Published online Jul 24, 2019. doi: 10.5306/wjco.v10.i7.247
Peer-review started: March 4, 2019
First decision: April 11, 2019
Revised: July 8, 2019
Accepted: July 16, 2019
Article in press: July 17, 2019
Published online: July 24, 2019
Checkpoint-Inhibition has revolutionized the treatment for several entities such as melanoma and renal cell carcinoma. The first encouraging experience in ovarian cancer was reported for nivolumab, a fully humanized anti-programmed death-1 antibody. Pseudoprogression is a new phenomenon associated with these novel immuno-oncologic agents. It can be explained by infiltrating leucocytes and edema that result in a temporary increase in tumor size and delayed subsequent shrinkage due to tumor cell destruction.
We report on a 47-year old patient with platinum-resistant ovarian cancer that was treated off-label with nivolumab 3mg/kg iv d1q14d. She first experienced classic pseudoprogression with inguinal lymph node swelling after cycle two and subsequent shrinkage. After 6 cycles she presented with rectal bleeding and progressive disease was diagnosed due to new tumor infiltration into the rectum.
Clinicians should be aware of pseudoprogression, its underlying mechanisms and strategies to discriminate pseudo- from real progression in ovarian cancer.
Core tip: Clinicians have to be aware of the phenomenon of pseudoprogression despite its rather rare occurrence. As both- pseudo-progression and real progression present with an increase in tumor size, the only certain way to differentiate between them is the occurrence of infiltrating growth. While the increase of tumor size in pseudoprogression can be explained by benign growth due to immune cell infiltration and edema, only malign growth of a real progression has the ability to infiltrate other tissues. When in doubt whether a pseudoprogression has occurred, we suggest cautious continuation of checkpoint-inhibition paired with corticoids to lower adverse effects if necessary.
- Citation: Passler M, Taube ET, Sehouli J, Pietzner K. Pseudo- or real progression? An ovarian cancer patient under nivolumab: A case report. World J Clin Oncol 2019; 10(7): 247-255
- URL: https://www.wjgnet.com/2218-4333/full/v10/i7/247.htm
- DOI: https://dx.doi.org/10.5306/wjco.v10.i7.247
Cancer has different techniques to evade the immune system, one of those being Programmed death-1 (PD-1) signaling. PD-1 plays an important role in antitumor immunity as it is a vital part of a set of activating and inhibitory T cell receptors called “the immune checkpoint”. By binding to its ligand PD-L1, which is expressed on the tumor cell, PD-1 inhibits antigen-specific cancer immune reactions and aggravates progression of ovarian cancer by inducing host immuno-suppression[1,2]. If PD-1 and PD-L1 bind, T cell proliferation and cytokine secretion are inhibited. The regulatory T-cells (Treg) increase and so called self-tolerance is maintained[3,4].
Nivolumab is a fully- humanized immunoglobulin G4, which targets PD-1. It prevents PD-1 from binding with its ligands and blocks signaling[2]. Thus, it increases the antitumor activity of T cells[5].
Checkpoint inhibitors have shown impressive results in the treatment of melanoma and non-small-cell-lung cancer and therefore, they have become the gold standard in the management of these entities[6]. Up to this point, only sparse data exist for checkpoint-inhibition in ovarian cancer. The first experience with nivolumab in ovarian cancer patients was reported by Hamanishi and colleagues. Nivolumab as a monotherapy was proven to be active in ovarian cancer- contrary to all other approaches of immune therapies like interleukines, vaccination or dendritic cell therapy. Acknowledging these positive results, it is important to mention that these first results on the efficacy of nivolumab in ovarian cancer are not as ground-breaking as in other entities[7]. Despite these first encouraging results, the rather low response rates of 15%-25% suggest that further effort is needed to increase efficacy of this novel substance in ovarian cancer. Strategies to improve efficacy could include patient selection, combination with chemotherapy and treatment at an earlier timepoint in the management (e.g., early platinum-sensible situation).
Pietzner et al[8] hypothesize that identifying specific patients with an immunogenic profile like BRCA mutation might lead to a better outcome. The BRCA mutation results in a DNA repair deficiency, mainly because a repair mechanism called “Homologous Recombination” is impaired, which leads to a higher mutational load. The higher the mutational load of the cancer - which includes a higher presentation of neoantigens and an overall immunogenetic profile - the higher the likelihood of success using a checkpoint inhibition therapy (CIT)[8-10].
This hypothesis is supported by data from patients with Lynch-Syndrome in colorectal cancer, which is a mismatch repair deficiency comparable to the BRCA mutation. Le et al[10] showed a strong correlation between mismatch repair deficiency and positive results under CIT.
