|
1
|
Noruzi S, Mohammadi R, Jamialahmadi K. CRISPR/Cas9 system: a novel approach to overcome chemotherapy and radiotherapy resistance in cancer. NAUNYN-SCHMIEDEBERG'S ARCHIVES OF PHARMACOLOGY 2025; 398:3373-3408. [PMID: 39560750 DOI: 10.1007/s00210-024-03480-2] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Subscribe] [Scholar Register] [Received: 07/24/2024] [Accepted: 09/21/2024] [Indexed: 11/20/2024]
Abstract
Cancer presents a global health challenge with rising incidence and mortality. Despite treatment advances in cancer therapy, radiotherapy and chemotherapy remained the most common treatments for all types of cancers. However, resistance phenotype in cancer cells leads to unsatisfactory results in the efficiency of therapeutic strategies. Therefore, researchers strive to propose effective solutions to overcome treatment failure, which requires a deep knowledge of treatment-resistant mechanisms. The progression and occurrence of tumors can be attributed to gene mutation. Over the past decade, the emergence of clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9 (CRISPR/Cas9) genome editing has revolutionized cancer research. This versatile technology enables cancer modeling, manipulation of specific DNA sequences, and genome-wide screening. CRISPR/Cas9 is an effective tool for identifying radio- and chemoresistance genes and offering potential adjunctive treatments to overcome tumor recurrence after chemo- and radiotherapy. This article aims to explain the potential of the CRISPR/Cas9 system in improving the effectiveness of chemo- and radiotherapy and ultimately overcoming treatment failure.
Collapse
Affiliation(s)
- Somaye Noruzi
- Department of Medical Biotechnology and Nanotechnology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Rezvan Mohammadi
- Student Research Committee, Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
| | - Khadijeh Jamialahmadi
- Biotechnology Research Center, Pharmaceutical Technology Institute, Mashhad University of Medical Sciences, Mashhad, Iran.
| |
Collapse
|
|
2
|
Gupta S, Silveira DA, Lorenzoni PR, Mombach JCM, Hashimoto RF. LncRNA PTENP1/miR-21/PTEN Axis Modulates EMT and Drug Resistance in Cancer: Dynamic Boolean Modeling for Cell Fates in DNA Damage Response. Int J Mol Sci 2024; 25:8264. [PMID: 39125832 PMCID: PMC11311614 DOI: 10.3390/ijms25158264] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/05/2024] [Revised: 07/21/2024] [Accepted: 07/23/2024] [Indexed: 08/12/2024] Open
Abstract
It is well established that microRNA-21 (miR-21) targets phosphatase and tensin homolog (PTEN), facilitating epithelial-to-mesenchymal transition (EMT) and drug resistance in cancer. Recent evidence indicates that PTEN activates its pseudogene-derived long non-coding RNA, PTENP1, which in turn inhibits miR-21. However, the dynamics of PTEN, miR-21, and PTENP1 in the DNA damage response (DDR) remain unclear. Thus, we propose a dynamic Boolean network model by integrating the published literature from various cancers. Our model shows good agreement with the experimental findings from breast cancer, hepatocellular carcinoma (HCC), and oral squamous cell carcinoma (OSCC), elucidating how DDR activation transitions from the intra-S phase to the G2 checkpoint, leading to a cascade of cellular responses such as cell cycle arrest, senescence, autophagy, apoptosis, drug resistance, and EMT. Model validation underscores the roles of PTENP1, miR-21, and PTEN in modulating EMT and drug resistance. Furthermore, our analysis reveals nine novel feedback loops, eight positive and one negative, mediated by PTEN and implicated in DDR cell fate determination, including pathways related to drug resistance and EMT. Our work presents a comprehensive framework for investigating cellular responses following DDR, underscoring the therapeutic potential of targeting PTEN, miR-21, and PTENP1 in cancer treatment.
Collapse
Affiliation(s)
- Shantanu Gupta
- Instituto de Matemática e Estatística, Departamento de Ciência da Computação, Universidade de São Paulo, Rua do Matão 1010, São Paulo 05508-090, SP, Brazil;
| | | | - Pedro R. Lorenzoni
- Departamento de Física, Universidade Federal de Santa Maria, Santa Maria 97105-900, RS, Brazil; (P.R.L.); (J.C.M.M.)
| | - Jose Carlos M. Mombach
- Departamento de Física, Universidade Federal de Santa Maria, Santa Maria 97105-900, RS, Brazil; (P.R.L.); (J.C.M.M.)
| | - Ronaldo F. Hashimoto
- Instituto de Matemática e Estatística, Departamento de Ciência da Computação, Universidade de São Paulo, Rua do Matão 1010, São Paulo 05508-090, SP, Brazil;
| |
Collapse
|
|
3
|
Batool A, Rashid W, Fatima K, Khan SU. Mechanisms of Cancer Resistance to Various Therapies. DRUG RESISTANCE IN CANCER: MECHANISMS AND STRATEGIES 2024:31-75. [DOI: 10.1007/978-981-97-1666-1_2] [Citation(s) in RCA: 7] [Impact Index Per Article: 7.0] [Reference Citation Analysis] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 01/03/2025]
|
|
4
|
Gasimli R, Kayabasi C, Ozmen Yelken B, Asik A, Sogutlu F, Celebi C, Yilmaz Susluer S, Kamer S, Biray Avci C, Haydaroglu A, Gunduz C. The effects of PKI-402 on breast tumor models' radiosensitivity via dual inhibition of PI3K/mTOR. Int J Radiat Biol 2023; 99:1961-1970. [PMID: 37389464 DOI: 10.1080/09553002.2023.2232019] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/03/2022] [Accepted: 06/25/2023] [Indexed: 07/01/2023]
Abstract
PURPOSE PI3K/Akt/mTOR pathway activation causes relapse and resistance after radiotherapy in breast cancer (BC). We aimed to radiosensitize BC cell lines to irradiation (IR) by PKI-402, a dual PI3K/mTOR inhibitor. METHODS We performed cytotoxicity, clonogenicity, hanging drop, apoptosis and double-strand break detection, and phosphorylation of 16 essential proteins involved in the PI3K/mTOR pathway. RESULTS Our findings showed that PKI-402 has cytotoxic efficiency in all cell lines. Clonogenic assay results showed that PKI-402 plus IR inhibited the colony formation ability of MCF-7 and breast cancer stem cell lines. Results showed that PKI-402 plus IR causes more apoptotic cell death than IR alone in the MCF-7 cells but did not cause significant changes in the MDA-MB-231. γ-H2AX levels were increased in MDA-MB-231 in PKI-402 plus IR groups, whereas we did not observe any apoptotic and γ-H2AX induction in BCSCs and MCF-10A cells in all treatment groups. Some pivotal phosphorylated proteins of the PI3K/AKT pathway decreased, several proteins increased and others did not change. CONCLUSION In conclusion, if the combined use of PKI-402 with radiation is supported by in vivo studies, it can contribute to the treatment options and the course of the disease.
Collapse
Affiliation(s)
- Roya Gasimli
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Cagla Kayabasi
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Besra Ozmen Yelken
- Department of Medical Biology, Faculty of Medicine, Bakircay University, Izmir, Turkey
| | - Aycan Asik
- Department of Medical Biology, Faculty of Medicine, Mugla Sitki Kocman University, Mugla, Turkey
| | - Fatma Sogutlu
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Caglar Celebi
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Sunde Yilmaz Susluer
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Serra Kamer
- Department of Radiation Oncology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Cigir Biray Avci
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Ayfer Haydaroglu
- Department of Radiation Oncology, Faculty of Medicine, Ege University, Izmir, Turkey
| | - Cumhur Gunduz
- Department of Medical Biology, Faculty of Medicine, Ege University, Izmir, Turkey
| |
Collapse
|
|
5
|
Imaizumi H, Minami K, Hieda M, Narihiro N, Koizumi M. The linker of nucleoskeleton and cytoskeleton complex is required for X-ray-induced epithelial-mesenchymal transition. JOURNAL OF RADIATION RESEARCH 2023; 64:358-368. [PMID: 36694940 PMCID: PMC10036107 DOI: 10.1093/jrr/rrac104] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 08/07/2022] [Revised: 11/13/2022] [Indexed: 06/17/2023]
Abstract
The linker of nucleoskeleton and cytoskeleton (LINC) complex has been implicated in various functions of the nuclear envelope, including nuclear migration, mechanotransduction and DNA repair. We previously revealed that the LINC complex component Sad1 and UNC84 domain containing 1 (SUN1) is required for sublethal-dose X-ray-enhanced cell migration and invasion. This study focused on epithelial-mesenchymal transition (EMT), which contributes to cell migration. Hence, the present study aimed to examine whether sublethal-dose X-irradiation induces EMT and whether LINC complex component SUN1 is involved in low-dose X-ray-induced EMT. This study showed that low-dose (0.5 Gy or 2 Gy) X-irradiation induced EMT in human breast cancer MDA-MB-231 cells. Additionally, X-irradiation increased the expression of SUN1. Therefore, SUN1 was depleted using siRNA. In SUN1-depleted cells, low-dose X-irradiation did not induce EMT. In addition, although the SUN1 splicing variant SUN1_916-depleted cells (containing 916 amino acids [AA] of SUN1) were induced EMT by low-dose X-irradiation like as non-transfected control cells, SUN1_888-depleted cells (which encodes 888 AA) were not induced EMT by low-dose X-irradiation. Moreover, since the Wnt/β-catenin signaling pathway regulates E-cadherin expression via the expression of the E-cadherin repressor Snail, the expression of β-catenin after X-irradiation was examined. After 24 hours of irradiation, β-catenin expression increased in non-transfected cells or SUN1_916-depleted cells, whereas β-catenin expression remained unchanged and did not increase in SUN1- or SUN1_888-depleted cells. Therefore, in this study, we found that low-dose X-irradiation induces EMT, and LINC complex component SUN1, especially SUN1_888, is required for X-ray-induced EMT via activation of the Wnt/β-catenin signaling pathway.
