Copyright
©The Author(s) 2023.
World J Clin Oncol. Jul 24, 2023; 14(7): 265-284
Published online Jul 24, 2023. doi: 10.5306/wjco.v14.i7.265
Published online Jul 24, 2023. doi: 10.5306/wjco.v14.i7.265
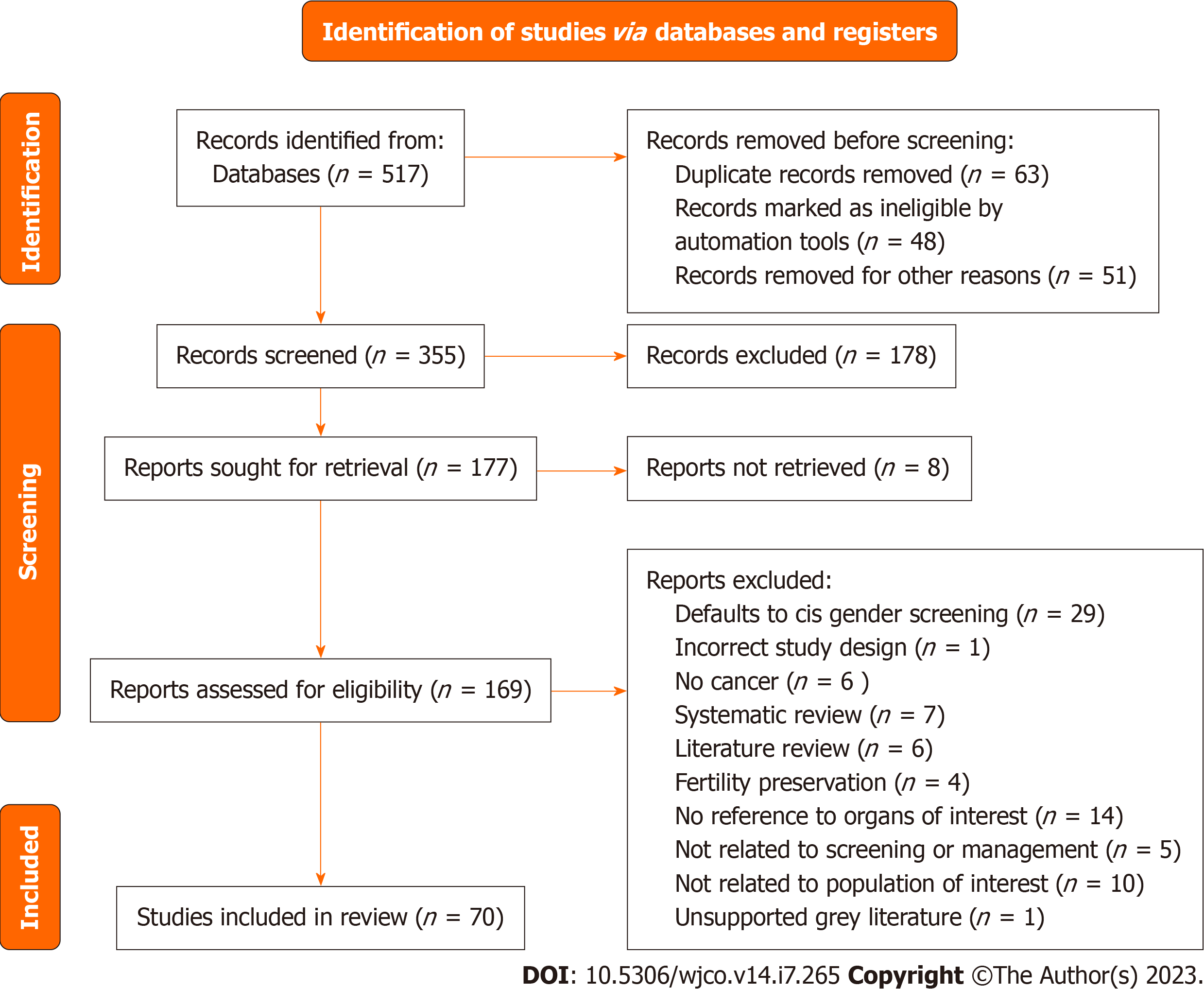
Figure 1 The preferred reporting items for systematic reviews and meta-analyses flowchart for overall cancer screening and manage-ment in the gender affirming surgery population.