Up until the introduction of CIT, the evaluation of therapy response followed a simple rule: If a new lesion is detected or the tumor growth increases, this process is classified as progression, and clinicians are used to stop the ongoing treatment (chemotherapy or targeted therapy) as it seems to be inefficient. This rule does not apply to the novel substance group of checkpoint inhibitors, because of a phen-omenon known as pseudoprogression.
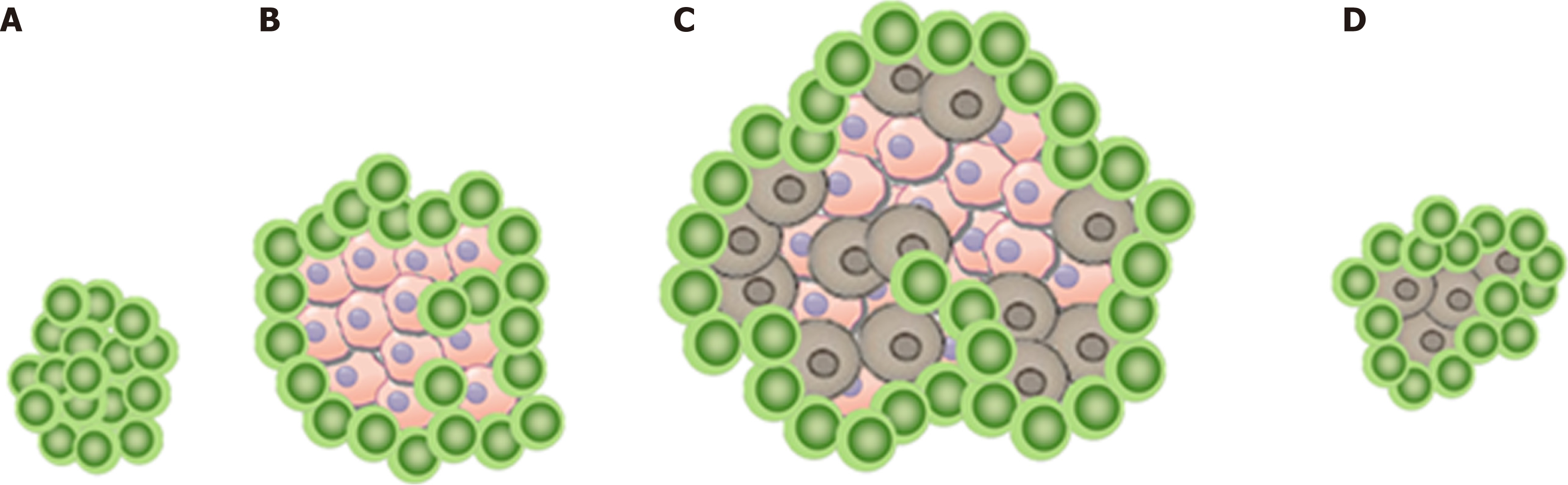
In this scenario, the increase in tumor size or the appearance of a new lesion is not related to tumor cell growth as shown in Figure 1A. Instead, it can be explained by the infiltration of immune cells into a preexisting tumor cell conglomerate as well as the consecutive edema as a response to the immune reaction[11]. Therefore, pseudo-progression initially appears like a classic progression, with subsequent decrease in size without additional treatment[6,12].
The number of patients with solid tumors undergoing immune checkpoint inhibitor therapy is rapidly growing, while pseudoprogression remains a challenge for the clinician. The estimated occurrence of pseudoprogression ranges from 1.5% to 17% depending on the tumor entity and the study[6,13].
Hodi et al[14] conducted a study on advanced melanoma patients treated with pembrolizumab, another PDL-1 inhibitor. They were able to show pseudoprogression in 7% of the patients and found that pseudoprogression has a tendency to occur relatively early - mostly within 12 wk of treatment (62.5%, 15/24 patients), whereas pseudoprogression later than 12 wk after the beginning of PDL-1 inhibitor treatment - so called delayed pseudoprogression – was only found in 37.5% (9/24 patients)[14]. In one remarkable case early and delayed pseudoprogression could be described in one patient[15].
Under Ipilimumab, another checkpoint inhibitor, 9.7% of pseudoprogression could be found[12].