Collapse
Affiliation(s)
- Hiromasa Imaizumi
- Corresponding author. Department of Radiological Technology, Faculty of Health Science and Technology, Kawasaki University of Medical Welfare, 288 Matsushima, Kurashiki, Okayama 701-0193, Japan. E-mail: ; Tel: +81-86-462-1111; Fax: +81-86-464-1109
| | - Kazumasa Minami
- Department of Medical Physics and Engineering, Graduate School of Medicine and Health Science, Osaka University, 1-7 Yamadaoka, Suita, Osaka 565-0871, Japan
| | - Miki Hieda
- Graduate School of Health Sciences, Ehime Prefectural University of Health Sciences, 543 Takoda, Tobe-cho, Iyo-gun, Ehime 791-2101, Japan
| | - Naomasa Narihiro
- Department of Radiological Technology, Faculty of Health Science and Technology, Kawasaki University of Medical Welfare, 288 Matsushima, Kurashiki, Okayama 701-0193, Japan
| | - Masahiko Koizumi
- Department of Medical Physics and Engineering, Graduate School of Medicine and Health Science, Osaka University, 1-7 Yamadaoka, Suita, Osaka 565-0871, Japan
| |
Collapse
|
|
6
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 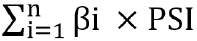 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
7
|
Sminia P, Guipaud O, Viktorsson K, Ahire V, Baatout S, Boterberg T, Cizkova J, Dostál M, Fernandez-Palomo C, Filipova A, François A, Geiger M, Hunter A, Jassim H, Edin NFJ, Jordan K, Koniarová I, Selvaraj VK, Meade AD, Milliat F, Montoro A, Politis C, Savu D, Sémont A, Tichy A, Válek V, Vogin G. Clinical Radiobiology for Radiation Oncology. RADIOBIOLOGY TEXTBOOK 2023:237-309. [DOI: 10.1007/978-3-031-18810-7_5] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Subscribe] [Scholar Register] [Indexed: 09/30/2023]
Abstract
AbstractThis chapter is focused on radiobiological aspects at the molecular, cellular, and tissue level which are relevant for the clinical use of ionizing radiation (IR) in cancer therapy. For radiation oncology, it is critical to find a balance, i.e., the therapeutic window, between the probability of tumor control and the probability of side effects caused by radiation injury to the healthy tissues and organs. An overview is given about modern precision radiotherapy (RT) techniques, which allow optimal sparing of healthy tissues. Biological factors determining the width of the therapeutic window are explained. The role of the six typical radiobiological phenomena determining the response of both malignant and normal tissues in the clinic, the 6R’s, which are Reoxygenation, Redistribution, Repopulation, Repair, Radiosensitivity, and Reactivation of the immune system, is discussed. Information is provided on tumor characteristics, for example, tumor type, growth kinetics, hypoxia, aberrant molecular signaling pathways, cancer stem cells and their impact on the response to RT. The role of the tumor microenvironment and microbiota is described and the effects of radiation on the immune system including the abscopal effect phenomenon are outlined. A summary is given on tumor diagnosis, response prediction via biomarkers, genetics, and radiomics, and ways to selectively enhance the RT response in tumors. Furthermore, we describe acute and late normal tissue reactions following exposure to radiation: cellular aspects, tissue kinetics, latency periods, permanent or transient injury, and histopathology. Details are also given on the differential effect on tumor and late responding healthy tissues following fractionated and low dose rate irradiation as well as the effect of whole-body exposure.
Collapse
|
|
8
|
Qiu R, Wang W, Li J, Wang Y. Roles of PTEN inactivation and PD-1/PD-L1 activation in esophageal squamous cell carcinoma. Mol Biol Rep 2022; 49:6633-6645. [PMID: 35301651 DOI: 10.1007/s11033-022-07246-y] [Citation(s) in RCA: 4] [Impact Index Per Article: 1.3] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/13/2021] [Revised: 02/04/2022] [Accepted: 02/08/2022] [Indexed: 02/07/2023]
Abstract
Esophageal squamous cell carcinoma (ESCC) is the most common type of esophageal cancer in China and developing countries. The purpose of this review is to summarize the roles of inactivation of the tumor suppressor gene, phosphatase and tensin homolog (PTEN), and activation of the programmed cell death protein 1 (PD-1) upon binding to its ligand (PD-L1) in the promotion of ESCC. Studies of ESCC performed in vitro and in vivo indicated that PTEN and PD-L1 function in the regulation of cell proliferation, invasion, and migration; the epithelial-mesenchymal transition; resistance to chemotherapy and radiotherapy; and the PI3K/AKT signaling pathway. Certain genetic variants of PTEN are related to susceptibility to ESCC, and PTEN and PD-L1 also function in ESCC progression and affect the prognosis of patients with ESCC. There is also evidence that the expression of PD-L1 and PTEN are associated with the progression of certain other cancers. Future studies should further examine the relationship of PD-L1 and PTEN and their possible interactions in ESCC.
Collapse
Affiliation(s)
- Rong Qiu
- Department of Radiation Oncology, Fourth Hospital of Hebei Medical University, No. 12 Jian Kang Road, Shijiazhuang, Hebei Province, P. R. China
| | - Wenxi Wang
- Department of Oncology, Xiangya Hospital, Central South University, 410008, Changsha, Hunan Province, China
| | - Juan Li
- Department of Radiation Oncology, Fourth Hospital of Hebei Medical University, No. 12 Jian Kang Road, Shijiazhuang, Hebei Province, P. R. China
| | - Yuxiang Wang
- Department of Radiation Oncology, Fourth Hospital of Hebei Medical University, No. 12 Jian Kang Road, Shijiazhuang, Hebei Province, P. R. China.
- , No.12, Jiankang Road, 050011, Shijiazhuang, Hebei Province, China.
| |
Collapse
|
|
9
|
Liu Y, Zheng C, Huang Y, He M, Xu WW, Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm (Beijing) 2021; 2:315-340. [PMID: 34766149 PMCID: PMC8554658 DOI: 10.1002/mco2.55] [Citation(s) in RCA: 167] [Impact Index Per Article: 41.8] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/26/2020] [Revised: 12/25/2020] [Accepted: 12/28/2020] [Indexed: 12/24/2022] Open
Abstract
Cancer is a leading cause of death worldwide. Surgery is the primary treatment approach for cancer, but the survival rate is very low due to the rapid progression of the disease and presence of local and distant metastasis at diagnosis. Adjuvant chemotherapy and radiotherapy are important components of the multidisciplinary approaches for cancer treatment. However, resistance to radiotherapy and chemotherapy may result in treatment failure or even cancer recurrence. Radioresistance in cancer is often caused by the repair response to radiation-induced DNA damage, cell cycle dysregulation, cancer stem cells (CSCs) resilience, and epithelial-mesenchymal transition (EMT). Understanding the molecular alterations that lead to radioresistance may provide new diagnostic markers and therapeutic targets to improve radiotherapy efficacy. Patients who develop resistance to chemotherapy drugs cannot benefit from the cytotoxicity induced by the prescribed drug and will likely have a poor outcome with these treatments. Chemotherapy often shows a low response rate due to various drug resistance mechanisms. This review focuses on the molecular mechanisms of radioresistance and chemoresistance in cancer and discusses recent developments in therapeutic strategies targeting chemoradiotherapy resistance to improve treatment outcomes.
Collapse
Affiliation(s)
- Ya‐Ping Liu
- MOE Key Laboratory of Tumor Molecular Biology and Key Laboratory of Functional Protein Research of Guangdong Higher Education InstitutesInstitute of Life and Health EngineeringJinan UniversityGuangzhouP. R. China
| | - Can‐Can Zheng
- MOE Key Laboratory of Tumor Molecular Biology and Key Laboratory of Functional Protein Research of Guangdong Higher Education InstitutesInstitute of Life and Health EngineeringJinan UniversityGuangzhouP. R. China
| | - Yun‐Na Huang
- MOE Key Laboratory of Tumor Molecular Biology and Guangdong Provincial Key Laboratory of Bioengineering MedicineNational Engineering Research Center of Genetic MedicineInstitute of BiomedicineCollege of Life Science and TechnologyJinan UniversityGuangzhouP. R. China
| | - Ming‐Liang He
- Department of Biomedical SciencesCity University of Hong KongHong KongChina
| | - Wen Wen Xu
- MOE Key Laboratory of Tumor Molecular Biology and Guangdong Provincial Key Laboratory of Bioengineering MedicineNational Engineering Research Center of Genetic MedicineInstitute of BiomedicineCollege of Life Science and TechnologyJinan UniversityGuangzhouP. R. China
| | - Bin Li
- MOE Key Laboratory of Tumor Molecular Biology and Key Laboratory of Functional Protein Research of Guangdong Higher Education InstitutesInstitute of Life and Health EngineeringJinan UniversityGuangzhouP. R. China
| |
Collapse
|
|
10
|
Oweida A, Paquette B. Reconciling two opposing effects of radiation therapy: stimulation of cancer cell invasion and activation of anti-cancer immunity. Int J Radiat Biol 2021; 99:951-963. [PMID: 34264178 DOI: 10.1080/09553002.2021.1956005] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 10/20/2022]
Abstract
PURPOSE The damage caused by radiation therapy to cancerous and normal cells inevitably leads to changes in the secretome profile of pro and anti-inflammatory mediators. The inflammatory response depends on the dose of radiation and its fractionation, while the inherent radiosensitivity of each patient dictates the intensity and types of adverse reactions. This review will present an overview of two apparently opposite reactions that may occur after radiation treatment: induction of an antitumor immune response and a protumoral response. Emphasis is placed on the molecular and cellular mechanisms involved. CONCLUSIONS By understanding how radiation changes the balance between anti- and protumoral effects, these forces can be manipulated to optimize radiation oncology treatments.
Collapse
Affiliation(s)
- Ayman Oweida
- Department of Nuclear Medicine and Radiobiology, Faculty of Medicine and Health Sciences, Universite de Sherbrooke, Sherbrooke, Canada
| | - Benoit Paquette
- Department of Nuclear Medicine and Radiobiology, Faculty of Medicine and Health Sciences, Universite de Sherbrooke, Sherbrooke, Canada
| |
Collapse
|
|
11
|
Zhang X, Bobeica M, Unger M, Bednarz A, Gerold B, Patties I, Melzer A, Landgraf L. Focused ultrasound radiosensitizes human cancer cells by enhancement of DNA damage. Strahlenther Onkol 2021; 197:730-743. [PMID: 33885910 PMCID: PMC8292237 DOI: 10.1007/s00066-021-01774-5] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/11/2020] [Accepted: 03/23/2021] [Indexed: 12/19/2022]
Abstract
Purpose High-intensity focused ultrasound (HIFU/FUS) has expanded as a noninvasive quantifiable option for hyperthermia (HT). HT in a temperature range of 40–47 °C (thermal dose CEM43 ≥ 25) could work as a sensitizer to radiation therapy (RT). Here, we attempted to understand the tumor radiosensitization effect at the cellular level after a combination treatment of FUS+RT. Methods An in vitro FUS system was developed to induce HT at frequencies of 1.147 and 1.467 MHz. Human head and neck cancer (FaDU), glioblastoma (T98G), and prostate cancer (PC-3) cells were exposed to FUS in ultrasound-penetrable 96-well plates followed by single-dose X‑ray irradiation (10 Gy). Radiosensitizing effects of FUS were investigated by cell metabolic activity (WST‑1 assay), apoptosis (annexin V assay, sub-G1 assay), cell cycle phases (propidium iodide staining), and DNA double-strand breaks (γH2A.X assay). Results The FUS intensities of 213 (1.147 MHz) and 225 W/cm2 (1.467 MHz) induced HT for 30 min at mean temperatures of 45.20 ± 2.29 °C (CEM43 = 436 ± 88) and 45.59 ± 1.65 °C (CEM43 = 447 ± 79), respectively. FUS improves the effect of RT significantly by reducing metabolic activity in T98G cells 48 h (RT: 96.47 ± 8.29%; FUS+RT: 79.38 ± 14.93%; p = 0.012) and in PC-3 cells 72 h (54.20 ± 10.85%; 41.01 ± 11.17%; p = 0.016) after therapy, but not in FaDu cells. Mechanistically, FUS+RT leads to increased apoptosis and enhancement of DNA double-strand breaks compared to RT alone in T98G and PC-3 cells. Conclusion Our in vitro findings demonstrate that FUS has good potential to sensitize glioblastoma and prostate cancer cells to RT by mainly enhancing DNA damage. Supplementary Information The online version of this article (10.1007/s00066-021-01774-5) contains supplementary material, which is available to authorized users.
