Published online Jun 24, 2022. doi: 10.5306/wjco.v13.i6.529
Peer-review started: January 7, 2022
First decision: April 13, 2022
Revised: April 16, 2022
Accepted: May 28, 2022
Article in press: May 28, 2022
Published online: June 24, 2022
Carcinoembryonic antigen (CEA) is an important serum tumour marker with a substantial role in diagnosis and monitoring of various solid tumours. About 36%-70% of breast cancers have elevated serum CEA. And the available studies show discrepancy in addressing the prognostic significance of CEA in advanced breast cancer.
To estimate the serum CEA level in our metastatic breast cancer patients and correlate it with response to treatment and clinical outcome.
This was a prospective clinical study conducted on 50 metastatic breast cancer patients treated at breast clinic, with newly diagnosed metastatic breast cancer planned for palliative chemotherapy, targeted therapy, and hormonal treatment. We estimated the proportion of patients with elevated serum CEA level at baseline and after palliative treatment and also studied the association of serum CEA levels with known prognostic factors. The response to treatment was correlated with the serum CEA levels in the context of responders and non-responders.
The median pre-treatment and post-treatment CEA levels were 7.9 (1.8-40.7) ng/mL and 4.39 (1.4-12.15) ng/mL, respectively, in the whole study population (P = 0.032). No statistically significant difference was seen in baseline serum CEA between responders and non-responders. Even in the luminal group, pre-treatment serum CEA was not a predictor of response, but post-treatment CEA was a significant predictor of tumour progression. In patients with liver and lung metastases, post-treatment CEA level difference was not statistically significant in both responders and non-responders though the values were higher in non-responders. Among those with bone metastases, 69.5% had elevated post-treatment serum CEA, and only 37.5% had elevated serum CEA in those with no bone metastases.
Elevated post-treatment serum CEA levels are associated with disease progression and poor response to therapy. Persistently elevated post-treatment serum CEA levels are significantly associated with bone metastases. Elevated serum CEA and hormonal status are significant predictors of treatment response.
Core Tip: In breast cancer patients, elevated serum carcinoembryonic antigen (CEA) levels are particularly noted in advanced disease. Our study suggested that serum CEA has potential clinical value in monitoring the treatment response of metastatic breast cancer patients, especially in those with bone metastasis.
- Citation: Anoop TM, Joseph P R, Soman S, Chacko S, Mathew M. Significance of serum carcinoembryonic antigen in metastatic breast cancer patients: A prospective study. World J Clin Oncol 2022; 13(6): 529-539
- URL: https://www.wjgnet.com/2218-4333/full/v13/i6/529.htm
- DOI: https://dx.doi.org/10.5306/wjco.v13.i6.529
Breast cancer, one of the leading causes of malignancy related morbidity and mortality among women, comprises of a spectrum of clinically and histologically heterogeneous group of diseases with distinct molecular portraits[1]. In spite of increasing awareness, advanced screening, and diagnostic methodologies, we still witness a significant proportion of patients who present with advanced stage disease. Deciding optimal treatment and monitoring strategies for patients with metastatic and recurrent disease remains a diagnostic challenge for physicians.
Carcinoembryonic antigen (CEA) is an important serum tumour marker with a substantial role in diagnosis and monitoring of colorectal cancer. Globally, cancer antigen 15-3 (CA15-3) and CEA are used serum tumor markers in breast cancer[2-4]. In breast cancer patients, elevated serum CEA levels are particularly noted in metastatic and recurrent disease. Studies have reported a varying incidence of serum CEA positivity ranging from 36%-70%[5]. Elevated levels are known to positively correlate with tumour burden, grade of tumour, and site of metastasis, and they also translate into poor overall survival (OS) and progression-free survival[6]. The clinical utility of serial tumour marker measurements is not indicated in asymptomatic women for surveillance after treatment of breast cancer[7-9]. The main applications are used in metastatic disease monitoring during treatment, especially CA15-3. Among serum tumour markers in breast cancer, CA15–3 and CEA have been the commonly used ones[10-13]. Hence, serum CEA estimation can be proposed as an auxiliary tool for response assessment, monitoring, and gaining prognostic information. In spite of these, due to discordant results, their clinical utility remains unclear[14-16]. There are very few studies addressing the prognostic significance of CEA and the available studies show discrepancy. Hence, we conducted this study to estimate the serum CEA level in our metastatic breast cancer (MBC) patients and correlate it with response to treatment and clinical outcome.
This was a prospective experimental study conducted on 50 MBC patients treated at Breast Clinic, Department of Medical Oncology during the period December 2019 to November 2020. Patients with newly diagnosed MBC planned for palliative chemotherapy, targeted therapy, and hormonal treatment were included. Routine protocol for MBC work-up included biopsy from breast lump or metastatic lesion, histopathology and immunohistochemistry for oestrogen, progesterone, and Her2 receptors, computed tomography of the chest, abdomen, and pelvis, bone scan, and serum biochemistry. Patients with inflammatory breast cancer and active inflammatory conditions were excluded in this study due to the fact that they could cause elevation of serum CEA levels. Five milliliters of venous blood was drawn from MBC patients who consented for study participation and serum was isolated after centrifugation at 3000 rpm for 10 min, transported into new disposable tubes, and stored at -20 ℃. In patients with hormone positive MBC with visceral crisis and triple negative breast cancer (TNBC) patients, sample for serum CEA levels was collected before initiation of first cycle of palliative chemotherapy and after completion of six cycles of chemotherapy. In patients with hormone positive MBC without visceral crisis, serum CEA sample was collected before initiation of endocrine agents and at 6 mo after initiation. In patients with HER2 positive MBC, blood sample was collected before initiation of first cycle of palliative chemotherapy plus trastuzumab and after completion of six cycles of chemotherapy plus trastuzumab.