Several older manuscripts report on patients who responded to CIT after progressive disease was diagnosed: After an initial progression, they benefited from the continued CIT. As these reports were published before the introduction of the immune-related Response Evaluation Criteria in Solid Tumors (ir-RECIST) criteria, we believe that those cases report on the phenomenon we now define to be pseudoprogression, proving that this phenomenon has challenged physicians for a long time. The mechanisms behind CIT are complex and dependent on the patients’ individual immunological answer, therefore the kinetics of CIT seem to be variable[16,17].
Nevertheless, it is crucial to be informed about pseudoprogression as indicates a high likelihood of > 1 year survival[18].
When considering different treatment options, practitioners and patients need to be informed about the possible occurrence of a pseudoprogression imitating real progression[6].
We present a platinum-resistant ovarian cancer patient, treated with nivolumab, who experienced both: A pseudoprogression and a real progression. We feel that this rare occurrence of both response patterns in the same patient makes this case ideal to illustrate both phenomenona and the difficulties to differentiate them.
We report on a 47-year-old-patient with recurrent ovarian cancer. She presented to the Emergency Department of our hospital with a swollen lymph node in her left groin.
Nivolumab was administered at a dosage of 3.0 mg/kg iv every three weeks for four cycles, starting December 2015 based on results from the Hamanishi et al[7] study. She responded with rash and pruritus to the first cycle of nivolumab which lessened under local corticoid-therapy. After the second cycle, the patient presented with typical inflammatory signs in her left groin: swelling, heat, redness and pain of an inguinal lymph node.
The patient was first diagnosed with high grade adenocarcinoma stage pT2b, G3, pN0(0/29), R0, at a different institution in February 2010. She underwent radical cytoreductive surgery with hysterectomy, bilateral salpingoovarectomy, pelvine and paraaortal lymphadenectomy, omentectomy and deperitonealisation. She was treated with adjuvant chemotherapy consisting of six cycles of Carboplatin and Paclitaxel.
In June 2011, the disease relapsed for the first time and the patient was referred to our institution. Over the next four years, the patient was treated with Carbo-platin/Gemcitabine as second line, pegylated liposomal Doxorubicin biweekly as third line, Carboplatin/Topotecan (Phase III “HECTOR” study) as fourth line and Paclitaxel/Bevacizumab as fifth line.