Collapse
Affiliation(s)
- Xinrui Zhang
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany.
| | - Mariana Bobeica
- Institute for Medical Science and Technology (IMSaT), University of Dundee, Wilson House, 1 Wurzburg Loan, Dundee MediPark, Dundee, DD2 1FD, UK.,Extreme Light Infrastructure - Nuclear Physics ELI-NP, "Horia Hulubei" National Institute for Physics and Nuclear Engineering, 30 Reactorului Street, Bucharest-Magurele, 077125, Romania
| | - Michael Unger
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany
| | - Anastasia Bednarz
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany
| | - Bjoern Gerold
- Institute for Medical Science and Technology (IMSaT), University of Dundee, Wilson House, 1 Wurzburg Loan, Dundee MediPark, Dundee, DD2 1FD, UK.,Theraclion, 102 Rue Etienne Dolet, Malakoff, 92240, France
| | - Ina Patties
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany.,Department of Radiation Oncology, University of Leipzig, Stephanstr. 9a, Leipzig, 04103, Germany
| | - Andreas Melzer
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany. .,Institute for Medical Science and Technology (IMSaT), University of Dundee, Wilson House, 1 Wurzburg Loan, Dundee MediPark, Dundee, DD2 1FD, UK.
| | - Lisa Landgraf
- Innovation Center Computer Assisted Surgery (ICCAS), University of Leipzig, Semmelweisstr. 14, Haus 14, Leipzig, 04103, Germany
| |
Collapse
|
|
12
|
Zhou S, Zhang M, Zhou C, Wang W, Yang H, Ye W. The role of epithelial-mesenchymal transition in regulating radioresistance. Crit Rev Oncol Hematol 2020; 150:102961. [PMID: 32361589 DOI: 10.1016/j.critrevonc.2020.102961] [Citation(s) in RCA: 57] [Impact Index Per Article: 11.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/30/2019] [Revised: 04/06/2020] [Accepted: 04/08/2020] [Indexed: 12/16/2022] Open
Abstract
Cancer patients with different stages can benefit from radiotherapy, but there are still limited due to inherent or acquired radioresistance. The epithelial-mesenchymal transition (EMT) is a complex biological process that is implicated in malignant characteristics of cancer, such as radioresistance. Although the possible mechanisms of EMT-dependent radioresistance are being extensively studied, there is a lack of a clear picture of the overall signaling of EMT-mediated radioresistance. In this review, we highlight the role and possible molecular mechanisms of EMT in cancer radioresistance, in particular to EMT-associated signaling pathway, EMT-inducing transcription factors (EMT-TFs), EMT-related non-coding RNAs. The knowledge of EMT-associated mechanisms of radioresistance will offer more potent therapy targets to improve the radiotherapy responses.
Collapse
Affiliation(s)
- Suna Zhou
- Department of Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China; Laboratory of Cellular and Molecular Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China.
| | - Mingxin Zhang
- Department of Gastroenterology, The First Affiliated Hospital of Xi'an Medical University, Xi'an 710077, Shaanxi, China
| | - Chao Zhou
- Department of Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China; Laboratory of Cellular and Molecular Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China
| | - Wei Wang
- Department of Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China; Laboratory of Cellular and Molecular Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China
| | - Haihua Yang
- Department of Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China; Laboratory of Cellular and Molecular Radiation Oncology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China
| | - Wenguang Ye
- Department of Gastroenterology, The Affiliated Taizhou Hospital, Wenzhou Medical University, Taizhou 317000, Zhejiang, China.
| |
Collapse
|
|
13
|
Labbé M, Hoey C, Ray J, Potiron V, Supiot S, Liu SK, Fradin D. microRNAs identified in prostate cancer: Correlative studies on response to ionizing radiation. Mol Cancer 2020; 19:63. [PMID: 32293453 PMCID: PMC7087366 DOI: 10.1186/s12943-020-01186-6] [Citation(s) in RCA: 20] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/13/2019] [Accepted: 03/12/2020] [Indexed: 12/13/2022] Open
Abstract
As the most frequently diagnosed non-skin cancer in men and a leading cause of cancer-related death, understanding the molecular mechanisms that drive treatment resistance in prostate cancer poses a significant clinical need. Radiotherapy is one of the most widely used treatments for prostate cancer, along with surgery, hormone therapy, and chemotherapy. However, inherent radioresistance of tumor cells can reduce local control and ultimately lead to poor patient outcomes, such as recurrence, metastasis and death. The underlying mechanisms of radioresistance have not been fully elucidated, but it has been suggested that miRNAs play a critical role. miRNAs are small non-coding RNAs that regulate gene expression in every signaling pathway of the cell, with one miRNA often having multiple targets. By fine-tuning gene expression, miRNAs are important players in modulating DNA damage response, cell death, tumor aggression and the tumor microenvironment, and can ultimately affect a tumor's response to radiotherapy. Furthermore, much interest has focused on miRNAs found in biofluids and their potential utility in various clinical applications. In this review, we summarize the current knowledge on miRNA deregulation after irradiation and the associated functional outcomes, with a focus on prostate cancer. In addition, we discuss the utility of circulating miRNAs as non-invasive biomarkers to diagnose, predict response to treatment, and prognosticate patient outcomes.
Collapse
Affiliation(s)
- Maureen Labbé
- CRCINA, INSERM, Université d'Angers, Université de Nantes, Nantes, France
| | - Christianne Hoey
- Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada
- Biological Sciences, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
| | - Jessica Ray
- Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada
- Biological Sciences, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
| | - Vincent Potiron
- CRCINA, INSERM, Université d'Angers, Université de Nantes, Nantes, France
- Institut de Cancérologie de L'Ouest René Gauducheau, Saint-Herblain, France
| | - Stéphane Supiot
- CRCINA, INSERM, Université d'Angers, Université de Nantes, Nantes, France
- Institut de Cancérologie de L'Ouest René Gauducheau, Saint-Herblain, France
| | - Stanley K Liu
- Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada.
- Biological Sciences, Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
- Department of Radiation Oncology, University of Toronto and Odette Cancer Centre, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
| | - Delphine Fradin
- CRCINA, INSERM, Université d'Angers, Université de Nantes, Nantes, France.
| |
Collapse
|
|
14
|
Wang H, Wang Z, Li Y, Lu T, Hu G. Silencing Snail Reverses Epithelial-Mesenchymal Transition and Increases Radiosensitivity in Hypopharyngeal Carcinoma. Onco Targets Ther 2020; 13:497-511. [PMID: 32021293 PMCID: PMC6970617 DOI: 10.2147/ott.s237410] [Citation(s) in RCA: 18] [Impact Index Per Article: 3.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/06/2019] [Accepted: 01/01/2020] [Indexed: 12/11/2022] Open
Abstract
Purpose Radioresistance in response to radiotherapy leads to cancer recurrence and poor survival in hypopharyngeal carcinoma patients. Previous studies indicate that ionizing radiation (IR) can induce epithelial–mesenchymal transition (EMT) that promotes the radioresistance, migration and invasiveness of tumors. The aim of this study was to explore the role of Snail in EMT and acquired radioresistance in hypopharyngeal carcinoma. Methods Radioresistance human hypopharyngeal carcinoma cells (FaduRR) were previously established from the Fadu cell line. Radiosensitivity was measured by colony forming assay. Western blot and Quantitative real-time PCR were used to detect the expression of EMT phenotypes and AKT/GSK-3β/Snail signaling pathway related proteins in Fadu+4Gy and FaduRR cells. Transwell and wound-healing assays were used to measure cell migration and invasiveness. EMT-related proteins and Snail expression were assessed in 80 hypopharyngeal carcinoma patient samples from radiosensitive and radioresistance groups using immunohistochemistry. Snail was silenced to evaluate its effects on EMT, radioresistance, migration, and invasiveness of FaduRR cells. Results The molecular characteristics of EMT were observed following radiation treatment, with migration, invasiveness and radioresistance enhanced in Fadu+4Gy and FaduRR cells. Moreover, we demonstrated that IR-induced EMT by activating the AKT/GSK-3β/Snail signaling pathway and that Snail silencing reversed EMT and attenuated radioresistance in FaduRR cells. Significant differences in EMT-related proteins and Snail expression were observed between radiosensitive and resistant group. Conclusion We demonstrate that IR can trigger EMT and enhance the migration, invasiveness, and radioresistance of FaduRR cells through the AKT/GSK-3β/Snail axis. Snail silencing could attenuate these effects and represents a novel therapeutic target for EMT-induced radioresistance in hypopharyngeal carcinoma.
Collapse
Affiliation(s)
- HaiYan Wang
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
| | - ZhiHai Wang
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
| | - YanShi Li
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
| | - Tao Lu
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
| | - GuoHua Hu
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, People's Republic of China
| |
Collapse
|
|
15
|
Dong Y, Sun Y, Huang Y, Fang X, Sun P, Dwarakanath B, Kong L, Lu JJ. Depletion of MLKL inhibits invasion of radioresistant nasopharyngeal carcinoma cells by suppressing epithelial-mesenchymal transition. ANNALS OF TRANSLATIONAL MEDICINE 2019; 7:741. [PMID: 32042757 PMCID: PMC6990020 DOI: 10.21037/atm.2019.11.104] [Citation(s) in RCA: 23] [Impact Index Per Article: 3.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Received: 10/11/2019] [Accepted: 10/25/2019] [Indexed: 11/06/2022]
Abstract
BACKGROUND To examine whether MLKL participated in the invasion of radiosensitive nasopharyngeal carcinoma (NPC) cell (CNE-2) and radioresistant NPC cell (CR) through regulating epithelial-mesenchymal transition (EMT). METHODS siRNA and CRISPR/Cas9 technique were used to decrease MLKL expression in NPC cell (CNE-2 and CR). Trans-well assay was conducted to evaluate invasion. Gene expression profiling was performed using Human U133 2.0 plus arrays (Affymetrix). Kyoto Encyclopedia of Genes and Genomes (KEGG) was adopted to analyze gene expression profiling. Hub genes at a functional level were accessed by protein-to-protein network (PPI). Quantitative real-time PCR and Western blot were used to access EMT markers. RESULTS Invasion of CR was about 3~fold change higher than that of CNE-2. Silencing MLKL by siRNA inhibited invasion of CR, not CNE-2. Further, depleting MLKL by CRISPR-Cas9 in CR (CR-MLKL KO) also inhibited its invasion. KEGG pathway analysis showed invasion-related pathways were altered, such as adherent junction, TGF-β signaling pathway. PPI demonstrated that compared with CNE-2, CR showed 9 elevated hub genes including EGFR, JUN, CD44, SPP1, VIM, IL-8, BCL2, WDFY2, PIK3CD and 1 downregulated hub gene CDH1. After MLKL depletion, 8 hub genes were downregulated (EGFR, JUN, CD44, SPP1, VIM, FGF13, PLAU, MMP1) and 2 hub genes were upregulated (MMP9, CDH1). Quantitative real-time PCR results showed that compared with CNE-2, CR displayed decreased epithelial markers significantly (E-Cadherin) and increased mesenchymal markers significantly (Vimentin, N-Cadherin, Zeb1), indicating irradiation-induced EMT. After depletion of MLKL in CR, the expression of E-Cadherin, Vimentin, N-Cadherin, Zeb1 was reversed to the level of CNE-2. Western blot confirmed the results from qRT-PCR. CONCLUSIONS Depletion of MLKL efficiently inhibits invasion of radioresistant NPC by suppressing EMT. MLKL may be an important target to suppress distant metastasis of NPC patients who relapsed after radiotherapy.