Concentrations of the serum tumour marker CEA were measured with an automated sandwich ELISA test system using the manufacturer’s recommended kits (ELISA 2010, Roche Company). CEA concentrations were recorded in nanogram per millilitre. CEA value more than 3.8 ng/mL was considered positive. Patient treatment and response evaluation were as per the institutional protocol. Treatment and follow-up details of the patients were noted from the medical case records. We estimated the proportion of patients with elevated serum CEA level in MBC and also studied the association of serum CEA levels with known prognostic factors. The radiological response was assessed using Response Evaluation Criteria in Solid tumours (RECIST1.1). The response to treatment were correlated with the serum CEA levels in the context of responders and non-responders.
Statistical calculations were performed using the SPSS for Windows, version 15.0 (SPSS, Inc., Chicago, United States). Categorical variables are expressed using frequencies and percentages. Continuous variables are presented in terms of the mean and standard deviation. Association between two categorical variables was analyzed using Chi square or fisher's exact test. Non-parametric tests were used for finding the statistical significance. Wilcoxon signed rank test was used for comparing pre- and post-treatment serum CEA in different categories. Comparison of serum CEA in different clinical categories was carried out using Mann-Whitney test and Kruskal-Wallis test.
The optimal cut-off values of the CEA were determined using receiver operator characteristic (ROC) curve. A P value < 0.05 was considered significant.
The median age of diagnosis was 57.5 (48.7-63.2) years. Median duration of symptoms was 4 (1.75-6.0) mo. About 24% (12/50) of the patients were premenopausal and 76% (38/50) were post-menopausal. The main comorbidities were diabetes mellitus (24%; 12/50), hypertension (28%; 14/50), and coronary heart disease (4%; 2/50). About 64% (32/50) of the patients had distant nodal metastases, 50% (25/50) had bone metastases, 72% (36/50) had lung metastases, 36% (18/50) had liver metastases, and 6% (3/50) had oligometastatic diseases. About 96% (48/50) had invasive ductal carcinoma (IDC) and 4% (2/50) had other histology. Approximately 72% (36/50) were hormone positive and 38% (19/50) were HER2 positive. Grade 2 IDC accounted for 24% (12/50) and grade 3 IDC accounted for 76% (38/50). Among the study population, luminal type was seen in 70% (35/50), HER2 positive type in 8% (4/50), and TNBC in 22% (11). The pre-chemotherapy CEA levels were more than 3.8 in 72% (36/50) of the patients. About 82% (41/50) were treated with chemotherapy and 18% (9/50) treated with hormonal agents. Anti-Her2 treatment was received by 16% (8/50) of the patients. The median number of cycles of chemotherapy was 6 (4-6). The main palliative chemotherapy agents were docetaxel (68%; 34/50), paclitaxel (4%; 2/50), capecitabine (2%; 1/50), doxorubicin plus cyclophosphamide (2%; 1/50), carboplatin (2%; 1/50), and paclitaxel plus carboplatin (4%; 2/50). About 6% (3/50) of the patients received palliative radiation to their painful bone metastases.
About 36% (18/50) of the patients progressed on treatment while 64% (32/50) had responded to palliative systemic treatment. Among responders (64%), 2% (1/50) had complete remission, 32% (16/50) had partial response, and 30% (15/50) had stable disease. About 36% (18/50) had progressive disease.
Serum CEA value more than 3.8 ng/mL was considered positive. Baseline serum CEA and its correlation with other variables in MBC are given in Table 1. None of the factors like menstrual status, grade of the tumour, number and sites of metastases, presence or absence of metastases, HER2 status, and TNBC status showed any statistical significance except luminal type (P = 0.016).