She experienced another relapse with intraperitoneal (rectum, bladder, etc.) and extraperitoneal manifestation (brain).
The tumor conference suggested the off-label-use of nivolumab in October 2015. The patients’ health care provider granted permission for the off-label-use because of the limited options in this platinum-resistant situation and the good general health of the patient.
The patient is married and lives with her husband and two children. Molecular analysis revealed BRCA-1 mutation (p. His 1707 Arg).
In the physical examination we saw a cardiopulmonary stable patient with a swollen lymph node in the left groin. In this location, a known lymph node metastasis was located, but the lymph-node nearly doubled in size initially suggesting classic progression.
The laboratory examinations were unremarkable, including a stable tumor marker CA125.
No imaging examinations were done.
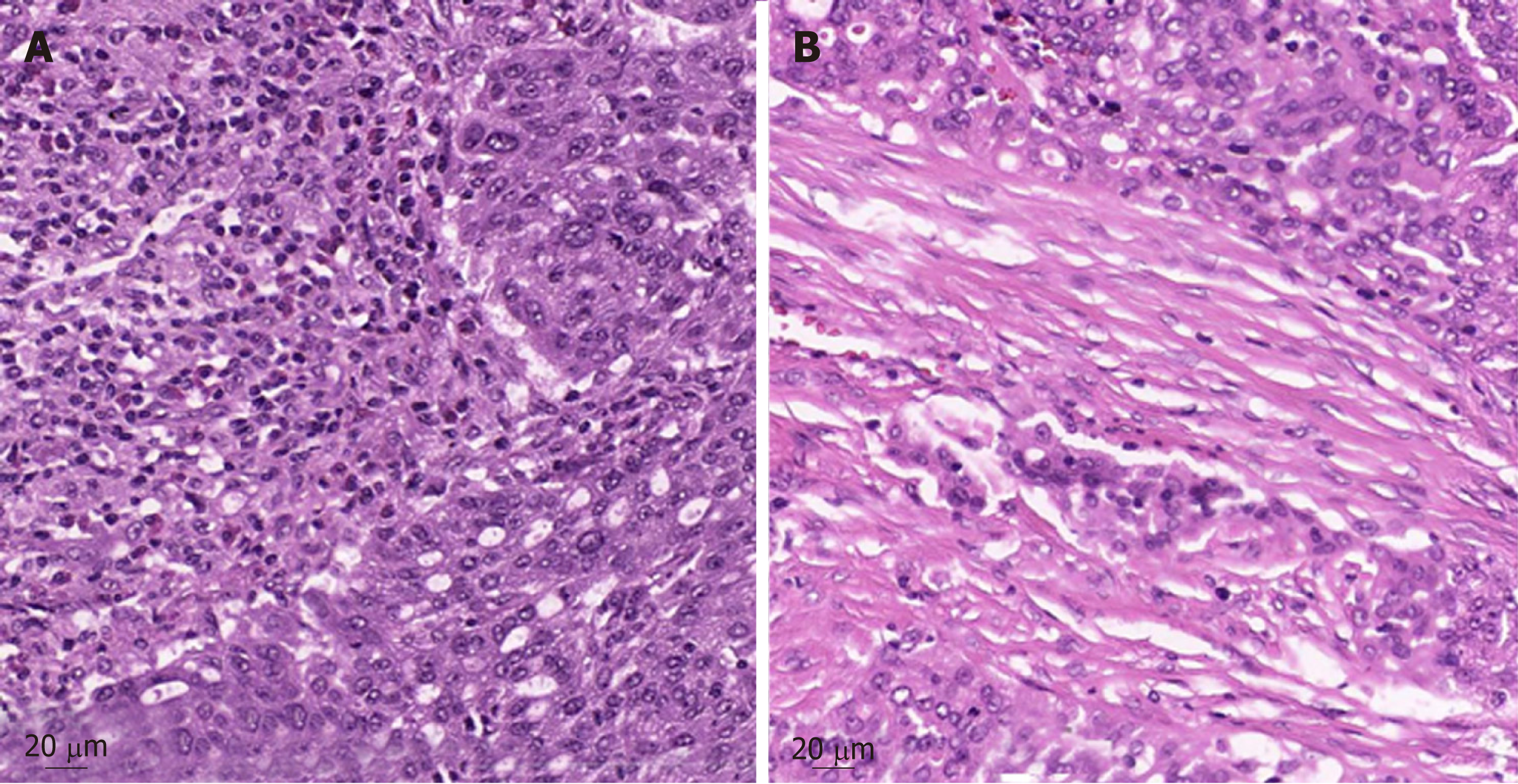
Further pathologic characteristics are shown in Figure 2 and 3. Figure 3 shows the histomorphology of the patients’ high-grade serous ovarian carcinoma pretreatment with solid growth pattern and pleomorphism of the tumor cells as well as frequent mitotic activity. An interesting factor are the increased tumor infiltrating lymphocytes (TILS) which have been shown to be associated with better prognosis[19]. In the biopsy after treatment the tumor still shows general features of a high-grade serous carcinoma, while TILS seem to be slightly reduced.
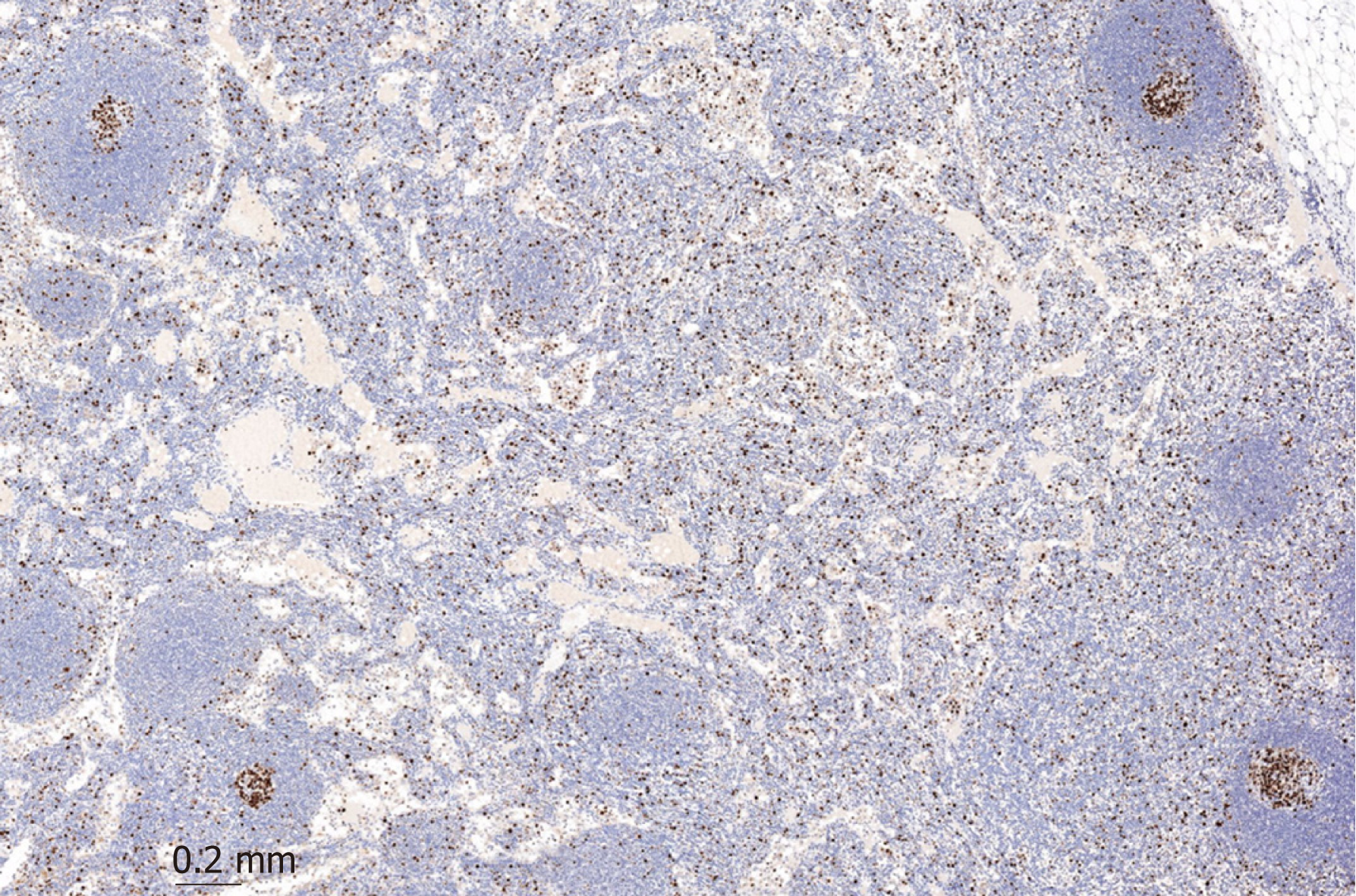
After the patient experienced progressive disease, lymph nodes were extracted. The mantle zone of the follicle can easily be distinguished from the increased Ki-67 positive interfollicular population, which indicates unspecific activation of the lymph node as seen in Figure 4.
This activation of the follicle combined with edema and dilated vessels are most likely caused by the nivolumab treatment.
A known lymph node metastasis was located in the patients’ left groin, but the lymph-node nearly doubled in size initially suggesting classic progression. But the lack of evidence for additional progression, the local inflammatory signs and the stable tumor marker CA125 made a pseudoprogression the most likely diagnosis.