Collapse
Affiliation(s)
- Yuanli Dong
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai 201321, China
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
| | - Yun Sun
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
- Division of Research and Development, Shanghai Proton and Heavy Ion Center, Shanghai 201321, China
| | - Yangle Huang
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai 201321, China
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
| | - Xumeng Fang
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai 201321, China
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
| | - Pian Sun
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai 201321, China
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
| | - Bilikere Dwarakanath
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
- Division of Research and Development, Shanghai Proton and Heavy Ion Center, Shanghai 201321, China
| | - Lin Kong
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Fudan University Cancer Hospital, Shanghai 201321, China
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
| | - Jiade Jay Lu
- Shanghai Engineering Research Center of Proton and Heavy Ion Radiation Therapy, Shanghai 201321, China
- Department of Radiation Oncology, Shanghai Proton and Heavy Ion Center, Shanghai 201321, China
| |
Collapse
|
|
16
|
Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers (Basel) 2019; 11:cancers11111781. [PMID: 31726667 PMCID: PMC6896076 DOI: 10.3390/cancers11111781] [Citation(s) in RCA: 12] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/04/2019] [Revised: 10/31/2019] [Accepted: 11/08/2019] [Indexed: 12/19/2022] Open
Abstract
Breast cancer is the most common cancer in women. Radiotherapy (RT) is one of the mainstay treatments for cancer but in some cases is not effective. Cancer stem cells (CSCs) within the tumor can be responsible for recurrence and metastasis after RT. Matrix metalloproteases (MMPs), regulated mainly by tissue inhibitors of metalloproteinases (TIMPs) and histone deacetylases (HDACs), may also contribute to tumor development by modifying its activity after RT. The aim of this work was to study the effects of RT on the expression of MMPs, TIMPs and HDACs on different cell subpopulations in MCF-7, MDA-MB-231 and SK-BR-3 cell lines. We assessed the in vitro expression of these genes in different 3D culture models and induced tumors in female NSG mice by orthotopic xenotransplants. Our results showed that gene expression is related to the cell subpopulation studied, the culture model used and the single radiation dose administered. Moreover, the crucial role played by the microenvironment in terms of cell interactions and CSC plasticity in tumor growth and RT outcome is also shown, supporting the use of higher doses (6 Gy) to achieve better control of tumor development.
Collapse
|
|
17
|
Wu SY, Chen CL, Tseng PC, Chiu CY, Lin YE, Lin CF. Fractionated ionizing radiation facilitates interferon-γ signaling and anticancer activity in lung adenocarcinoma cells. J Cell Physiol 2019; 234:16003-16010. [PMID: 30767202 DOI: 10.1002/jcp.28258] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/11/2018] [Revised: 12/11/2018] [Accepted: 01/10/2019] [Indexed: 01/24/2023]
Abstract
Fractionated ionizing radiation (FIR) is a radiotherapy regimen that is regularly performed as part of lung cancer treatment. In contrast to the growth inhibition caused by DNA damage, immunomodulation in post-irradiated cancer cells is not well documented. Interferon (IFN)-γ confers anticancer activity by triggering both growth inhibition and cytotoxicity. This study investigated the priming effects of FIR with immunomodulation on the anticancer IFN-γ. Cell morphology, cell growth, and cytotoxicity were observed in FIR-treated A549 lung adenocarcinoma. Induction of p53 and epithelial-mesenchymal transition (EMT) were monitored. Following FIR, activation of IFN-γ signaling pathways were detected. FIR caused changes in cell morphology, inhibited cell growth, and induced cytotoxicity. While p53 was induced by FIR, no epithelial-mesenchymal transition could be found. Following IFN-γ stimulation, FIR-induced p53-associated cell cytotoxicity was significantly enhanced. Additionally, FIR increased the downstream response to IFN-γ by facilitating IFN-γ-induced signal transducer and activator of transcription 1 (STAT1) signaling without affecting the receptor expression. FIR-facilitated STAT1 activation through the mechanism involving mitogen-activated protein kinase activation and Src-homology 2 domain-containing tyrosine phosphatase 2 inactivation. These results demonstrate the FIR-facilitated IFN-γ signaling and its anticancer activity.
Collapse
Affiliation(s)
- Szu-Yuan Wu
- Department of Radiation Oncology, Wan Fang Hospital, Taipei Medical University, Taipei, Taiwan.,Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
| | - Chia-Ling Chen
- Department of Respiratory Therapy, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
| | - Po-Chun Tseng
- Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.,Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan
| | - Chi-Yun Chiu
- Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
| | - Yung-En Lin
- Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
| | - Chiou-Feng Lin
- Department of Microbiology and Immunology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan.,Graduate Institute of Medical Sciences, College of Medicine, Taipei Medical University, Taipei, Taiwan
| |
Collapse
|
|
18
|
Yang L, Zhang X, Hou Q, Huang M, Zhang H, Jiang Z, Yue J, Wu S. Single-cell RNA-seq of esophageal squamous cell carcinoma cell line with fractionated irradiation reveals radioresistant gene expression patterns. BMC Genomics 2019; 20:611. [PMID: 31345182 PMCID: PMC6659267 DOI: 10.1186/s12864-019-5970-0] [Citation(s) in RCA: 23] [Impact Index Per Article: 3.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/06/2018] [Accepted: 07/11/2019] [Indexed: 01/10/2023] Open
Abstract
Background Esophageal squamous cell carcinoma (ESCC) cells are heterogeneous, easily develop radioresistance, and recur. Single-cell RNA-seq (scRNA-seq) is a next-generation sequencing method that can delineate diverse gene expression profiles of individual cells and mining their heterogeneous behaviors in response to irradiation. Our aim was using scRNA-seq to describe the difference between parental cells and cells that acquired radioresistance, and to investigate the dynamic changes of the transcriptome of cells in response to FIR. Results We sequenced ESCC cell lines KYSE180 with and without fractionated irradiation (FIR). A total of 218 scRNA-seq libraries were obtained from 88 cells exposed to 12 Gy (KYSE-180-12 Gy), 89 exposed to 30 Gy (KYSE-180-30 Gy), and 41 parental KYSE-180 cells not exposed to FIR. Dynamic gene expression patterns were determined by comprehensive consideration of genes and pathways. Biological experiments showed that KYSE-180 cells became radioresistant after FIR. PCA analysis of scRNA-seq data showed KYSE-180, KYSE-180-12 Gy and KYSE-180-30 Gy cells were discrete away from each other. Two sub-populations found in KYSE-180-12 Gy and only one remained in KYSE-180-30 Gy. This sub-population genes exposure to FIR through 12 Gy to 30 Gy were relevant to the PI3K-AKT pathway, pathways evading apoptosis, tumor cell migration, metastasis, or invasion pathways, and cell differentiation and proliferation pathways. We validated DEGs, such as CFLAR, LAMA5, ITGA6, ITGB4, and SDC4 genes, in these five pathways as radioresistant genes in bulk cell RNA-seq data from ESCC tissue of a ESCC patient treated with radiotherapy and from KYSE-150 cell lines. Conclusions Our results delineated the divergent gene expression patterns of individual ESCC cells exposure to FIR, and displayed genes and pathways related to development of radioresistance. Electronic supplementary material The online version of this article (10.1186/s12864-019-5970-0) contains supplementary material, which is available to authorized users.
Collapse
Affiliation(s)
- Ling Yang
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Xiaoyan Zhang
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Qiang Hou
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Ming Huang
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Hongfang Zhang
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Zhenzhen Jiang
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Jing Yue
- Hangzhou Cancer Institute, Hangzhou Cancer Hospital, Hangzhou, Zhejiang Province, 310002, People's Republic of China
| | - Shixiu Wu
- National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.113 Baohe Street Longgang District, Shenzhen, China.
| |
Collapse
|
|
19
|
Yadav P, Shankar BS. Radio resistance in breast cancer cells is mediated through TGF-β signalling, hybrid epithelial-mesenchymal phenotype and cancer stem cells. Biomed Pharmacother 2019; 111:119-130. [DOI: 10.1016/j.biopha.2018.12.055] [Citation(s) in RCA: 24] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/03/2018] [Revised: 12/11/2018] [Accepted: 12/14/2018] [Indexed: 12/20/2022] Open
|
|
20
|
Yu D, An X, Fan W, Wang X, He Y, Li B. PNUTS mediates ionizing radiation-induced CNE-2 nasopharyngeal carcinoma cell migration, invasion, and epithelial-mesenchymal transition via the PI3K/AKT signaling pathway. Onco Targets Ther 2019; 12:1205-1214. [PMID: 30863088 PMCID: PMC6388972 DOI: 10.2147/ott.s188571] [Citation(s) in RCA: 6] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/06/2023] Open
Abstract
Purpose Ionizing radiation (IR) is widely used for treating nasopharyngeal carcinoma (NPC). However, recent studies indicate that IR can also promote the migration and invasion of malignant tumors. Phosphatase 1 nuclear-targeting subunit (PNUTS), a novel interacting protein, was recently demonstrated to be involved in tumorigenesis and metastasis formation. This protein was hypothesized to take part in IR-induced migration and invasion in NPC cells in this study. Materials and methods Western blotting was used to detect how PNUTS was expressed in NPC cells with or without IR treatment. Wound-healing and Transwell assays were used to measure cell migration and invasion. Quantitative real-time PCR and Western blotting were used to determine the expression levels of PNUTS and epithelial–mesenchymal transition (EMT) proteins, respectively, after CNE-2 cells were infected with an adenovirus vector, ad-PNUTS, or transfected with PNUTS-specific siRNA. Finally, the expression levels of PI3K/AKT signaling-related proteins were detected by Western blotting. Results IR significantly promoted PNUTS expression and the migration and invasion in CNE-2 cells. Moreover, after exposure to IR, expression of the mesenchymal markers N-cadherin and vimentin increased, while that of the epithelial marker E-cadherin decreased. Silencing PNUTS remarkably attenuated IR-induced increases in cell migration and invasion and reversed the EMT process. Additionally, the overexpression of PNUTS restored the mobility and invasiveness of CNE-2 cells, which regained EMT characteristics. Furthermore, we found that PNUTS regulated IR-induced EMT via the PI3K/AKT signaling pathway. Conclusion Our research illustrates a relationship between PNUTS and IR-induced cell migration and invasion and provides a novel therapeutic target for preventing radiotherapy-induced metastasis in NPC patients.