| CEA level | Less than or equal to 3.8 | More than 3.8 | P value |
| Pre-menopausal | 3 | 9 | 0.79 |
| Post-menopausal | 11 | 27 | |
| Grade 2 | 3 | 9 | 0.79 |
| Grade 3 | 11 | 27 | |
| Luminal | 6 | 30 | 0.016 |
| Her2 Neu | 1 | 2 | |
| TNBC | 7 | 4 | |
| Luminal | 6 | 30 | 0.012 |
| Non luminal | 8 | 6 | |
| Bone metastases | 6 | 19 | 0.682 |
| No bone metastases | 7 | 17 | |
| Lung metastases | 11 | 25 | 0.487 |
| No lung metastases | 2 | 11 | |
| Liver metastases | 3 | 15 | 0.392 |
| No liver metastases | 10 | 21 | |
| Less than 5 metastases | 1 | 2 | 0.78 |
| More than 5 | 12 | 34 | |
| PR/SD/CR | 9 | 23 | 0.79 |
| Progression | 5 | 13 |
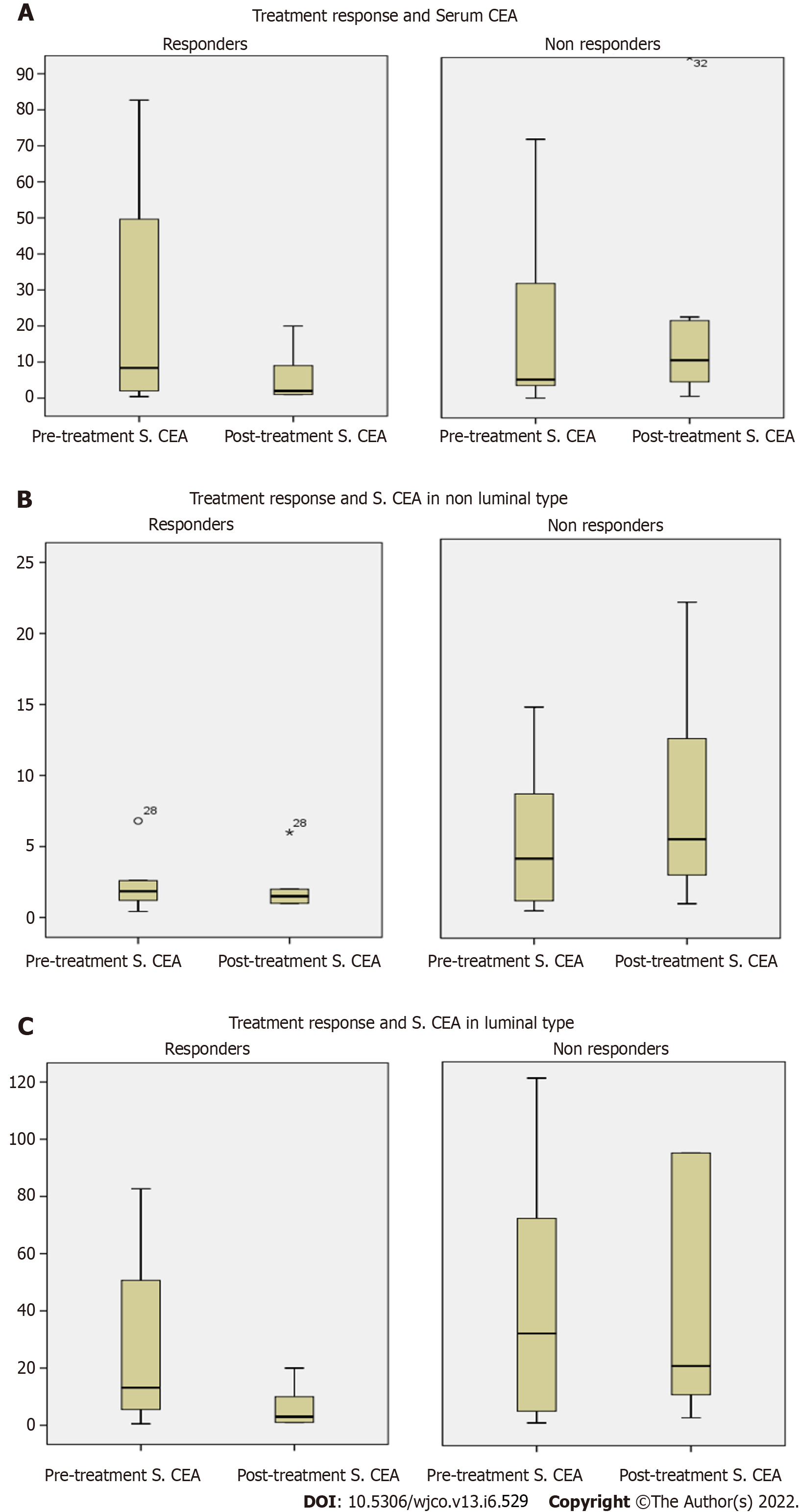
The median pre-treatment and post-treatment CEA levels were 7.9 (1.8-40.7) ng/mL and 4.39 (1.4-12.15) ng/mL, respectively, in the whole study population (P = 0.032). Serum CEA and response to treatment in responders and non-responders are given in Table 2. Among responders, median pre-treatment CEA was 8.87 (2-49.6) ng/mL and post-treatment CEA was 2.07 (1-8.7) ng/mL (P = 0.001). Among non-responders, median pre-treatment CEA was 5.4 (1.7-36.01) ng/mL and post-treatment CEA was 11 (4.65-22.5) ng/mL (P = 0.06). Since there was no statistically significant difference between responders and non-responders in baseline serum CEA, it cannot be taken as a predictor of response but post-treatment increase in CEA was associated with non-response or progression.