Because the RECIST do not provide a complete assessment of immune-therapeutic agents, ir-RECIST were defined by Wolchok et al[12]. In this adapted recommendation, the increase in tumor size or even the appearance of a new lesion, does not automatically translate to the classification as progressive disease. While taking the potential toxicity of the treatment into consideration, continuation with the immune related therapy while persistently performing follow-up examinations to ensure the patients’ safety is recommended in ir-RECIST[12]. The recommendation on the frequency of follow-up exams is four weeks, but if a rapid decline of the patients’ status is observed, an earlier follow up is necessary.
Therefore, we proceeded with nivolumab treatment and the lymph node decreased in size. The shrinkage was interpreted as confirmation of pseudoprogression.
Three weeks after the fourth cycle of nivolumab, she presented with rectal bleeding. A cysto-rectoscopy was performed, which demonstrated new tumor infiltration into the rectum. A biopsy was taken and the pathological analysis verified new relapse with infiltration into the rectum. Three days after the cysto-rectoscopy, an operation using laparotomy by longitudinal incision was performed without any complications in order to remove the tumor. The histological findings of the biopsy of the bladder showed necrosis and atypical cells. A colostomy was done during the same procedure.
Nivolumab has been shown to be active in ovarian cancer, but the possibility of pseudoprogression imitating real progression remains[11]. This case report highlights the possibility of pseudoprogression in ovarian cancer patients undergoing nivolumab treatment and shows the challenges differentiating between pseudoprogression and real progression.
Immune-related-RECIST (ir-RC) were defined by Wolchok et al[12] but is important to notice that the recommended follow-up after four weeks is not evidence based and it remains unclear if another frequency of the follow-up-examinations is more beneficial.
Pseudoprogression emerges to be a challenge not only for the attending physician, but also for the radiologist: Wang et al[20] describe the two main differences between the ir-RC and the RECIST system: On the one hand, new lesions need to be interpreted taking into consideration the total tumor burden. On the other hand, this increase in total tumor burden has to be controlled and confirmed at least four weeks after the first event indicating possible progression[20].