Collapse
Affiliation(s)
- Dan Yu
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| | - Xiang An
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| | - Wanlin Fan
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| | - Xin Wang
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| | - Yuxing He
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| | - Bing Li
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chongqing Medical University, Chongqing 400016, China,
| |
Collapse
|
|
21
|
Yao K, Zhao YF, Zu HB. Melatonin receptor stimulation by agomelatine prevents Aβ-induced tau phosphorylation and oxidative damage in PC12 cells. DRUG DESIGN DEVELOPMENT AND THERAPY 2019; 13:387-396. [PMID: 30718944 PMCID: PMC6345325 DOI: 10.2147/dddt.s182684] [Citation(s) in RCA: 26] [Impact Index Per Article: 4.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Indexed: 12/12/2022]
Abstract
Purpose As a novel antidepressant drug, agomelatine has good therapeutic effect on the mood disorder and insomnia in Alzheimer's disease (AD). Recent studies have shown the neuroprotective function of agomelatine, including anti-oxidative and anti-apoptosis effect. However, it remains unclear whether agomelatine exerts neuroprotection in AD. Thus, the neuroprotective effect of agomelatine against amyloid beta 25-35 (Aβ25-35)-induced toxicity in PC12 cells was evaluated in this study. Methods The concentration of malondialdehyde (MDA), LDH, and ROS was investigated to evaluate oxidative damage. The expression of P-tau, tau, PTEN, P-Akt, Akt, P-GSK3β, and GSK3β proteins was assessed by Western blotting. Our results demonstrated that Aβ25-35 significantly increased the content of MDA, LDH, and ROS. Meanwhile, Aβ25-35 upregulated the expression of P-tau and PTEN as well as downregulated P-Akt and P-GSK3β expression. These effects could be blocked by agomelatine pretreatment. Furthermore, luzindole, the melatonin receptor (MT) antagonist, could reverse the neuroprotective effect of agomelatine. Conclusion The results demonstrated that antidepressant agomelatine might prevent the tau protein phosphorylation and oxidative damage induced by Aβ25-35 in PC12 cells by activating MT-PTEN/Akt/GSK3β signaling. This study provided a novel therapeutic target for AD in the future.
Collapse
Affiliation(s)
- Kai Yao
- Department of Neurology, Jinshan Hospital Affiliated to Fudan University, Shanghai 201508, China,
| | - Yong-Fei Zhao
- Department of Neurology, Jinshan Hospital Affiliated to Fudan University, Shanghai 201508, China,
| | - Heng-Bing Zu
- Department of Neurology, Jinshan Hospital Affiliated to Fudan University, Shanghai 201508, China,
| |
Collapse
|
|
22
|
Li J, Qi Z, Hu YP, Wang YX. Possible biomarkers for predicting lymph node metastasis of esophageal squamous cell carcinoma: a review. J Int Med Res 2019; 47:544-556. [PMID: 30616477 PMCID: PMC6381495 DOI: 10.1177/0300060518819606] [Citation(s) in RCA: 8] [Impact Index Per Article: 1.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 02/06/2023] Open
Abstract
Esophageal cancer is the eighth most common form of cancer worldwide, and esophageal squamous cell carcinoma (ESCC) is a major type of esophageal cancer that arises from epithelial cells of the esophagus. Local lymph node metastasis (LNM) is a typical sign of failure for ESCC clinical treatments, and a link has been established between LNM and the aberrant expression of specific biomarkers. In this review, we summarize what is known about nine factors significantly associated with LNM in ESCC patients: phosphatase and tensin homolog (PTEN), mucin 1, vascular endothelial growth factor-C, tumor necrosis factor alpha-induced protein 8 (TNFAIP8), Raf-1 kinase inhibitory protein, stathmin (STMN1), metastasis-associated protein 1, caveolin-1, and interferon-induced transmembrane protein 3. The function of these nine proteins involves four major mechanisms: tumor cell proliferation, tumor cell migration and invasion, epithelium–mesenchymal transition, and chemosensitivity. The roles of PTEN, STMN1, and TNFAIP8 involve at least two of these mechanisms, and we suggest that they are possible biomarkers for predicting LNM in ESCC. However, further retrospective research into PTEN, STMN1, and TNFAIP8 is needed to test their possibilities as indicators.
Collapse
Affiliation(s)
- Juan Li
- 1 Department of Radiotherapy, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, P. R. China
| | - Zhan Qi
- 2 Department of Thoracic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, P. R. China
| | - Yuan-Ping Hu
- 1 Department of Radiotherapy, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, P. R. China
| | - Yu-Xiang Wang
- 1 Department of Radiotherapy, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, P. R. China
| |
Collapse
|
|
23
|
Leith JT, Mousa SA, Hercbergs A, Lin HY, Davis PJ. Radioresistance of cancer cells, integrin αvβ3 and thyroid hormone. Oncotarget 2018; 9:37069-37075. [PMID: 30651936 PMCID: PMC6319341 DOI: 10.18632/oncotarget.26434] [Citation(s) in RCA: 18] [Impact Index Per Article: 2.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/30/2018] [Accepted: 11/16/2018] [Indexed: 02/06/2023] Open
Abstract
Radioresistance is a substantial barrier to success in cancer management. A number of molecular mechanisms support radioresistance. We have shown experimentally that the thyroid hormone analogue receptor on the extracellular domain of integrin αvβ3 may modulate the state of radiosensitivity of tumor cells. Specifically, tetraiodothyroacetic acid (tetrac), a derivative of L-thyroxine (T4), can reduce radioresistance in cancer cells. In this review, we list a number of intrinsic signal transduction molecules and other host factors that have been reported to support/induce radioresistance in cancer cells and that are also subject to control by T4 through actions primarily initiated at integrin αvβ3. Additional preclinical evidence is needed to support these radioresistance-relevant actions of thyroid hormone.
Collapse
Affiliation(s)
- John T Leith
- Rhode Island Nuclear Science Center, Narragansett, RI, USA
| | - Shaker A Mousa
- Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, Rensselaer, NY, USA
| | - Aleck Hercbergs
- Department of Radiation Oncology, Cleveland Clinic, Cleveland, OH, USA
| | - Hung-Yun Lin
- Taipei Cancer Center, Taipei Medical University, Taipei, Taiwan.,PhD Program for Cancer Molecular Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan.,Traditional Herbal Medicine Research Center, Taipei Medical University, Taipei, Taiwan.,TMU Research Center of Cancer Translational Medicine, Taipei Medical University, Taipei, Taiwan
| | - Paul J Davis
- Pharmaceutical Research Institute, Albany College of Pharmacy and Health Sciences, Rensselaer, NY, USA.,Department of Medicine, Albany Medical College, Albany, NY, USA
| |
Collapse
|
|
24
|
Nguemgo Kouam P, Rezniczek GA, Kochanneck A, Priesch-Grzeszkowiak B, Hero T, Adamietz IA, Bühler H. Robo1 and vimentin regulate radiation-induced motility of human glioblastoma cells. PLoS One 2018; 13:e0198508. [PMID: 29864155 PMCID: PMC5986140 DOI: 10.1371/journal.pone.0198508] [Citation(s) in RCA: 19] [Impact Index Per Article: 2.7] [Reference Citation Analysis] [Abstract] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/02/2017] [Accepted: 05/21/2018] [Indexed: 11/18/2022] Open
Abstract
Glioblastoma is a primary brain tumor with a poor prognosis despite of many treatment regimens. Radiotherapy significantly prolongs patient survival and remains the most common treatment. Slit2 and Robo1 are evolutionarily conserved proteins involved in axon guidance, migration, and branching of neuronal cells. New studies have shown that Slit2 and Robo1 could play important roles in leukocyte chemotaxis and glioblastoma cell migration. Therefore, we investigated whether the Slit2/Robo1 complex has an impact on the motility of glioblastoma cells and whether irradiation with therapeutic doses modulates this effect. Our results indicate that photon irradiation increases the migration of glioblastoma cells in vitro. qPCR and immunoblotting experiments in two different glioblastoma cell lines (U-373 MG and U-87 MG) with different malignancy revealed that both Slit2 and Robo1 are significantly lower expressed in the cell populations with the highest motility and that the expression was reduced after irradiation. Overexpression of Robo1 significantly decreased the motility of glioblastoma cells and inhibited the accelerated migration of wild-type cells after irradiation. Immunoblotting analysis of migration-associated proteins (fascin and focal adhesion kinase) and of the epithelial-mesenchymal-transition-related protein vimentin showed that irradiation affected the migration of glioblastoma cells by increasing vimentin expression, which can be reversed by the overexpression of Slit2 and Robo1. Our findings suggest that Robo1 expression might counteract migration and also radiation-induced migration of glioblastoma cells, a process that might be connected to mesenchymal-epithelial transition.
Collapse
Affiliation(s)
- Pascaline Nguemgo Kouam
- Institute for Molecular Oncology, Radio-Biology and Experimental Radiotherapy, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Günther A. Rezniczek
- Department of Obstetrics and Gynecology, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Anja Kochanneck
- Institute for Molecular Oncology, Radio-Biology and Experimental Radiotherapy, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Bettina Priesch-Grzeszkowiak
- Institute for Molecular Oncology, Radio-Biology and Experimental Radiotherapy, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Thomas Hero
- Department of Radiotherapy and Radio-Oncology, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Irenäus A. Adamietz
- Department of Radiotherapy and Radio-Oncology, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| | - Helmut Bühler
- Institute for Molecular Oncology, Radio-Biology and Experimental Radiotherapy, Ruhr-Universität Bochum, Medical Research Center, Marien Hospital Herne, Herne, Germany
| |
Collapse
|
|
25
|
Xiu G, Sui X, Wang Y, Zhang Z. FOXM1 regulates radiosensitivity of lung cancer cell partly by upregulating KIF20A. Eur J Pharmacol 2018; 833:79-85. [PMID: 29704495 DOI: 10.1016/j.ejphar.2018.04.021] [Citation(s) in RCA: 38] [Impact Index Per Article: 5.4] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/31/2018] [Revised: 04/17/2018] [Accepted: 04/20/2018] [Indexed: 12/25/2022]
Abstract
Forkhead box protein M1 (FOXM1), an important regulator of tumorigenesis in various human tumors, has recently been reported to play a role in the modulation of radiosensitivity in glioma and breast cancer cells. The present study aimed to investigate the effects of FOXM1 on radiotherapy resistance in human lung cancer and to explore the related molecular mechanisms. The results revealed that FOXM1 expression was upregulated in A549 and H1299 cells after IR (Ionizing radiation). FOXM1 inhibition impeded survival fractions, impeded proliferation, and triggered apoptosis after IR. Moreover, the silencing of FOXM1 dampened cell migration, invasion, and EMT (epithelial-mesenchyman transition) in A549 and H1299 cells treated by IR. In addition, KIF20A was also highly expressed in IR-treated A549 cells and downregulated by FOXM1 inhibition. Knockdown of KIF20A inhibited the survival fraction. Reintroduction of KIF20A partly reversed the effects of FOXM1 on the proliferation, apoptosis, and metastasis of A549 cells. Taken together, these results indicated that FOXM1 might enhance radioresistance partly through the induction of KIF20A expression.
Collapse
Affiliation(s)
- Guanghong Xiu
- No.1 Radiotherapy Department, Yantaishan Hospital, Yantai City, China.
| | - Xiujie Sui
- No.1 Radiotherapy Department, Yantaishan Hospital, Yantai City, China
| | - Yirong Wang
- No.1 Radiotherapy Department, Yantaishan Hospital, Yantai City, China
| | - Ze Zhang
- No.1 Radiotherapy Department, Yantaishan Hospital, Yantai City, China
| |
Collapse
|
|
26
|
Abstract
Radiotherapy remains one of the corner stones in the treatment of various malignancies and often leads to an improvement in overall survival. Nonetheless, pre-clinical evidence indicates that radiation can entail pro-metastatic effects via multiple pathways. Via direct actions on cancer cells and indirect actions on the tumor microenvironment, radiation has the potential to enhance epithelial-to-mesenchymal transition, invasion, migration, angiogenesis and metastasis. However, the data remains ambiguous and clinical observations that unequivocally prove these findings are lacking. In this review we discuss the pre-clinical and clinical data on the local and systemic effect of irradiation on the metastatic process with an emphasis on the molecular pathways involved.