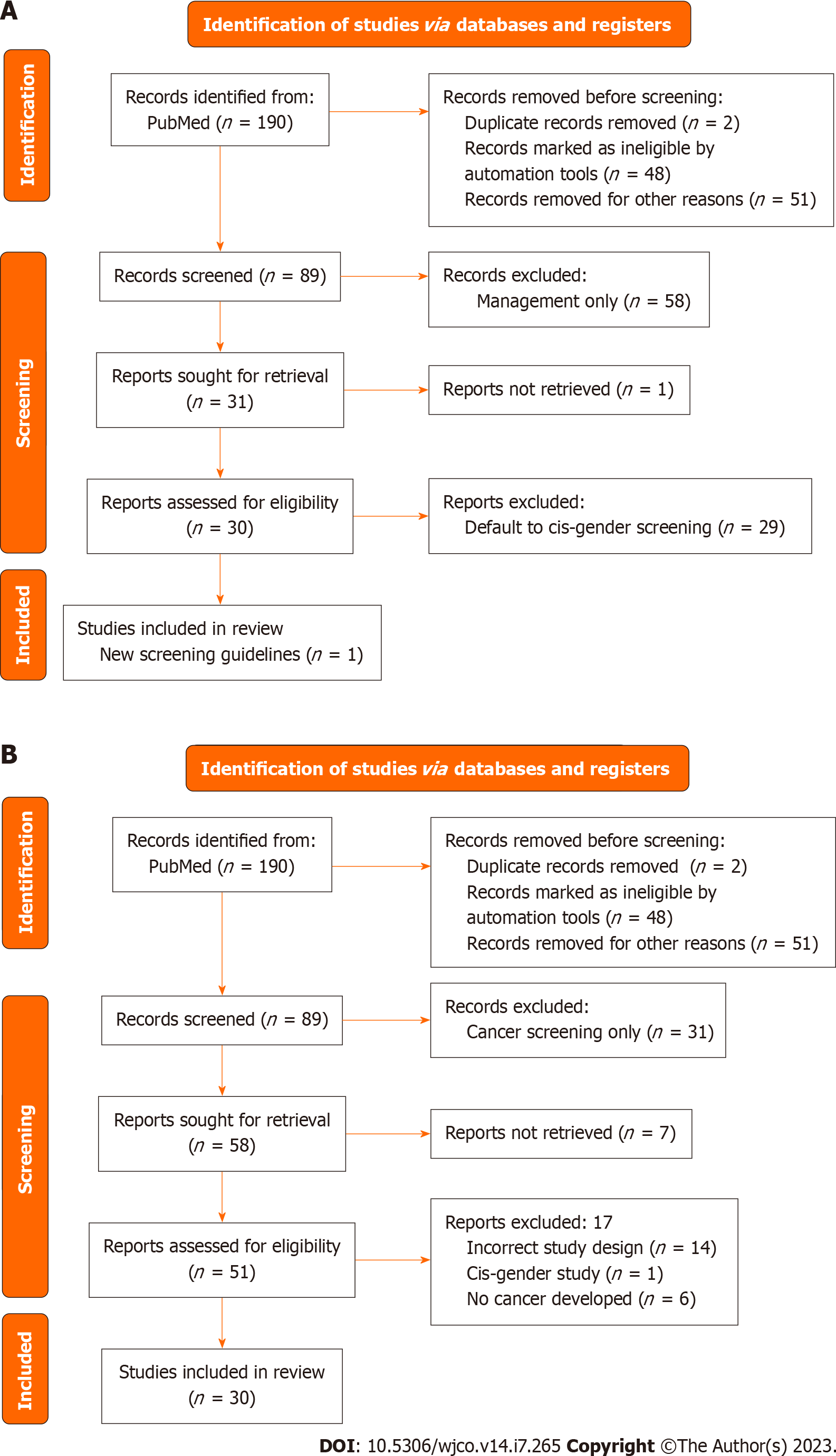
Figure 2 The preferred reporting items for systematic reviews and meta-analyses charts for the breast screening and management.