| Serum CEA | Responders | Non-responders | P value |
| Median pre-treatment serum CEA | 8.87 (2-49.6) | 5.4 (1.7-36.01) | 0.527 |
| Median post-treatment serum CEA | 2.07 (1-8.7) | 11 (4.65-22.5) | 0.002 |
| P value | 0.001 | 0.06 |
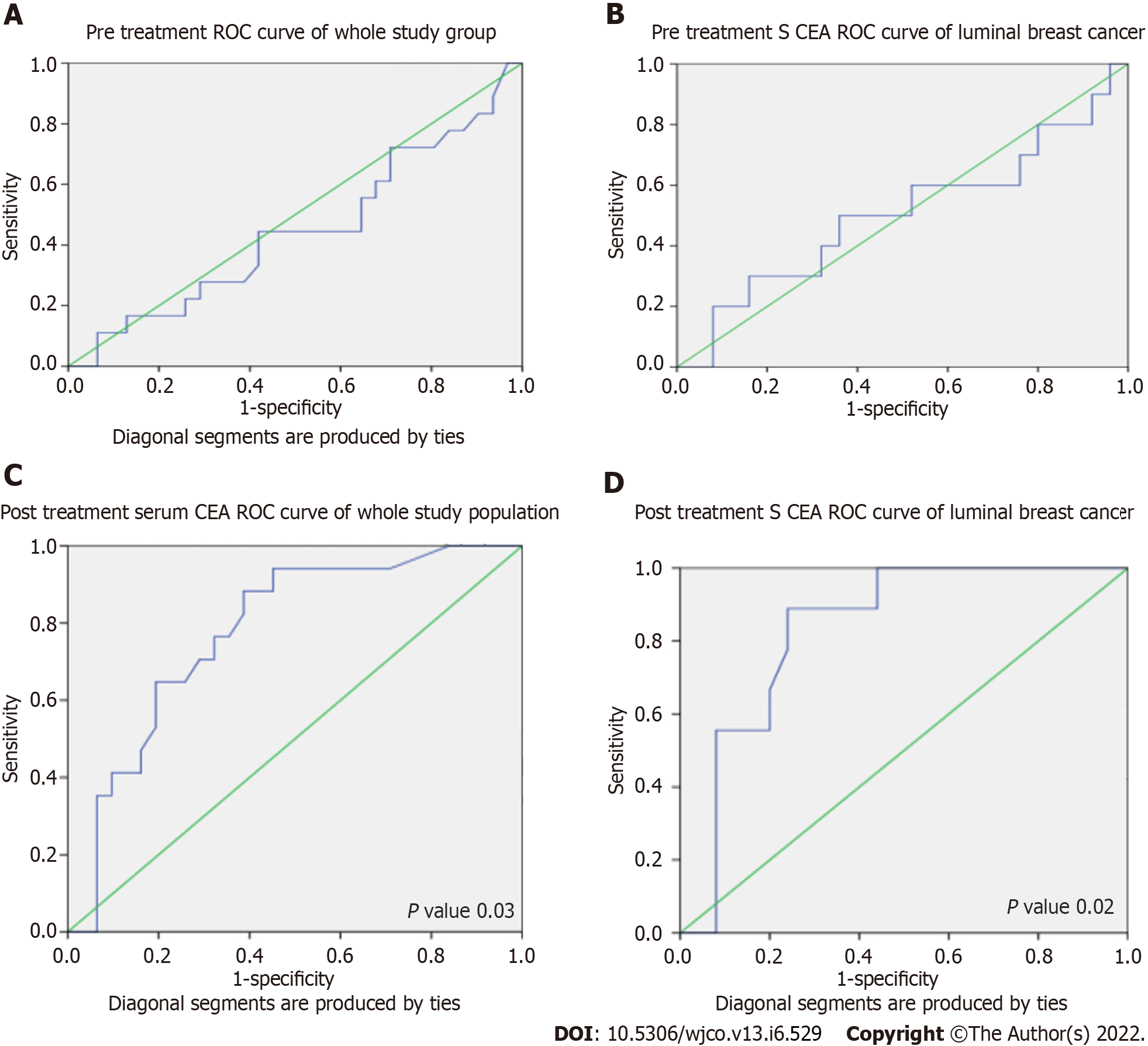
Pre-treatment and post-treatment ROC curves for the whole study population and luminal type breast cancer are given in Figure 1. We tried to find optimal pre-treatment cut-off for serum CEA in luminal breast cancer using ROC curve. The cut-off can be taken as 29.7 ng/mL as a predictor of tumour progression, with a sensitivity of 50% and specificity of 64%, but that cut-off was not statistically significant. ROC curve analysis for finding the cut-off for post-treatment CEA was also done. Post-treatment CEA for predicting the progression was taken as 2.16 ng/mL, with a sensitivity of 94.1% and specificity of 54.8%. For hormone positive tumours, post-treatment cut-off can be taken as 9.46 ng/mL with a sensitivity of 88.9% and specificity of 75.9% (P = 0.02). With a cut-off of 9.41, we found statistical significance in the whole group of patients (P = 0.006).
Table 3 shows serum CEA and response to treatment in responders and non-responders according to breast cancer type. Among responders, median pre-treatment CEA for luminal type was 14.7 (5.4-50.6) ng/mL and post-treatment CEA was 3.0 (1-10) ng/mL (P = 0. 001). Even in the luminal group, pre-treatment serum CEA was not a predictor of response, but post-treatment CEA was a significant predictor of tumour progression (Figure 2).