Collapse
|
|
27
|
Jin Y, Xu K, Chen Q, Wang B, Pan J, Huang S, Wei Y, Ma H. Simvastatin inhibits the development of radioresistant esophageal cancer cells by increasing the radiosensitivity and reversing EMT process via the PTEN-PI3K/AKT pathway. Exp Cell Res 2017; 362:362-369. [PMID: 29208461 DOI: 10.1016/j.yexcr.2017.11.037] [Citation(s) in RCA: 40] [Impact Index Per Article: 5.0] [Reference Citation Analysis] [Abstract] [Key Words] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/08/2017] [Revised: 11/13/2017] [Accepted: 11/29/2017] [Indexed: 02/07/2023]
Abstract
Acquired radioresistance compromises the efficacy of radiotherapy for carcinomas including esophageal cancer (EC), thus resulting in recurrence and poor survival. Recent research corroborated radiosensitive function of simvastatin in stem-like breast cancer cells. However, its role in EC radioresistance remains poorly elucidated. Here, we developed a radioresistant EC cell line Ec9706-R with higher resistance to irradiation relative to control Ec9706 cells. Intriguingly, Ec9706-R cells exhibited epithelial-mesenchymal transition (EMT) characteristics with high invasion and migration ability. Simvastatin sensitized radioresistance of Ec9706-R cells and suppressed cell proliferation, but aggravated radiation-induced apoptosis and caspase-3 activity. Furthermore, simvastatin reversed EMT and inhibited cell invasion and migration of Ec9706-R cells. Mechanism assay confirmed the activation of PI3K/AKT pathway after radiation, which was inhibited by simvastatin. After restoring this pathway by its activator, IGF-1, simvastatin-mediated radiosensitivity and EMT reversion were abrogated. Further assay substantiated the PTEN suppression after irradiation, which was elevated following simvastatin pre-treatment. Moreover, PTEN cessation attenuated the inhibitory effect of simvastatin on PI3K/AKT activation, and subsequently antagonized simvastatin-induced radiosensitivity and EMT reversion. Additionally, simvastatin aggravated radiation-mediated Ec9706-R tumor growth inhibition. Together, simvastatin inhibits the development of Ec9706-R cells by increasing radiosensitivity and reversing EMT via PTEN-PI3K/AKT pathway, implying a promising strategy against EC radioresistance.
Collapse
Affiliation(s)
- Yingying Jin
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China.
| | - Kun Xu
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China
| | - Qingjuan Chen
- Department of Oncology, Xianyang Center Hospital, Xianyang 610041, Shaanxi Province, China
| | - Baofeng Wang
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China
| | - Jiyuan Pan
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China
| | - Shan Huang
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China
| | - Yang Wei
- Laboratory of Scientific Research Center, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China
| | - Hongbing Ma
- Department of Oncology, The Second Affiliated Hospital of Xi'an Jiaotong University, Xi'an 710004, Shaanxi Province, China.
| |
Collapse
|
|
28
|
Desai S, Barai A, Bukhari AB, De A, Sen S. α-Actinin-4 confers radioresistance coupled invasiveness in breast cancer cells through AKT pathway. BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR CELL RESEARCH 2017; 1865:196-208. [PMID: 29055790 DOI: 10.1016/j.bbamcr.2017.10.006] [Citation(s) in RCA: 20] [Impact Index Per Article: 2.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Subscribe] [Scholar Register] [Received: 04/21/2017] [Revised: 10/09/2017] [Accepted: 10/13/2017] [Indexed: 12/18/2022]
Abstract
Acquired radioresistance accompanied with increased metastatic potential is a major hurdle in effective radiotherapy of breast cancers. However, the nature of their inter-dependence and the underlying mechanism remains largely intangible. By employing radioresistant (RR) cell lines, we herein demonstrate that MCF-7 RR cells display phenotypic and molecular alterations evocative of epithelial to mesenchymal transition (EMT) with increased traction forces and membrane ruffling culminating in boosted invasiveness. We then show that these changes can be attributed to overexpression of alpha-actinin-4 (ACTN4), with ACTN4 knockdown near-completely abrogating both radioresistance and EMT-associated changes. We further found that in MCF-7 RR cells, ACTN4 mediates the observed effects by activating AKT, and downstream AKT/GSK3β signalling. Though ACTN4 plays a similar role in mediating radioresistance and invasiveness in MDA-MB-231 RR cells, co-immunoprecipitation studies reveal that these changes are effected through increased association with AKT and not by overexpression of AKT. Taken together, our study identifies ACTN4/AKT/GSK3β as a novel pathway regulating radioresistance coupled invasion which can be further explored to improve the radiotherapeutic gain.
Collapse
Affiliation(s)
- Sejal Desai
- Biosciences and Bioengineering Department, IIT Bombay, Mumbai, India
| | - Amlan Barai
- Biosciences and Bioengineering Department, IIT Bombay, Mumbai, India
| | | | - Abhijit De
- ACTREC, Tata Memorial Centre, Kharghar, Navi Mumbai, India.
| | - Shamik Sen
- Biosciences and Bioengineering Department, IIT Bombay, Mumbai, India.
| |
Collapse
|
|
29
|
Yuan DY, Meng Z, Xu K, Li QF, Chen C, Li KY, Zhang B. Betulinic acid increases radiosensitization of oral squamous cell carcinoma through inducing Sp1 sumoylation and PTEN expression. Oncol Rep 2017; 38:2360-2368. [DOI: 10.3892/or.2017.5872] [Citation(s) in RCA: 12] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/22/2016] [Accepted: 06/02/2017] [Indexed: 11/05/2022] Open
|
|
30
|
Zang C, Liu X, Li B, He Y, Jing S, He Y, Wu W, Zhang B, Ma S, Dai W, Li S, Peng Z. IL-6/STAT3/TWIST inhibition reverses ionizing radiation-induced EMT and radioresistance in esophageal squamous carcinoma. Oncotarget 2017; 8:11228-11238. [PMID: 28061440 PMCID: PMC5355260 DOI: 10.18632/oncotarget.14495] [Citation(s) in RCA: 57] [Impact Index Per Article: 7.1] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/25/2016] [Accepted: 12/27/2016] [Indexed: 01/06/2023] Open
Abstract
The acquisition of radioresistance by esophageal squamous carcinoma (ESC) cells during radiotherapy may lead to cancer recurrence and poor survival. Previous studies have demonstrated that ionizing radiation (IR) induces epithelial–mesenchymal transition (EMT) of ESC cells accompanied by increased migration, invasion, and radioresistance. However, the underlying molecular mechanisms of IR-induced EMT and radioresistance are not well established, hampering the development of potential solutions. To address this issue, we investigated the role of the IL-6/STAT3/TWIST signaling pathway in IR-induced EMT. We found not only the pathway was activated during IR-induced EMT but also STAT3 inhibition or Twist depletion reversed the EMT process and attenuated radioresistance. These results improve our understanding of the underlying mechanisms involved in IR-induced EMT and suggest potential interventions to prevent EMT-induced acquisition of radioresistance.
Collapse
Affiliation(s)
- Chunbao Zang
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Xujie Liu
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Bing Li
- Department of Otorhinolaryngology, The First Affiliated Hospital of Chonqqing Medical University, Chongqing, China
| | - Yanqiong He
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Shen Jing
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Yujia He
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Wenli Wu
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Bingqian Zhang
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Shuhong Ma
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Weiwei Dai
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Shaolin Li
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| | - Zhiping Peng
- Department of Radiological Medicine, Chongqing Medical University, Chonging, China
| |
Collapse
|
|
31
|
Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, Farina NH, Lian JB, Tomasello L, Liu CG, Palamarchuk A, Hart JR, Bell C, Carosi M, Pescarmona E, Perracchio L, Diodoro M, Russo A, Antenucci A, Visca P, Ciardi A, Harris CC, Vogt PK, Pekarsky Y, Croce CM. tsRNA signatures in cancer. Proc Natl Acad Sci U S A 2017; 114:8071-8076. [PMID: 28696308 PMCID: PMC5544330 DOI: 10.1073/pnas.1706908114] [Citation(s) in RCA: 213] [Impact Index Per Article: 26.6] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/29/2022] Open
Abstract
Small, noncoding RNAs are short untranslated RNA molecules, some of which have been associated with cancer development. Recently we showed that a class of small RNAs generated during the maturation process of tRNAs (tRNA-derived small RNAs, hereafter "tsRNAs") is dysregulated in cancer. Specifically, we uncovered tsRNA signatures in chronic lymphocytic leukemia and lung cancer and demonstrated that the ts-4521/3676 cluster (now called "ts-101" and "ts-53," respectively), ts-46, and ts-47 are down-regulated in these malignancies. Furthermore, we showed that tsRNAs are similar to Piwi-interacting RNAs (piRNAs) and demonstrated that ts-101 and ts-53 can associate with PiwiL2, a protein involved in the silencing of transposons. In this study, we extended our investigation on tsRNA signatures to samples collected from patients with colon, breast, or ovarian cancer and cell lines harboring specific oncogenic mutations and representing different stages of cancer progression. We detected tsRNA signatures in all patient samples and determined that tsRNA expression is altered upon oncogene activation and during cancer staging. In addition, we generated a knocked-out cell model for ts-101 and ts-46 in HEK-293 cells and found significant differences in gene-expression patterns, with activation of genes involved in cell survival and down-regulation of genes involved in apoptosis and chromatin structure. Finally, we overexpressed ts-46 and ts-47 in two lung cancer cell lines and performed a clonogenic assay to examine their role in cell proliferation. We observed a strong inhibition of colony formation in cells overexpressing these tsRNAs compared with untreated cells, confirming that tsRNAs affect cell growth and survival.