A: The preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart for articles about breast cancer screening; B: PRISMA flowchart for breast cancer management.
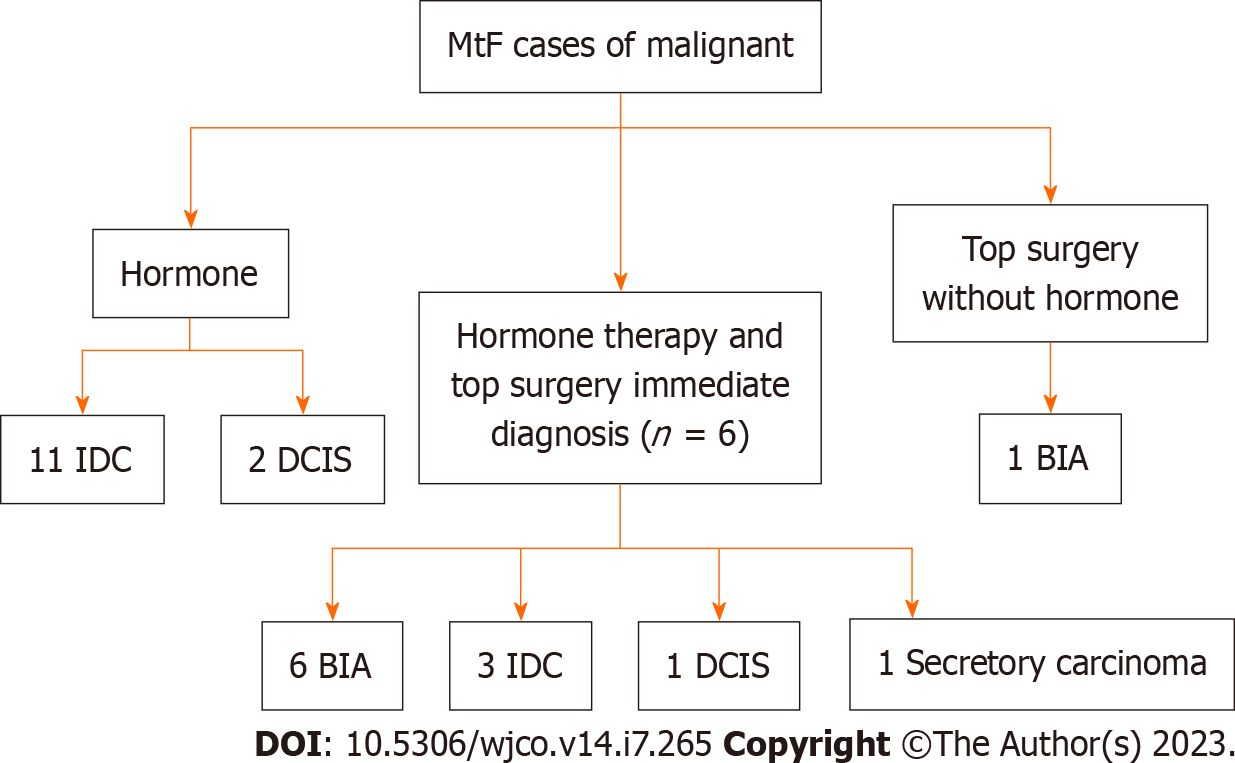
Figure 3 Study design for male to female gender affirming surgery patients.
MtF: Male to female; IDC: Invasive ductal carcinoma; DCIS: Ductal carcinoma in situ; BIA: Breast implant associated.