If pseudoprogression is not as unambiguous as in our case, ultimately only follow-up imaging can help differentiate between pseudo- and real progression as shown in Table 1[21].
| Progression | New measurable lesion | New non measurable lesion | |
| RECIST 1.1 | Increase in tumor burden on one examination | Represent progressive disease | Follow-up necessary |
| Ir- RECIST | Increase in tumor burden on two examinations > 4 wk apart | Are incorporated into tumor burden | Preclude complete response |
Imafuku et al[22] report on two cases of melanoma patients treated with nivolumab who experienced pseudoprogression. They performed sonographic imaging and computed tomography (CT) scans on both patients and found that the CT scans - in contrary to the sonographic imaging of the pseudoprogression - were not able to detect an association between tumor size and tumor blood flow. Interestingly, they describe that the lesions caused by pseudoprogression grew while simultaneously the blood flow within the lesion dropped. They therefore believe that sonographic imaging could be helpful in differentiating between pseudo- and real progression, which is intriguing because CT-imaging - especially if it has to be performed several times as the ir-RECIST requests - puts the oftentimes heavily pretreated patients at risk[22].
The patient discussed in this report is BRCA 1 positive. This mutation possibly results in a high mutational load linked to higher treatment success similar to patients with Lynch Syndrome[10]. We hypothesize that a higher immunogenic profile not only leads to higher rates of treatment success but subsequently also results in higher rates of pseudoprogression. Further investigation on patient selection, especially BRCA mutation and its underlying mechanism is crucial to fully understand CIT and pseudoprogression.
Apart from pseudoprogression, another new phenomenon was noticed with the introduction of immunooncologic agents: It is notable that the progression free survival (PFS) under CIT is oftentimes not significantly lengthened: The patients relapse after a similar time compared to those who did not receive CIT, but surprisingly the overall survival (OS) of CIT patients is often prolonged. This is remarkable as the majority of other agents prolong the PFS while the OS remains unchanged.
The better OS in CIT patients indicates that the number of unreported cases of pseudoprogression might be a lot higher than the 4% suggested by Chiou et al[6]. The prolonged OS could possibly be explained by patients, who had a pseudoprogression that was wrongly diagnosed as a real progression. It could be hypothesized, that even though these patients received a shortened CIT treatment, they profited from it, which resulted in a benefit of the OS.
Tanizaki et al[23] show another interesting aspect of the durable immune reaction after CIT: They report on a Non-small-call-lung-cancer patient, whose histological evaluation of a liver metastasis showed no viable tumor cells but fibrotic tissue infiltrated by CD 3, 4 and 8 positive lymphocytes.
Tumor markers can be used as an additional source of information to differentiate between real and pseudoprogression, but - as any inflammatory process can lead to a rise of the tumor marker - a moderate increase of the tumor marker occurs in both pseudo and real progression. A rapid increase of tumor markers suggests a real progression as the more likely diagnosis. But further predictive makers for the response to immune-checkpoint inhibitors are needed.
Interestingly, interleukine-8 levels (IL-8) were shown to decrease during pseudoprogression and increase during progression. Although this was only shown in three cases so far, IL-8 monitoring might be a promising and helpful tool in the future to differentiate between pseudo- and real progression[24].
From the pathologic point of view, the overall number of TILS could be evaluated to that aspect.
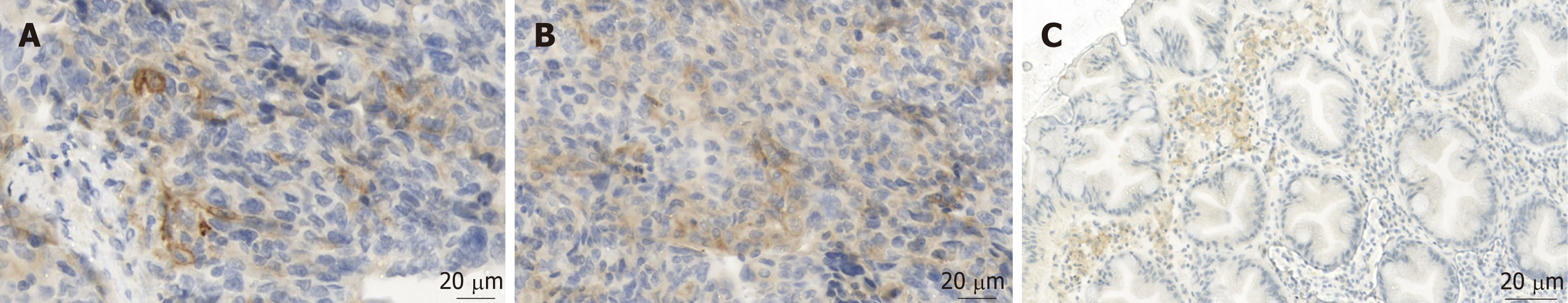
Pathologic evaluation of PD-L1 remains very challenging. PD-L1 is a positive prognostic marker in ovarian carcinoma, while the predictive value for therapy response still remains doubtful[19]. Therefore, the pathologic report usually includes the percentage of PD-L1 positive tumor cells, defined as cells with a strong membranous staining of PD-L1 (Figure 2A). Cytoplasmic staining of tumor cells is considered artificial (Figure 2B). A special problem consists in tumor infiltrating immune cells (TILS). Immune cells often express some PD-L1 - either membranous or cytoplasmic (Figure 2C) - and can easily be confused with tumor cells if being intermingled as TILS.