Collapse
Affiliation(s)
- Veronica Balatti
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | - Giovanni Nigita
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | - Dario Veneziano
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | - Alessandra Drusco
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | - Gary S Stein
- Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT 05405
- University of Vermont Cancer Center, College of Medicine, Burlington, VT 05405
| | - Terri L Messier
- Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT 05405
- University of Vermont Cancer Center, College of Medicine, Burlington, VT 05405
| | - Nicholas H Farina
- Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT 05405
- University of Vermont Cancer Center, College of Medicine, Burlington, VT 05405
| | - Jane B Lian
- Department of Biochemistry, University of Vermont College of Medicine, Burlington, VT 05405
- University of Vermont Cancer Center, College of Medicine, Burlington, VT 05405
| | - Luisa Tomasello
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | | | - Alexey Palamarchuk
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210
| | - Jonathan R Hart
- Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037
| | - Catherine Bell
- Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037
| | - Mariantonia Carosi
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Edoardo Pescarmona
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Letizia Perracchio
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Maria Diodoro
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Andrea Russo
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Anna Antenucci
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | - Paolo Visca
- Istituto di Ricovero e Cura a Carattere Scientifico, Regina Elena National Cancer Institute, 00144 Rome, Italy
| | | | - Curtis C Harris
- Laboratory of Human Carcinogenesis, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD 20892
| | - Peter K Vogt
- Department of Molecular Medicine, The Scripps Research Institute, La Jolla, CA 92037
| | - Yuri Pekarsky
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210;
| | - Carlo M Croce
- Department of Cancer Biology and Medical Genetics, The Ohio State University Comprehensive Cancer Center, Columbus, OH 43210;
| |
Collapse
|
|
32
|
Cancer stem cells with increased metastatic potential as a therapeutic target for esophageal cancer. Semin Cancer Biol 2017; 44:60-66. [DOI: 10.1016/j.semcancer.2017.03.010] [Citation(s) in RCA: 45] [Impact Index Per Article: 5.6] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/03/2017] [Revised: 03/12/2017] [Accepted: 03/15/2017] [Indexed: 02/07/2023]
|
|
33
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis 2017; 9:849-859. [PMID: 28449496 PMCID: PMC5394057 DOI: 10.21037/jtd.2017.03.23] [Citation(s) in RCA: 61] [Impact Index Per Article: 7.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/07/2016] [Accepted: 01/19/2017] [Indexed: 12/21/2022]
Abstract
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide. Surgery is the primary form of treatment, but the survival is poor, especially for patients with locally advanced esophageal cancer. Radiotherapy has been a critical treatment option that may be combined with chemotherapy in patients with unresectable esophageal cancer. However, resistance to chemoradiotherapy might result in treatment failures and cancer relapse. This review will mainly focus on the possible cellular mechanisms and tumor-associated microenvironmental (TAM) factors that result in radioresistance in patients with esophageal cancer. In addition, current strategies to increase radiosensitivity, including targeted therapy and the use of radiosensitive biomarkers in clinical treatment, are discussed in this review.
Collapse
Affiliation(s)
- Guang-Zong Chen
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Hong-Cheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Wang-Shu Dai
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xiao-Ning Zeng
- Department of Respiratory Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Jin-Hua Luo
- Department of Thoracic Surgery, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xin-Chen Sun
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| |
Collapse
|
|
34
|
Wang Y, Zhang C, Zhu H, Tang J, Zhang S, Luo J, Sun X. CD90 positive cells exhibit aggressive radioresistance in esophageal squamous cell carcinoma. J Thorac Dis 2017; 9:610-620. [PMID: 28449469 PMCID: PMC5394075 DOI: 10.21037/jtd.2017.03.28] [Citation(s) in RCA: 11] [Impact Index Per Article: 1.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/08/2016] [Accepted: 01/20/2017] [Indexed: 01/06/2023]
Abstract
BACKGROUND Cancer stem cells (CSCs) are widely abundant and considered to be an important factor in therapy resistance. They are also promising potential targets for conquering tumors. We explored the effects of radiation on stem cell-like tumor cells via the candidate marker CD90 to provide new ideas for the comprehensive treatment of esophageal squamous cell carcinoma (ESCC). METHODS We constructed CD90 overexpression ESCC cells by lentiviral transfection and observed differences of toxicity, proliferation, clone number, apoptosis, migration, invasion, and in vivo tumor formation after irradiation. RESULTS We found that the population of CD90 positive cells showed CSC-like characteristics, including increased tumorigenicity and migration in ESCC cells. We discovered that these capacities were strengthened to varying degrees in remaining cells after irradiation. Further exploration revealed that the genes of ETS-1 and its downstream target MMPs changed significantly, which are correlated with the epithelial mesenchymal transition (EMT). These effects lead to enhanced tumor growth and resistance to radiation. CONCLUSIONS We show that CD90 overexpressing ESCC cells exhibit CSC-like characteristics and radiation resistance. From a clinical perspective, ESCC patients with tumors that have high CD90 expression, which inhibits apoptosis, could exhibit more local invasion as well as distant metastasis, indicating a poorer prognosis. Research on the mechanism of CD90 may provide a new perspective to therapeutic strategies for patients with ESCC.
Collapse
Affiliation(s)
- Yuandong Wang
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Chi Zhang
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Hongcheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Junwei Tang
- Liver Transplantation Center, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Shu Zhang
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Jinhua Luo
- Department of Thoracic Surgery, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xinchen Sun
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| |
Collapse
|
|
35
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY, Kim CH, Park HG, Han SI, Kang HS. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer 2017; 16:10. [PMID: 28137309 PMCID: PMC5282724 DOI: 10.1186/s12943-016-0577-4] [Citation(s) in RCA: 388] [Impact Index Per Article: 48.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/04/2016] [Accepted: 12/25/2016] [Indexed: 12/12/2022] Open
Abstract
Radiation therapy is one of the major tools of cancer treatment, and is widely used for a variety of malignant tumours. Radiotherapy causes DNA damage directly by ionization or indirectly via the generation of reactive oxygen species (ROS), thereby destroying cancer cells. However, ionizing radiation (IR) paradoxically promotes metastasis and invasion of cancer cells by inducing the epithelial-mesenchymal transition (EMT). Metastasis is a major obstacle to successful cancer therapy, and is closely linked to the rates of morbidity and mortality of many cancers. ROS have been shown to play important roles in mediating the biological effects of IR. ROS have been implicated in IR-induced EMT, via activation of several EMT transcription factors—including Snail, HIF-1, ZEB1, and STAT3—that are activated by signalling pathways, including those of TGF-β, Wnt, Hedgehog, Notch, G-CSF, EGFR/PI3K/Akt, and MAPK. Cancer cells that undergo EMT have been shown to acquire stemness and undergo metabolic changes, although these points are debated. IR is known to induce cancer stem cell (CSC) properties, including dedifferentiation and self-renewal, and to promote oncogenic metabolism by activating these EMT-inducing pathways. Much accumulated evidence has shown that metabolic alterations in cancer cells are closely associated with the EMT and CSC phenotypes; specifically, the IR-induced oncogenic metabolism seems to be required for acquisition of the EMT and CSC phenotypes. IR can also elicit various changes in the tumour microenvironment (TME) that may affect invasion and metastasis. EMT, CSC, and oncogenic metabolism are involved in radioresistance; targeting them may improve the efficacy of radiotherapy, preventing tumour recurrence and metastasis. This study focuses on the molecular mechanisms of IR-induced EMT, CSCs, oncogenic metabolism, and alterations in the TME. We discuss how IR-induced EMT/CSC/oncogenic metabolism may promote resistance to radiotherapy; we also review efforts to develop therapeutic approaches to eliminate these IR-induced adverse effects.
Collapse
Affiliation(s)
- Su Yeon Lee
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea
| | - Eui Kyong Jeong
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea
| | - Min Kyung Ju
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea
| | - Hyun Min Jeon
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea
| | - Min Young Kim
- Research Center, Dongnam Institute of Radiological and Medical Science (DIRAMS), Pusan, 619-953, Korea
| | - Cho Hee Kim
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea.,DNA Identification Center, National Forensic Service, Seoul, 158-707, Korea
| | - Hye Gyeong Park
- Nanobiotechnology Center, Pusan National University, Pusan, 609-735, Korea
| | - Song Iy Han
- The Division of Natural Medical Sciences, College of Health Science, Chosun University, Gwangju, 501-759, Korea
| | - Ho Sung Kang
- Department of Molecular Biology, College of Natural Sciences, Pusan National University, Pusan, 609-735, Korea.
| |
Collapse
|
|
36
|
Nakayama A, Ninomiya I, Harada S, Tsukada T, Okamoto K, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Oyama K, Miyashita T, Tajima H, Takamura H, Fushida S, Ohta T. Metformin inhibits the radiation-induced invasive phenotype of esophageal squamous cell carcinoma. Int J Oncol 2016; 49:1890-1898. [PMID: 27599468 DOI: 10.3892/ijo.2016.3676] [Citation(s) in RCA: 15] [Impact Index Per Article: 1.7] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/15/2016] [Accepted: 08/04/2016] [Indexed: 11/06/2022] Open
Abstract
Esophageal cancer is one of the most aggressive tumor types because of its invasiveness and metastatic potential. Several reports have described an association between increased invasiveness after ionizing radiation (IR) treatment and epithelial-to-mesenchymal transition (EMT). The biguanide metformin is reported to prevent transforming growth factor-β (TGF-β)-induced EMT and proliferation of cancer. This study examined whether IR induces EMT and promotes the invasive potential of TE-9 esophageal squamous cell carcinoma cells and the effect of metformin on IR-induced EMT. After IR exposure, TE-9 cells showed a spindle-shaped morphology and lost cell-cell adhesion. Immunoblotting showed that IR induced expression of mesenchymal markers (vimentin and N-cadherin), transcription factors (Slug, Snail, and Twist), and matrix metalloproteinases. A scratch wound assay and Matrigel invasion assay showed that IR enhanced the invasive potential and migratory capacity of TE-9 cells. Expression of hypoxia-related factor-1α and TGF-β was increased after IR. IR also induced phosphorylation of Smad2 and Smad3. Metformin inhibited radiation-induced EMT-like morphological changes, and enhanced invasion and migration of TE-9 cells. Metformin inhibited IR-induced phosphorylation of Smad2 and Smad3. Although phosphorylation of AMP-activated protein kinase was enhanced by IR and metformin, phosphorylation of mammalian target of rapamycin was enhanced by IR and suppressed by metformin. These results indicated that metformin suppressed IR-induced EMT via suppression of the TGF-β-Smad phosphorylation pathway, and a part of the non-Smad pathway. Metformin might be useful to prevent IR-induced invasion and metastasis of esophageal squamous cell carcinoma.
Collapse
Affiliation(s)
- Akira Nakayama
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Itasu Ninomiya
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Shinichi Harada
- Center for Biomedical Research and Education, School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Tomoya Tsukada
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Koichi Okamoto
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Shinichi Nakanuma
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Seisho Sakai
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Isamu Makino
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Jun Kinoshita
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Hironori Hayashi
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Katsunobu Oyama
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Tomoharu Miyashita
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Hidehiro Tajima
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Hiroyuki Takamura
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Sachio Fushida
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| | - Tetsuo Ohta
- Department of Gastroenterological Surgery, Division of Cancer Medicine, Graduate School of Medical Science, Kanazawa University, Kanazawa 920-8641, Japan
| |
Collapse
|
|
37
|
Kanamoto A, Ninomiya I, Harada S, Tsukada T, Okamoto K, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi H, Oyama K, Miyashita T, Tajima H, Takamura H, Fushida S, Ohta T. Valproic acid inhibits irradiation-induced epithelial-mesenchymal transition and stem cell-like characteristics in esophageal squamous cell carcinoma. Int J Oncol 2016; 49:1859-1869. [PMID: 27826618 PMCID: PMC5063503 DOI: 10.3892/ijo.2016.3712] [Citation(s) in RCA: 22] [Impact Index Per Article: 2.4] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/19/2016] [Accepted: 08/10/2016] [Indexed: 12/14/2022] Open
Abstract
Esophageal carcinoma is one of the most aggressive malignancies, and is characterized by poor response to current therapy and a dismal survival rate. In this study we investigated whether irradiation induces epithelial-mesenchymal transition (EMT) in esophageal squamous cell carcinoma (ESCC) TE9 cells and whether the classic histone deacetylase (HDAC) inhibitor valproic acid (VPA) suppresses these changes. First, we showed that 2 Gy irradiation induced spindle cell-like morphologic changes, decreased expression of membranous E-cadherin, upregulated vimentin expression, and altered the localization of β-catenin from its usual membrane-bound location to cytoplasm in TE9 cells. Irradiation induced upregulation of transcription factors including Slug, Snail, and Twist, which regulate EMT. Stimulation by irradiation resulted in increased TGF-β1 and HIF-1α expression and induced Smad2 and Smad3 phosphorylation. Furthermore, irradiation enhanced CD44 expression, indicating acquisition of cancer stem-like cell properties. In addition, irradiation enhanced invasion and migration ability with upregulation of matrix metalloproteinases. These findings indicate that single-dose irradiation can induce EMT in ESCC cells. Second, we found that treatment with 1 mM VPA induced reversal of EMT caused by irradiation in TE9 cells, resulting in attenuated cell invasion and migration abilities. These results suggest that VPA might have clinical value to suppress irradiation-induced EMT. The reversal of EMT by HDAC inhibitors may be a new therapeutic strategy to improve the effectiveness of radiotherapy in ESCC by inhibiting the enhancement of invasion and metastasis.