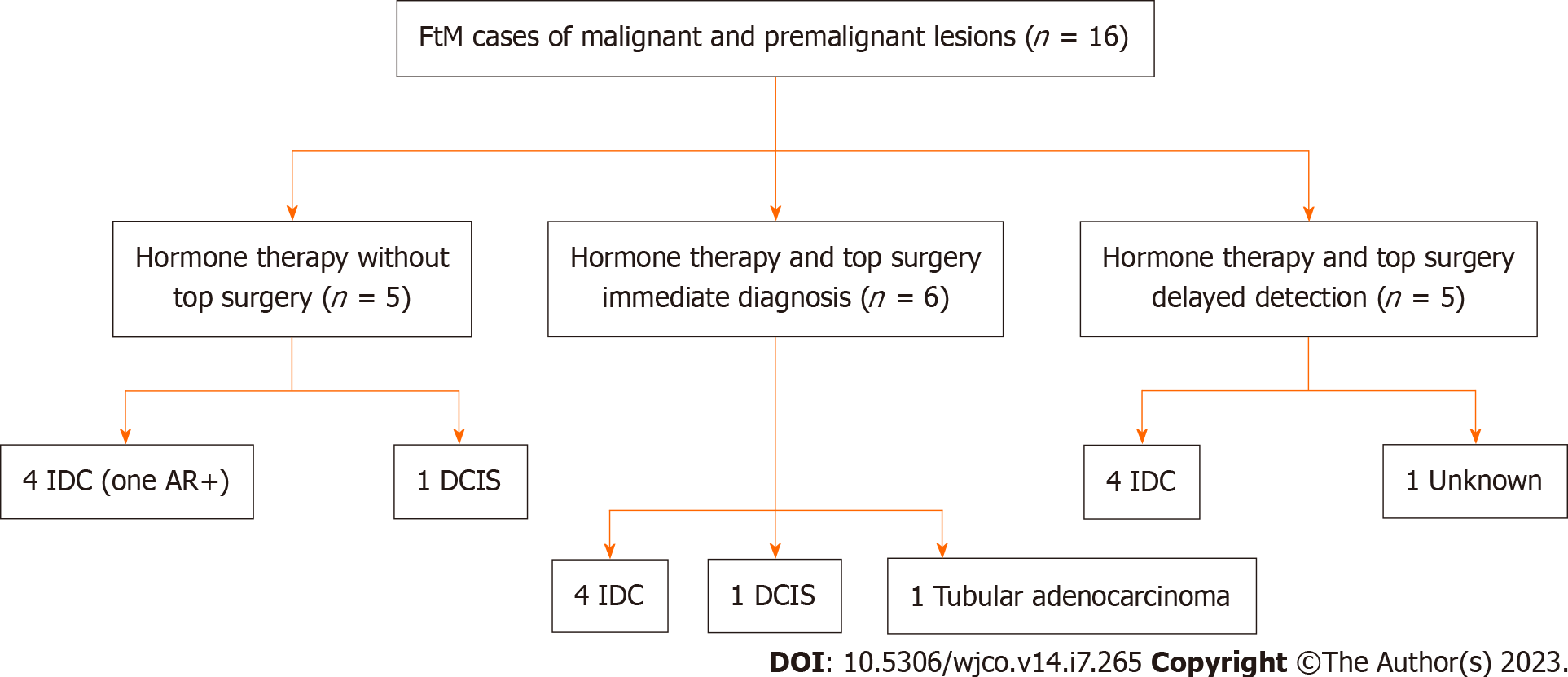
Figure 4 Study design for female to male gender affirming surgery patients.
FtM: Female to male; IDC: Invasive ductal carcinoma; DCIS: Ductal carcinoma in situ; AR: Androgen receptor.
Figure 5 The preferred reporting items for systematic reviews and meta-analyses flow diagram indicated database search records and inclusion decision steps.
