Copyright
©2014 Baishideng Publishing Group Co.
World J Clin Oncol. May 10, 2014; 5(2): 103-113
Published online May 10, 2014. doi: 10.5306/wjco.v5.i2.103
Published online May 10, 2014. doi: 10.5306/wjco.v5.i2.103
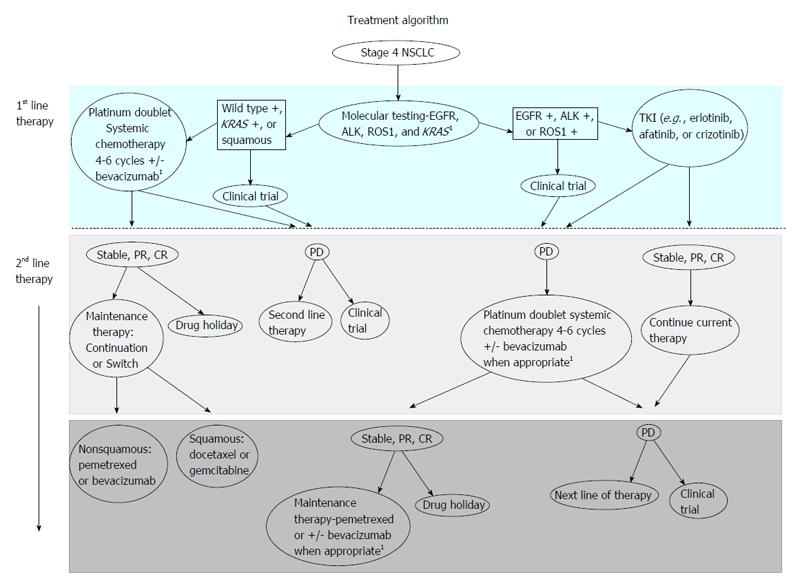
Figure 1 Treatment algorithm.
Stage 4 nonsquamous non-small cell lung cancer (NSCLC) should have their tumors analyzed for epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma viral oncogene homolog (KRAS) and ROS1 in a Clinical Laboratory Improvement Amendments-certified laboratory setting. For patients with EGFR mutation, we recommend first-line therapy is erlotinib or afatinib or a clinical trial. For patients with ALK or ROS1 rearrangements, we recommend first-line crizotinib therapy. We recommend continuation of targeted therapy until disease progression. Upon disease progression, provided the patient is eligible to receive additional therapy, we next recommend a clinical trial or platinum-doublet systemic chemotherapy for 4-6 cycles with or without bevacizumab (drug holiday). Upon disease progression, provided the patient is eligible to receive additional therapy, we next recommend a clinical trial or another National Comprehensive Cancer Network (NCCN) guideline recommended cytotoxic therapy. For patients with squamous, KRAS mutation, or wild-type for these 4 molecular phenotypes, we recommend platinum-doublet systemic chemotherapy for 4-6 cycles with or without bevacizumab or a clinical trial. Note: bevacizumab should not be administered to patients with squamous cell carcinoma. Those patients with stable disease or better can proceed on maintenance therapy or have a drug holiday. Those with disease progression on first-line therapy or developing disease progression during maintenance therapy or drug holiday, can be evaluated for a clinical trial or another NCCN guideline recommended cytotoxic therapy, provided the patient is eligible to receive additional therapy. 1Not for squamous cell lung cancer. TKI: Tyrosine kinase inhibitor; PD: Progressive disease; CR: Complete response.
- Citation: Dearing KR, Sangal A, Weiss GJ. Maintaining clarity: Review of maintenance therapy in non-small cell lung cancer. World J Clin Oncol 2014; 5(2): 103-113
- URL: https://www.wjgnet.com/2218-4333/full/v5/i2/103.htm
- DOI: https://dx.doi.org/10.5306/wjco.v5.i2.103