Our patient showed positive TILS in the pretreatment biopsy.
Considering these strict criteria, our patient was negative for PD-L1 expression before treatment with nivolumab (biopsy from 2012) and showed some 3% of PD-L1 positive tumor cells in the re-biopsy (2016) after treatment. Therefore, the absence of sufficient PD-L1 expression in the pre-treatment biopsy was not predictive for a negative therapy effect. Surprisingly, the tumor cells showed a positive rate of 3% after the treatment.
Although nivolumab inhibits immune checkpoints (especially PD-L1), a pretreatment evaluation is not yet required for treatment. Unfortunately, no pretreatment biomarker has been found, but it is likely that it will be necessary to also take into consideration factors like tumor genomic studies of mutational load and studies of T-cell receptors[25]. Further research is necessary to include pathologic findings as reliable markers for predictive therapeutic effects.
CIT imposes many opportunities on oncologic treatment, but also challenges our current understanding of cancer: The occurrence of pseudoprogression shows that we have to think outside the box in order to use CIT to its full potential: For decades, our understanding of cancer treatment was mainly based on data from cytotoxic agents and our definitions and statistical analysis are based on this knowledge. However, as it was necessary to introduce the ir-RECIST criteria in order to meet the novel requirements of CIT, it will likewise be necessary to adapt our statistical analysis. Possible methods to better incorporate pseudoprogression and additional new phenomena into statistics might include time-specific endpoints, immune-related endpoints, restricted mean survival time or generalized pairwise comparison[26].
As both- pseudoprogression and real progression- present with an increase in tumor size, the only certain way to differentiate between them is the occurrence of infiltrating growth. While the increase of tumor size in pseudoprogression can be explained by benign growth due to immune cell infiltration and edema, only malign growth of a real progression has the ability to infiltrate other tissues. This way to differentiate between pseudoprogression and real progression is vividly illustrated in our case report. A limitation of our case report is the lack of imaging of the left groin after pseudoprogression.
Although checkpoint inhibitor therapy is one of the most promising anti-tumor treatments yet, many questions remain unanswered: How long does the stabilizing effect after pseudoprogression last? Is pseudoprogression a predictor for progression or remission? Which symptoms are associated with pseudoprogression?
Further studies are necessary to fully characterize pseudoprogression not only translationally but also clinically and to understand its symptoms and clinical outcome.
This case illustrates not only pseudo-, but also real progression and vividly shows the main difference between the two: Only real progression has the ability to infiltrate other tissues. While the appearance of new lesions as well as the increase in size of a known lesion can be due to pseudoprogression, the new manifestation of infiltrative disease (such as the rectum infiltration in our case) is bound to be caused by real progression.
Risk factors for pseudoprogression and guidelines to diagnose pseudoprogression have yet to be investigated to ensure both the physician and the patient of the safety and efficacy of checkpoint-inhibition.