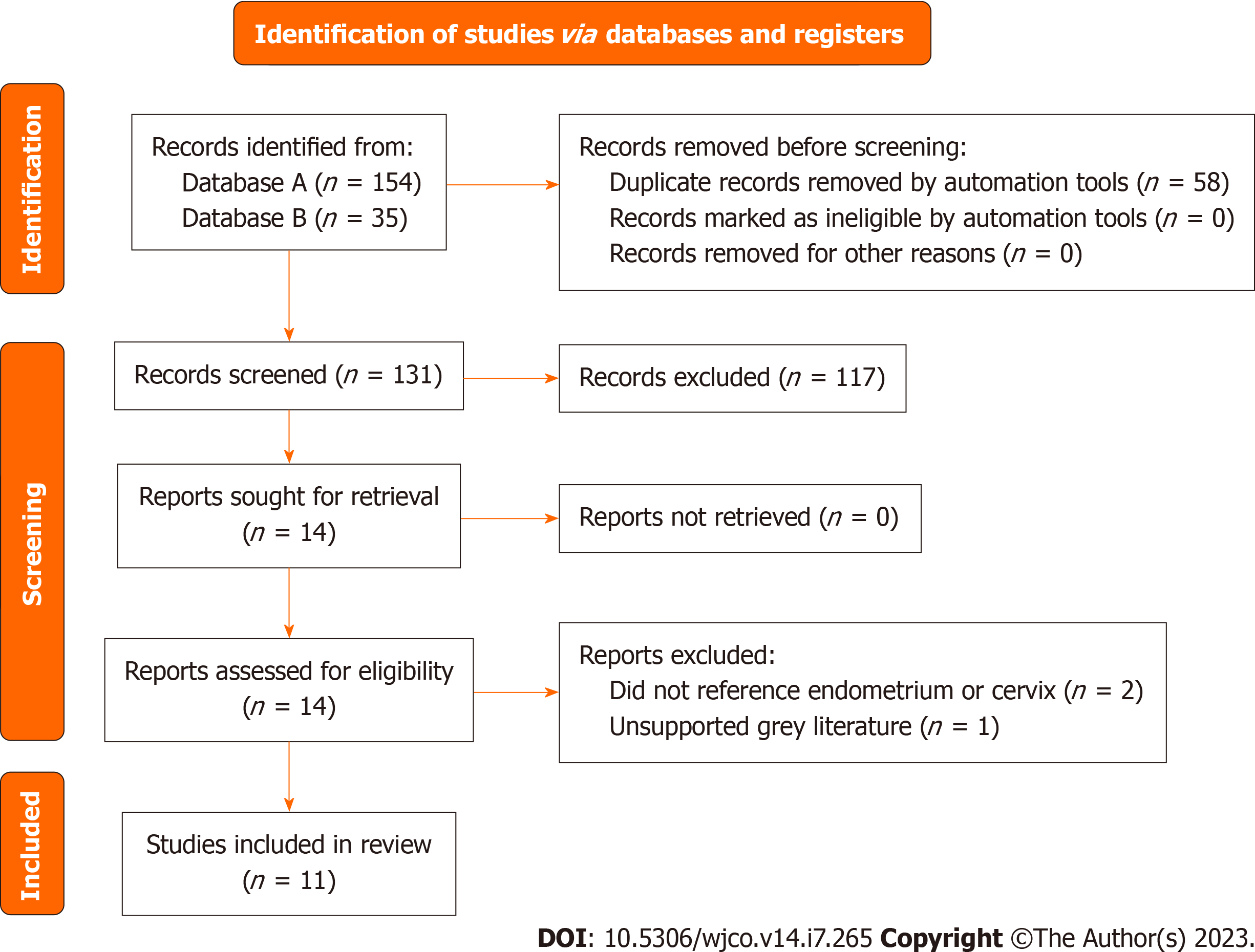
The diagram includes records regarding endometrial and cervical cancer studies.
Figure 6 The preferred reporting items for systematic reviews and meta-analyses flow diagram indicated database search records and inclusion decision steps.
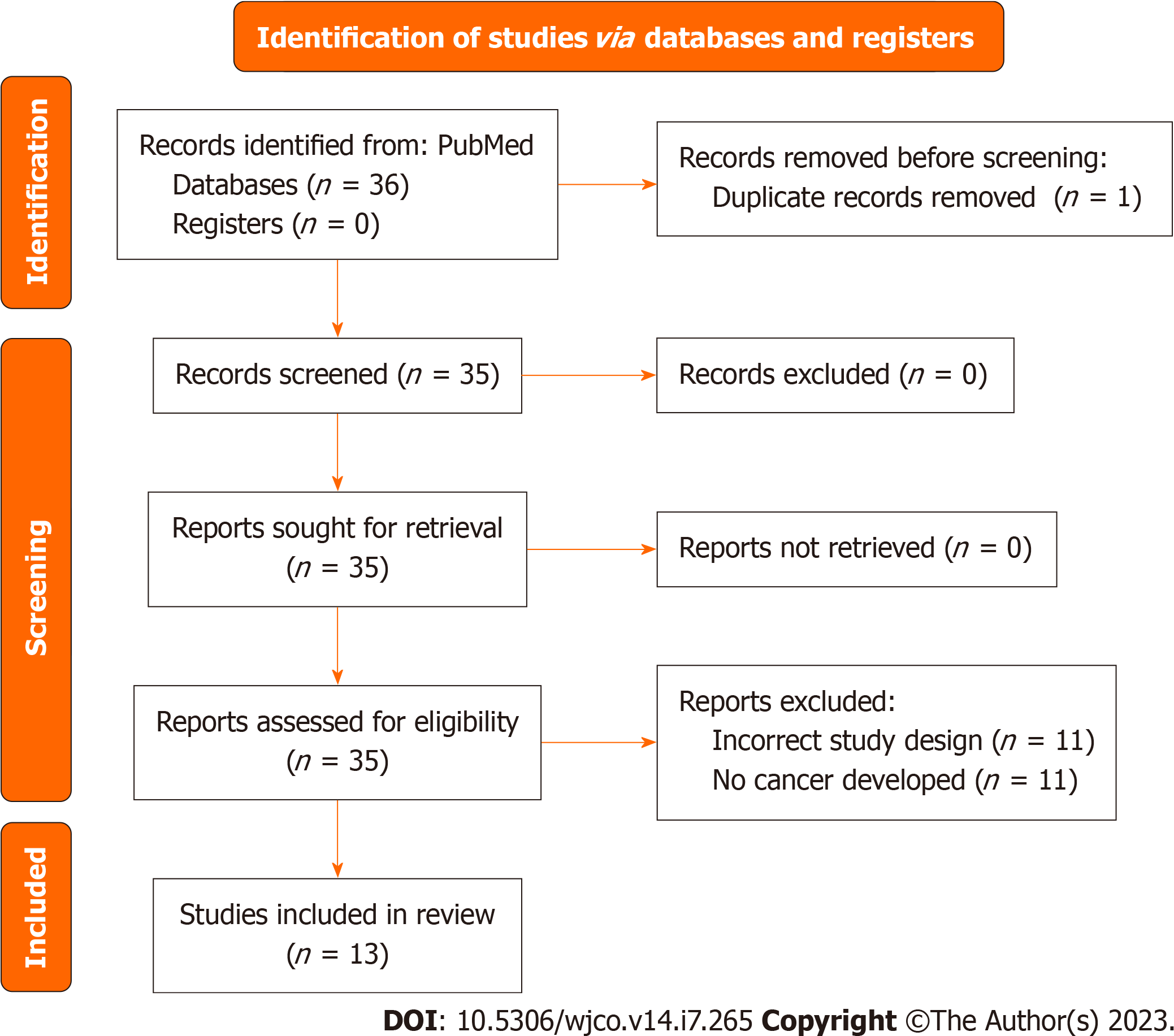
The diagram includes records regarding ovarian cancer studies.
Figure 7 The preferred reporting items for systematic reviews and meta-analyses flow diagram indicated database search records and inclusion decision steps.
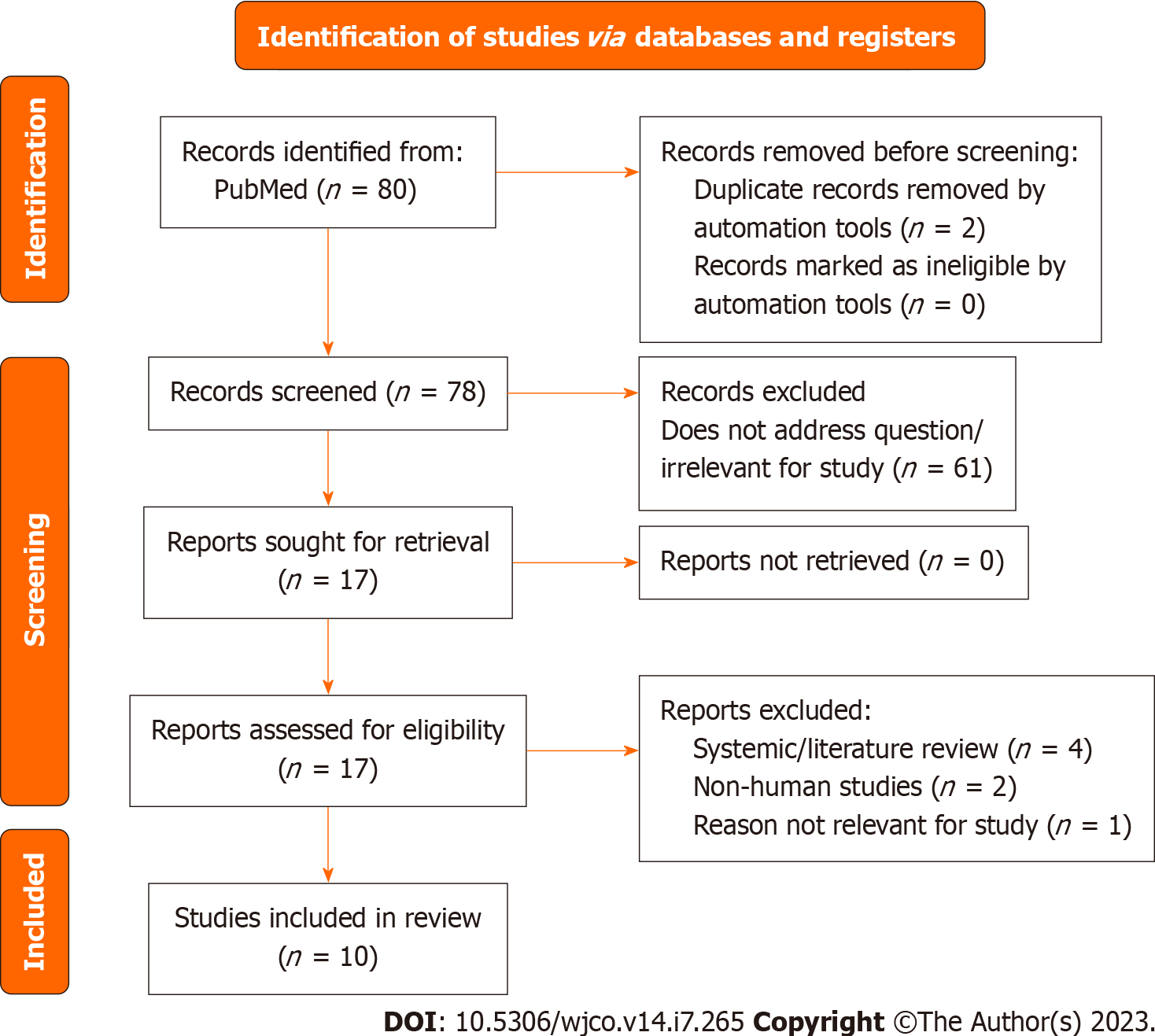
The diagram includes records regarding prostate cancer studies.
Figure 8 The preferred reporting items for systematic reviews and meta-analyses flow diagram indicated database search records and inclusion decision steps.
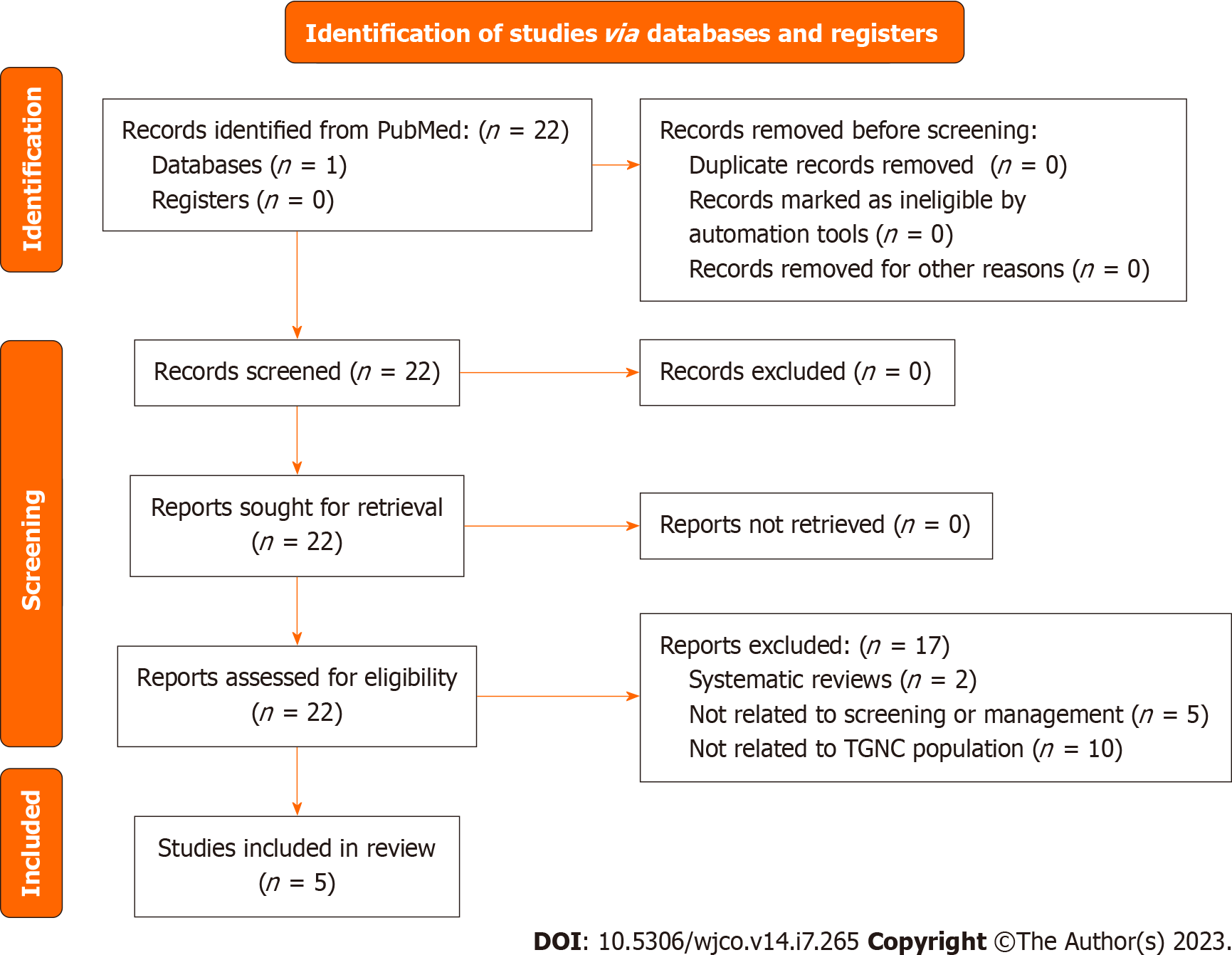
The diagram includes records regarding testicular cancer studies. TGNC: Transgender and gender nonconforming.
- Citation: Panichella JC, Araya S, Nannapaneni S, Robinson SG, You S, Gubara SM, Gebreyesus MT, Webster T, Patel SA, Hamidian Jahromi A. Cancer screening and management in the transgender population: Review of literature and special considerations for gender affirmation surgery. World J Clin Oncol 2023; 14(7): 265-284
- URL: https://www.wjgnet.com/2218-4333/full/v14/i7/265.htm
- DOI: https://dx.doi.org/10.5306/wjco.v14.i7.265