Collapse
Affiliation(s)
- Ayako Kanamoto
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Itasu Ninomiya
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Shinichi Harada
- Center for Biomedical Research and Education, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Tomoya Tsukada
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Koichi Okamoto
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Shinichi Nakanuma
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Seisho Sakai
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Isamu Makino
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Jun Kinoshita
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Hironori Hayashi
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Katsunobu Oyama
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Tomoharu Miyashita
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Hidehiro Tajima
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Hiroyuki Takamura
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Sachio Fushida
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| | - Tetsuo Ohta
- Department of Gastroenterological Surgery, Graduate School of Medical Science, Kanazawa University, Kanazawa, Ishikawa 920-8641, Japan
| |
Collapse
|
|
38
|
Increased MALAT1 expression predicts poor prognosis in esophageal cancer patients. Biomed Pharmacother 2016; 83:8-13. [DOI: 10.1016/j.biopha.2016.05.044] [Citation(s) in RCA: 34] [Impact Index Per Article: 3.8] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/23/2015] [Revised: 05/25/2016] [Accepted: 05/26/2016] [Indexed: 01/06/2023] Open
|
|
39
|
Jeong H, Bok S, Hong BJ, Choi HS, Ahn GO. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res 2016; 51:157-163. [PMID: 27722125 PMCID: PMC5054246 DOI: 10.5045/br.2016.51.3.157] [Citation(s) in RCA: 45] [Impact Index Per Article: 5.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/31/2016] [Revised: 09/13/2016] [Accepted: 09/13/2016] [Indexed: 01/22/2023] Open
Abstract
Recent advancement in the radiotherapy technology has allowed conformal delivery of high doses of ionizing radiation precisely to the tumors while sparing large volume of the normal tissues, which have led to better clinical responses. Despite this technological advancement many advanced tumors often recur and they do so within the previously irradiated regions. How could tumors recur after receiving such high ablative doses of radiation? In this review, we outlined how radiation can elicit anti-tumor responses by introducing some of the cytokines that can be induced by ionizing radiation. We then discuss how tumor hypoxia, a major limiting factor responsible for failure of radiotherapy, may also negatively impact the anti-tumor responses. In addition, we highlight how there may be other populations of immune cells including regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) that can be recruited to tumors interfering with the anti-tumor immunity. Finally, the impact of irradiation on tumor hypoxia and the immune responses according to different radiotherapy regimen is also delineated. It is indeed an exciting time to see that radiotherapy is being combined with immunotherapy in the clinic and we hope that this review can add an excitement to the field.
Collapse
Affiliation(s)
- Hoibin Jeong
- Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang, Korea
| | - Seoyeon Bok
- Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang, Korea
| | - Beom-Ju Hong
- Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang, Korea
| | - Hyung-Seok Choi
- Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang, Korea
| | - G-One Ahn
- Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology (POSTECH), Pohang, Korea
| |
Collapse
|
|
40
|
Li F, Zhou K, Gao L, Zhang B, Li W, Yan W, Song X, Yu H, Wang S, Yu N, Jiang Q. Radiation induces the generation of cancer stem cells: A novel mechanism for cancer radioresistance. Oncol Lett 2016; 12:3059-3065. [PMID: 27899964 PMCID: PMC5103903 DOI: 10.3892/ol.2016.5124] [Citation(s) in RCA: 67] [Impact Index Per Article: 7.4] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/10/2015] [Accepted: 08/19/2016] [Indexed: 12/13/2022] Open
Abstract
Radioresistance remains a major obstacle for the radiotherapy treatment of cancer. Previous studies have demonstrated that the radioresistance of cancer is due to the existence of intrinsic cancer stem cells (CSCs), which represent a small, but radioresistant cell subpopulation that exist in heterogeneous tumors. By contrast, non-stem cancer cells are considered to be radiosensitive and thus, easy to kill. However, recent studies have revealed that under conditions of radiation-induced stress, theoretically radiosensitive non-stem cancer cells may undergo dedifferentiation subsequently obtaining the phenotypes and functions of CSCs, including high resistance to radiotherapy, which indicates that radiation may directly result in the generation of novel CSCs from non-stem cancer cells. These findings suggest that in addition to intrinsic CSCs, non-stem cancer cells may also contribute to the relapse and metastasis of cancer following transformation into CSCs. This review aims to investigate the radiation-induced generation of CSCs, its association with epithelial-mesenchymal transition and its significance with regard to the radioresistance of cancer.
Collapse
Affiliation(s)
- Fengsheng Li
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Kunming Zhou
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Ling Gao
- Key Laboratory of Radiological Protection and Nuclear Emergency, National Institute for Radiological Protection, China Center for Disease Control and Prevention, Beijing 100088, P.R. China
| | - Bin Zhang
- Department of Colorectal Disease Surgery, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Wei Li
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Weijuan Yan
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Xiujun Song
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Huijie Yu
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Sinian Wang
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Nan Yu
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| | - Qisheng Jiang
- Central Laboratories, The Second Artillery General Hospital, Beijing 100088, P.R. China
| |
Collapse
|
|
41
|
Histamine prevents radiation-induced mesenchymal changes in breast cancer cells. Pharmacol Res 2016; 111:731-739. [DOI: 10.1016/j.phrs.2016.07.039] [Citation(s) in RCA: 10] [Impact Index Per Article: 1.1] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 04/01/2016] [Revised: 07/09/2016] [Accepted: 07/25/2016] [Indexed: 11/19/2022]
|
|
42
|
Chen X, Zhang H, Zhu H, Yang X, Yang Y, Yang Y, Min H, Chen G, Liu J, Lu J, Cheng H, Sun X. Endostatin combined with radiotherapy suppresses vasculogenic mimicry formation through inhibition of epithelial-mesenchymal transition in esophageal cancer. Tumour Biol 2015; 37:4679-88. [PMID: 26511968 DOI: 10.1007/s13277-015-4284-3] [Citation(s) in RCA: 15] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/08/2015] [Accepted: 10/19/2015] [Indexed: 02/08/2023] Open
Abstract
The growth of solid tumors requires angiogenesis to provide oxygen and nutrients and to support cell proliferation. The switch from an avascular to a vascular phenotype is typically related to acceleration of tumor growth. Anti-angiogenic therapy is becoming a very promising way for malignant tumors. Meanwhile, malignant tumor cells themselves were able to develop the formation of cell-lined vessels that contribute to tumor neovascularization and supply the nutrients and oxygen, which is called vasculogenic mimicry (VM). However, the molecular mechanism of VM remains unclear. The purpose of this study was to investigate the efficacy of the novel recombinant human endostatin (rh-Endo) protein combined with radiotherapy on human esophageal squamous cell carcinoma (ESCC) cell lines Eca-109 and TE13. Our results showed that rh-Endo combined with radiotherapy significantly inhibited the proliferation, migration, invasion, and VM of human esophageal cancer cells in a dose-dependent manner; however, it has no direct effect on apoptosis of carcinoma cells, which indicated that rh-Endo combined with radiotherapy significantly changed the microenvironment of esophageal carcinoma, and played an important role in preventing distant metastasis. Our findings suggested that rh-Endo inhibited the metastasis of esophageal cancer and the activation of AKT pathway, and the down-regulation of epithelial-mesenchymal transition (EMT) may be associated with such effect of rh-Endo. These results also supported the bright prospect of rh-Endo combined with radiotherapy for clinical applications in the future.
Collapse
Affiliation(s)
- Xiaochen Chen
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Hao Zhang
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Hongcheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Xi Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Yuehua Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Yan Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Hua Min
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Guangzong Chen
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Jia Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Jing Lu
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Hongyan Cheng
- Department of General Internal Medicine, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China
| | - Xinchen Sun
- Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, 210029, China.
| |
Collapse
|
|
43
|
Qi HL, Li CS, Qian CW, Xiao YS, Yuan YF, Liu QY, Liu ZS. The long noncoding RNA, EGFR-AS1, a target of GHR, increases the expression of EGFR in hepatocellular carcinoma. Tumour Biol 2015; 37:1079-89. [PMID: 26271667 DOI: 10.1007/s13277-015-3887-z] [Citation(s) in RCA: 28] [Impact Index Per Article: 2.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/01/2015] [Accepted: 08/03/2015] [Indexed: 12/30/2022] Open
Abstract
LncRNA has provided an important new perspective regarding gene regulation. Both the expression and activation of EGFR have been proven to be under the tight control of the GHR pathway. EGFR-AS1 has been found to inhibit the expression of EGFR. GHR-siRNA and EGFR-AS1-siRNA were transfected into HCC cell lines, and a series of WB, q-PCR, and IF experiments was conducted to evaluate whether EGFR-AS1 participated in the regulation of GHR and EGFR. We found that impeded expression of GHR decreased the expression of EGFR and EGFR-AS1 in vivo and in vitro. Then, it was verified that EGFR and EGFR-AS1 were relatively upregulated in HCC tissue, and they were significantly related to some clinical characteristics and patient prognosis. Furthermore, EGFR-AS1 was determined to promote HCC development by improving the ability of invasion and proliferation of HCC cells in vitro, and it was also found to affect the cell cycle. Our study identified that EGFR-AS1 may promote HCC genesis and development. EGFR-AS1 may act as a prognostic factor in HCC. More importantly, we observed that the inhibition of EGFR-AS1 in HCC cells significantly impeded cell proliferation and invasion in vivo, which might provide a potential possibility for targeted therapy of HCC.
Collapse
Affiliation(s)
- Hao-Long Qi
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China.
| | - Chang-Sheng Li
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| | - Chong-Wei Qian
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| | - Yu-Sha Xiao
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| | - Yu-Feng Yuan
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| | - Quan-Yan Liu
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| | - Zhi-Su Liu
- Department of General Surgery, Research Center of Digestive Diseases, Zhongnan Hospital, Wuhan University, Wuhan, 430071, People's Republic of China
| |
Collapse
|
|
44
|
He E, Pan F, Li G, Li J. Correction: Fractionated Ionizing Radiation Promotes Epithelial-Mesenchymal Transition in Human Esophageal Cancer Cells through PTEN Deficiency-Mediated Akt Activation. PLoS One 2015; 10:e0133097. [PMID: 26173079 PMCID: PMC4501689 DOI: 10.1371/journal.pone.0133097] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.2] [Reference Citation Analysis] [Track Full Text] [Download PDF] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/18/2022] Open
|