| Classification | Responders | Non-responders | |||||
| Median pre-CEA | Median post-CEA | P value | Median pre-CEA | Median post-CEA | P value | ||
| Hormonal classification | Luminal | 14.7 (5.4-50.6) | 3 (1-10) | 0.001 | 22.39 (3.9-84.4) | 21.00 (10.6-164.15) | 0.26 |
| Non-luminal | 1.85 (1-3.65) | 1.25 (0.5-3) | 0.046 | 4.15 (0.85-10.17) | 5.65 (2.65-12.05) | 0.161 | |
| Genomic classification | Luminal | 14.7 (5.4-50.6) | 3 (1-10) | 0.001 | 22.39 (3.9-84.47) | 20.67 (10.6-164.17) | 0.260 |
| HER2 | 4 (1.2-4) | 3.25 (0.5-3.25) | 0.18 | 11.7 | 13 | _ | |
| TNBC | 1.85 (0.74-2.4) | 1.25 (0.67-1.88) | 0.144 | 4 (0.5-5.6) | 5.3 (2.2-9.2) | 0.237 | |
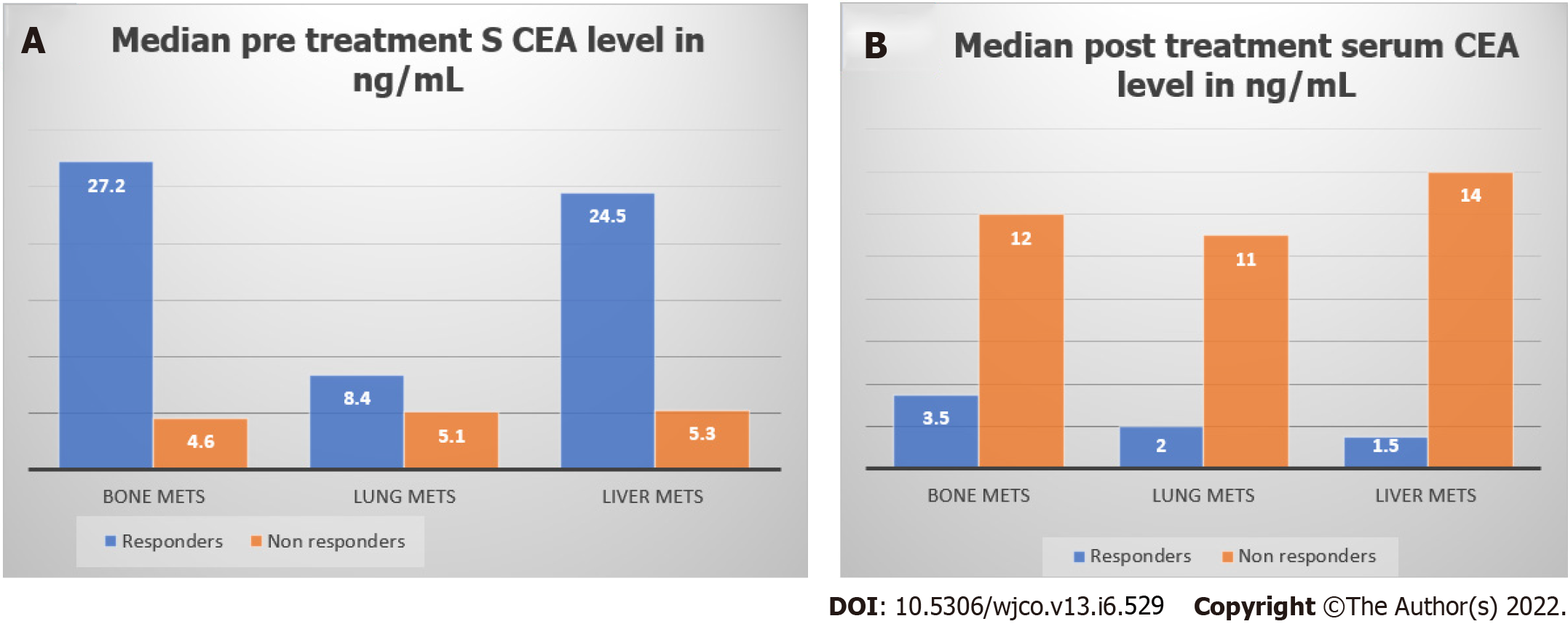
Figure 3 shows median pre-treatment and post-treatment serum CEA levels in responders and non-responders according to site of metastasis. Among responders, median pre-treatment serum CEA levels of patients with bone metastases, lung metastases, and liver metastases were 27.2 ng/mL, 8.4 ng/mL, and 24.5 ng/mL respectively. Among non-responders, median post-treatment serum CEA levels of patients with bone metastases, lung metastases, and liver metastases were 12 ng/mL, 11 ng/mL, and 14 ng/mL, respectively.
Table 4 shows serum CEA and response to treatment in bone, liver, and lung metastases. In patients with liver and lung metastases, post-treatment CEA level difference was not statistically significant in both responders and non-responders though the values were higher in non-responders.
| Serum CEA (ng/mL) | Bone metastases | No bone metastases | P value |
| Median pre-treatment serum CEA | 11.7 (2.9-48.4) | 6.8 (2-32.3) | 0.788 |
| Median post-treatment serum CEA | 9 (2-20) | 2 (1-9) | 0.063 |
| Liver metastases | No liver metastases | ||
| Median pre-treatment serum CEA | 11.7 (4.4-62.7) | 6.8 (1.9-22.7) | 0.244 |
| Median post-treatment serum CEA | 8 (1.2-19.75) | 3 (1.25-11.5) | 0.352 |
| Lung metastases | No lung metastases | ||
| Median pre-treatment serum CEA | 7.8 (1.9-31.3) | 9.78 (5.15-66.64) | 0.353 |
| Median post-treatment serum CEA | 3.5 (1-13.75) | 5 (1.5-10) | 0.93 |
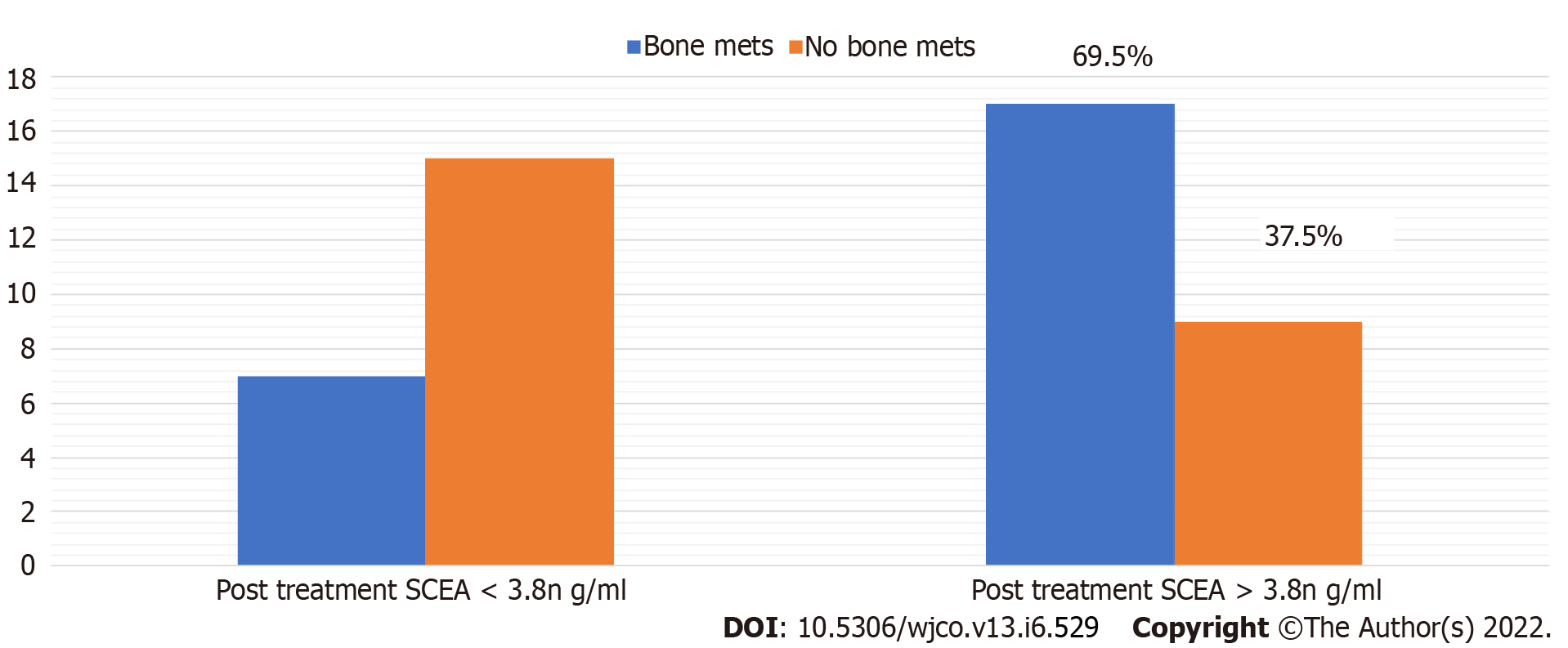
In non-responders, comparing patients with or without bone metastases, the median post-treatment serum CEA of patients with bone metastases was 12 ng/dL whereas median post-treatment serum CEA in those without bone metastases was 10 ng/mL; post-treatment CEA level difference was statistically significant (P = 0.063). Among those with bone metastases, 69.5% had elevated post-treatment serum CEA, and only 37.5% had elevated serum CEA in those with no bone metastases (Figure 4).