When in doubt whether a pseudoprogression has occurred, we suggest cautious continuation of checkpoint-inhibition paired with corticoids to lower adverse effects if necessary. Increased investigation of this phenomenon is crucial to improve the management of checkpoint-inhibitors such as nivolumab.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Karatza AA S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, Kosaka K, Konishi I. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 2. | Mittica G, Genta S, Aglietta M, Valabrega G. Immune Checkpoint Inhibitors: A New Opportunity in the Treatment of Ovarian Cancer? Int J Mol Sci. 2016;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 3. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3572] [Cited by in F6Publishing: 3735] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 4. | Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1346] [Cited by in F6Publishing: 1511] [Article Influence: 100.7] [Reference Citation Analysis (0)] |
| 5. | Taneja SS. Re: Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Urol. 2012;188:2149-2150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol. 2015;33:3541-3543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 622] [Article Influence: 69.1] [Reference Citation Analysis (0)] |
| 7. | Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. J Clin Oncol. 2015;33:4015-4022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 717] [Cited by in F6Publishing: 818] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 8. | Pietzner K, Nasser S, Alavi S, Darb-Esfahani S, Passler M, Muallem MZ, Sehouli J. Checkpoint-inhibition in ovarian cancer: rising star or just a dream? J Gynecol Oncol. 2018;29:e93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5919] [Cited by in F6Publishing: 5862] [Article Influence: 651.3] [Reference Citation Analysis (0)] |
| 10. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6096] [Cited by in F6Publishing: 6542] [Article Influence: 726.9] [Reference Citation Analysis (0)] |
| 11. | Patel AB, Pacha O. Skin Reactions to Immune Checkpoint Inhibitors. Adv Exp Med Biol. 2017;995:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbé C, Maio M, Binder M, Bohnsack O, Nichol G, Humphrey R, Hodi FS. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412-7420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2311] [Cited by in F6Publishing: 2361] [Article Influence: 157.4] [Reference Citation Analysis (0)] |
| 13. | Soria F, Beleni AI, D'Andrea D, Resch I, Gust KM, Gontero P, Shariat SF. Pseudoprogression and hyperprogression during immune checkpoint inhibitor therapy for urothelial and kidney cancer. World J Urol. 2018;36:1703-1709. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, Patnaik A, Ribas A, Robert C, Gangadhar TC, Joshua AM, Hersey P, Dronca R, Joseph R, Hille D, Xue D, Li XN, Kang SP, Ebbinghaus S, Perrone A, Wolchok JD. Evaluation of Immune-Related Response Criteria and RECIST v1.1 in Patients With Advanced Melanoma Treated With Pembrolizumab. J Clin Oncol. 2016;34:1510-1517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 15. | Ozaki Y, Shindoh J, Miura Y, Nakajima H, Oki R, Uchiyama M, Masuda J, Kinowaki K, Kondoh C, Tanabe Y, Tanaka T, Haruta S, Ueno M, Kitano S, Fujii T, Udagawa H, Takano T. Serial pseudoprogression of metastatic malignant melanoma in a patient treated with nivolumab: a case report. BMC Cancer. 2017;17:778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, Morton KE, Mavroukakis SA, Duray PH, Steinberg SM, Allison JP, Davis TA, Rosenberg SA. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372-8377. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1225] [Cited by in F6Publishing: 1166] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 17. | Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PubMed] [Cited in This Article: ] |
| 18. | Vikram K, Sullivan RJ, Gainor JF, Hodi FS, Gandhi L, Sadow CA, Harris GJ, Flaherty K, Lee S. Pseudoprogression in cancer immunotherapy: Rates, time course and patient outcomes. J Clin Oncol. 2016;34:6580–6580. [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, Braicu I, Jöhrens K. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7:1486-1499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 20. | Wang GX, Guo LQ, Gainor JF, Fintelmann FJ. Immune Checkpoint Inhibitors in Lung Cancer: Imaging Considerations. AJR Am J Roentgenol. 2017;209:567-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Wang GX, Kurra V, Gainor JF, Sullivan RJ, Flaherty KT, Lee SI, Fintelmann FJ. Immune Checkpoint Inhibitor Cancer Therapy: Spectrum of Imaging Findings. Radiographics. 2017;37:2132-2144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Imafuku K, Hata H, Kitamura S, Yanagi T, Shimizu H. Ultrasonographic findings can identify 'pseudoprogression' under nivolumab therapy. Br J Dermatol. 2017;177:1726-1731. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Tanizaki J, Hayashi H, Kimura M, Tanaka K, Takeda M, Shimizu S, Ito A, Nakagawa K. Report of two cases of pseudoprogression in patients with non-small cell lung cancer treated with nivolumab-including histological analysis of one case after tumor regression. Lung Cancer. 2016;102:44-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro C, Martín-Algarra S, Andueza MP, Gurpide A, Morgado M, Wang J, Bacchiocchi A, Halaban R, Kluger H, Chen L, Sznol M, Melero I. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28:1988-1995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 287] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 25. | Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139-2145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 1039] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 26. | Huang B. Some statistical considerations in the clinical development of cancer immunotherapies. Pharm Stat. 2018;17:49-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |