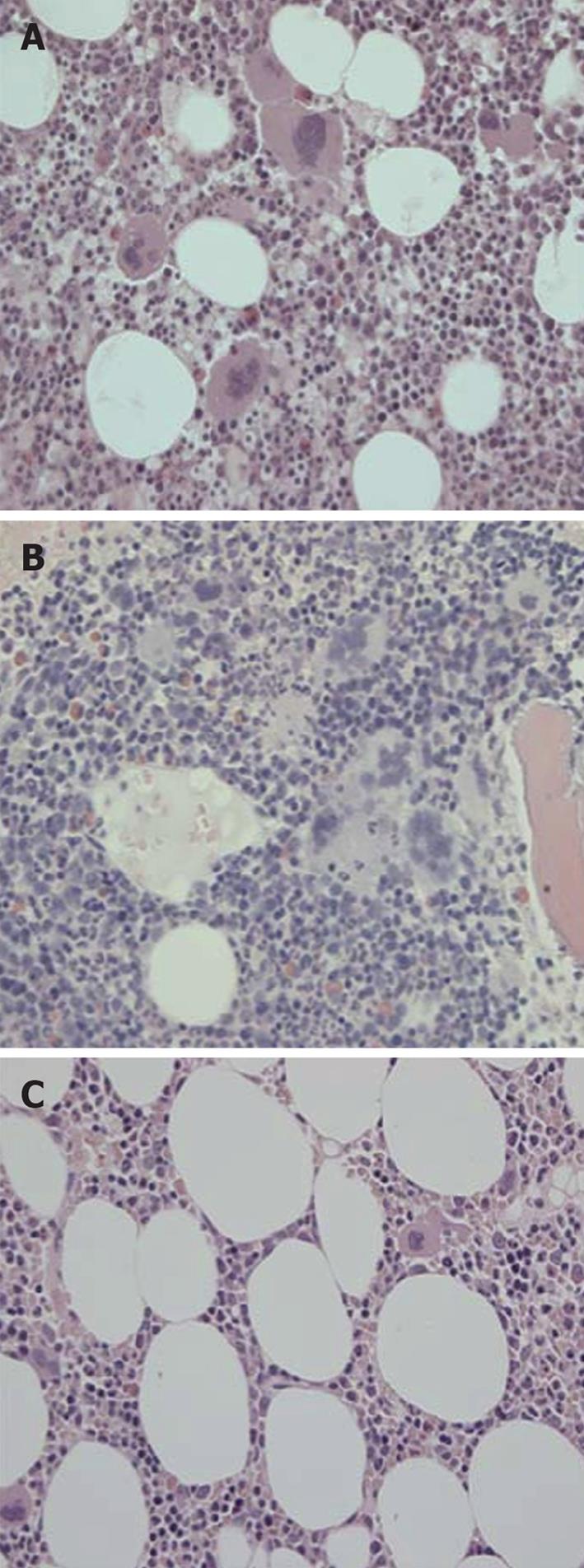
Figure 11 Bone marrow histomorphology from a patient with early prefibrotic polycythemia vera at diagnosis in 1996 (A) (hemoglobin 19.
7 g/dL, hematoctit 0.60. leukocytes normal, platelets 750 × 109/L, modest splenomegaly, JAKV617F > 54%) just prior to interferon α-2b treatment (B) showing typical hypercellular polycythemia vera pictures with clustered large pleiomorphic megakaryocytes and normal cellular bone marrow with megakaryocytes of normal size and morphology (JAK2V617F < 1%) after 8 years of treatment with interferon α-2b treatment (C). Courtesy of Hans Hasselbalch, Denmark. A and B: Pleiomorphic (normal sized and enlarged ) loosely clustered megakaryocytes with hyperploid nuclei and increased cellularity due to increased erythropoiesis in a case of polycythemia vera (PV) before start of interferon α-2b treatment in 1996; C: Megakaryocytes of normal size and morphology in a normocellular bone marrow in the case of PV (A, B) in complete hematological and molecular remission. Originated from Larsen et al[98].
- Citation: Michiels JJ, Ten Kate FW, Koudstaal PJ, Van Genderen PJ. Aspirin responsive platelet thrombophilia in essential thrombocythemia and polycythemia vera. World J Hematol 2013; 2(2): 20-43
- URL: https://www.wjgnet.com/2218-6204/full/v2/i2/20.htm
- DOI: https://dx.doi.org/10.5315/wjh.v2.i2.20