The measurement of serum tumour marker levels could provide useful information for earlier detection of recurrence or accurate prediction of outcomes after recurrence in various cancers. They are more useful when patients have elevated level at baseline. The commonly studied tumour markers in breast cancer are CA15-3 and CEA. The significance of these markers remains unclear[17,18]. Even though the prognostic value of CA15-3 in breast cancer had been documented in some studies, serum CEA is less widely investigated as a prognostic factor than CA15-3 because of its poor sensitivity and specificity[18,19]. Elevated serum levels of CA15-3 and CEA preoperatively were significantly associated with tumour size, axillary node metastasis, and advanced stage[20-23]. A recent meta-analysis investigated the prognostic value of these two markers (serum CA15-3 and CEA) in 12993 breast cancer patients and indicated that elevated CA15-3 level significantly corresponded with poor disease-free survival and OS of breast cancer[23].
In our study, no clinically meaningful significance was seen in factors like menstrual status, grade of the tumour, number and sites of metastases, presence or absence of metastases, HER2 status, and TNBC status except luminal type. This finding was consistent with a study by Geng et al[23]. Elevated CEA levels were significantly associated with breast cancer molecular subtypes and luminal subtypes exhibited a higher percentage of elevated CEA levels compared to non-luminal subtypes. The reason for this differential expression of CEA is that the expression patterns of luminal, HER2 positive, and basal-like tumours are closely associated with their maturation and differentiation. Luminal subtypes have high expression of hormone receptor related genes, whereas HER2 positive or basal-like tumours have low expression of hormone receptor related genes, which explains the association between CEA elevation and luminal subtype. Our study showed that pre-treatment serum CEA cannot be taken as a predictor of response even in luminal subtype but post-treatment CEA was a significant predictor of tumour progression. Hence, we can conclude that monitoring CEA levels in luminal MBC at the end of treatment is a significant predictor of treatment response.
The correlation between tumour marker levels and various metastatic sites in MBC is poorly defined[24,25]. A study by Yerushalmi et al[26] identified that tumour marker elevation was documented in the majority of patients with MBC and luminal subtypes expressed more frequently compared with the non-luminal groups[26]. CEA elevation was not different between different sites of metastasis. Whereas in our study, in patients with liver and lung metastases, post-treatment CEA level difference was not statistically significant in both responders and non-responders even though the values were higher in non-responders.
A study by Yazdani et al[27] showed that age, menopausal status, number of axillary lymph node metastases, tumor size, and ALP were identified as prognostic factors for bone metastasis in patients with breast cancer, whereas significantly persistent elevated post-treatment serum CEA levels were seen with bone metastases in our study[27]. Kosaka et al[28] proposed that in hormone receptor positive breast cancer, nodal metastasis and elevated serum CEA were associated with a poor prognosis and there was a significant rate of recurrence in those with high serum CEA levels compared with those with low levels of CEA[28]. Elevated serum levels of HER2, BCL2, CA15-3, and CEA in breast cancer patients are useful markers for predicting aggressive behaviour and relapse[29,30].
One major limitation of our study is the small sample size (50 patients) and it limits the predictive power of these markers and needs larger studies to confirm the findings.
Pretreatment serum CEA is elevated in luminal subtype. With treatment, responders have a significant fall in serum CEA level but it is clinically significant in luminal breast cancer type. Elevated post-treatment serum CEA levels are associated with disease progression and poor response to therapy. Persistently elevated post treatment serum CEA levels are associated with bone metastases. Elevated serum CEA and hormonal status are significant predictors of treatment response.
In breast cancer patients, elevated serum carcinoembryonic antigen (CEA) levels are particularly noted in metastatic and recurrent disease and its significance in clinical practice is doubtful.
We aimed to estimate the serum CEA level in our metastatic breast cancer patients and correlate it with response to treatment and clinical outcome.
To evaluate the efficacy of serum CEA levels as a prognostic marker in metastatic breast cancer patients.
This is a prospective clinical study of 50 patients with metastatic breast cancer treated at a breast clinic with newly diagnosed metastatic breast cancer planned for palliative chemotherapy, targeted therapy, and hormone therapy. We estimated the proportion of patients with elevated serum CEA levels at baseline and after palliative care, and investigated the association of serum CEA levels with known prognostic factors. Response to treatment was correlated with serum CEA levels in both responders and non-responders.
Pretreatment serum CEA was elevated in luminal subtype. With treatment, responders had a significant fall in serum CEA level but it was clinically significant in luminal breast cancer type. Metastatic breast cancer patients with bone metastases had significantly elevated post-treatment serum CEA levels after treatment.
Based on our results, we suggest that serum CEA has potential clinical value in monitoring the treatment response of metastatic breast cancer patients, especially in patients with bone metastasis.
Serum CEA as a tumour marker warrants further studies in metastatic breast cancer especially with bone metastases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liang Z, China; Wang J, China A-Editor: Liu X, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Qi WW
| 1. | Turner KM, Yeo SK, Holm TM, Shaughnessy E, Guan JL. Heterogeneity within molecular subtypes of breast cancer. Am J Physiol Cell Physiol. 2021;321:C343-C354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Ma W, Wang X, Xu G, Liu Z, Yin Z, Xu Y, Wu H, Baklaushev VP, Peltzer K, Sun H, Kharchenko NV, Qi L, Mao M, Li Y, Liu P, Chekhonin VP, Zhang C. Distant metastasis prediction via a multi-feature fusion model in breast cancer. Aging (Albany NY). 2020;12:18151-18162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Imamura M, Morimoto T, Nomura T, Michishita S, Nishimukai A, Higuchi T, Fujimoto Y, Miyagawa Y, Kira A, Murase K, Araki K, Takatsuka Y, Oh K, Masai Y, Akazawa K, Miyoshi Y. Independent prognostic impact of preoperative serum carcinoembryonic antigen and cancer antigen 15-3 Levels for early breast cancer subtypes. World J Surg Oncol. 2018;16:26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Hing JX, Mok CW, Tan PT, Sudhakar SS, Seah CM, Lee WP, Tan SM. Clinical utility of tumour marker velocity of cancer antigen 15-3 (CA 15-3) and carcinoembryonic antigen (CEA) in breast cancer surveillance. Breast. 2020;52:95-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Yang Y, Zhang H, Zhang M, Meng Q, Cai L, Zhang Q. Elevation of serum CEA and CA15-3 Levels during antitumor therapy predicts poor therapeutic response in advanced breast cancer patients. Oncol Lett. 2017;14:7549-7556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Khushk M, Khan A, Rehman A, Sheraz S, Tunio YM, Rehman K, Rehman D, Ahmed M, Abbas K, Khan ME. The Role of Tumor Markers: Carcinoembryonic Antigen and Cancer Antigen 15-3 in Patients With Breast Cancer. Cureus. 2021;13:e16298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Puglisi F, Fontanella C, Numico G, Sini V, Evangelista L, Monetti F, Gori S, Del Mastro L. Follow-up of patients with early breast cancer: is it time to rewrite the story? Crit Rev Oncol Hematol. 2014;91:130-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Di Gioia D, Stieber P, Schmidt GP, Nagel D, Heinemann V, Baur-Melnyk A. Early detection of metastatic disease in asymptomatic breast cancer patients with whole-body imaging and defined tumour marker increase. Br J Cancer. 2015;112:809-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Rasmy A, Abozeed W, Elsamany S, Baiomy ME, Nashwa A, Amrallah A, Hasaan E, Alzahrani A, Faris M, Alsaleh K, AlFaraj A. Correlation of Preoperative Ki67 and Serum CA15.3 Levels with Outcome in Early Breast Cancers a Multi Institutional Study. Asian Pac J Cancer Prev. 2016;17:3595-3600. [PubMed] [Cited in This Article: ] |
| 10. | Bhindi B, Leibovich BC. Re: Robotic vs Open Level I-II Inferior Vena Cava Thrombectomy: A Matched Group Comparative Analysis: L. Gu, X. Ma, Y. Gao, H. Li, X. Li, L. Chen, B. Wang, Y. Xie, Y. Fan and X. Zhang J Urol 2017;198:1241-1246. J Urol. 2018;199:1351-1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Stieber P, Nagel D, Blankenburg I, Heinemann V, Untch M, Bauerfeind I, Di Gioia D. Diagnostic efficacy of CA 15-3 and CEA in the early detection of metastatic breast cancer-A retrospective analysis of kinetics on 743 breast cancer patients. Clin Chim Acta. 2015;448:228-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Maccio G, Goussot V, Berriolo-Riedinger A, Riedinger JM. Clinical value of CEA for detection of distant metastases in newly diagnosed breast cancer: comparison with CA 15-3. Ann Biol Clin (Paris). 2017;75:431-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Araz M, Beypinar I, Kazan S, Inci F, Celiker M, Uysal M. Are preoperative serum CA15-3 Levels different in breast cancer subgroups? Curr Probl Cancer. 2019;43:115-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Li J, Liu L, Feng Z, Wang X, Huang Y, Dai H, Zhang L, Song F, Wang D, Zhang P, Ma B, Li H, Zheng H, Chen K. Tumor markers CA15-3, CA125, CEA and breast cancer survival by molecular subtype: a cohort study. Breast Cancer. 2020;27:621-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 15. | Bidard FC, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, Cottu P, Beuzeboc P, Rolland E, Mathiot C, Pierga JY. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res. 2012;14:R29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Nam SE, Lim W, Jeong J, Lee S, Choi J, Park H, Jung YS, Jung SP, Bae SY. The prognostic significance of preoperative tumor marker (CEA, CA15-3) elevation in breast cancer patients: data from the Korean Breast Cancer Society Registry. Breast Cancer Res Treat. 2019;177:669-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Falzarano R, Viggiani V, Michienzi S, Longo F, Tudini S, Frati L, Anastasi E. Evaluation of a CLEIA automated assay system for the detection of a panel of tumor markers. Tumour Biol. 2013;34:3093-3100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Guadagni F, Ferroni P, Carlini S, Mariotti S, Spila A, Aloe S, D'Alessandro R, Carone MD, Cicchetti A, Ricciotti A, Venturo I, Perri P, Di Filippo F, Cognetti F, Botti C, Roselli M. A re-evaluation of carcinoembryonic antigen (CEA) as a serum marker for breast cancer: a prospective longitudinal study. Clin Cancer Res. 2001;7:2357-2362. [PubMed] [Cited in This Article: ] |
| 19. | Wang W, Xu X, Tian B, Wang Y, Du L, Sun T, Shi Y, Zhao X, Jing J. The diagnostic value of serum tumor markers CEA, CA19-9, CA125, CA15-3, and TPS in metastatic breast cancer. Clin Chim Acta. 2017;470:51-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 20. | Albuquerque KV, Price MR, Badley RA, Jonrup I, Pearson D, Blamey RW, Robertson JF. Pre-treatment serum levels of tumour markers in metastatic breast cancer: a prospective assessment of their role in predicting response to therapy and survival. Eur J Surg Oncol. 1995;21:504-509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Lee JS, Park S, Park JM, Cho JH, Kim SI, Park BW. Elevated levels of preoperative CA 15-3 and CEA serum levels have independently poor prognostic significance in breast cancer. Ann Oncol. 2013;24:1225-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Shao Y, Sun X, He Y, Liu C, Liu H. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS One. 2015;10:e0133830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Geng B, Liang MM, Ye XB, Zhao WY. Association of CA 15-3 and CEA with clinicopathological parameters in patients with metastatic breast cancer. Mol Clin Oncol. 2015;3:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Li X, Dai D, Chen B, Tang H, Xie X, Wei W. Clinicopathological and Prognostic Significance of Cancer Antigen 15-3 and Carcinoembryonic Antigen in Breast Cancer: A Meta-Analysis including 12,993 Patients. Dis Markers. 2018;2018:9863092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Zhang J, Wei Q, Dong D, Ren L. The role of TPS, CA125, CA15-3 and CEA in prediction of distant metastasis of breast cancer. Clin Chim Acta. 2021;523:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Yerushalmi R, Tyldesley S, Kennecke H, Speers C, Woods R, Knight B, Gelmon KA. Tumor markers in metastatic breast cancer subtypes: frequency of elevation and correlation with outcome. Ann Oncol. 2012;23:338-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Yazdani A, Dorri S, Atashi A, Shirafkan H, Zabolinezhad H. Bone Metastasis Prognostic Factors in Breast Cancer. Breast Cancer (Auckl). 2019;13:1178223419830978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Kosaka Y, Minatani N, Tanaka Y, Shida A, Kikuchi M, Nishimiya H, Waraya M, Katoh H, Sato T, Sengoku N, Tanino H, Yamashita K, Watanabe M. Lymph node metastasis and high serum CEA are important prognostic factors in hormone receptor positive and HER2 negative breast cancer. Mol Clin Oncol. 2018;9:566-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Samy N, Ragab HM, El Maksoud NA, Shaalan M. Prognostic significance of serum Her2/neu, BCL2, CA15-3 and CEA in breast cancer patients: a short follow-up. Cancer Biomark. 2010;6:63-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Oloomi M, Moazzezy N, Bouzari S. Comparing blood vs tissue-based biomarkers expression in breast cancer patients. Heliyon. 2020;6:e03728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |