Copyright
©The Author(s) 2015.
World J Gastrointest Pathophysiol. Nov 15, 2015; 6(4): 86-89
Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.86
Published online Nov 15, 2015. doi: 10.4291/wjgp.v6.i4.86
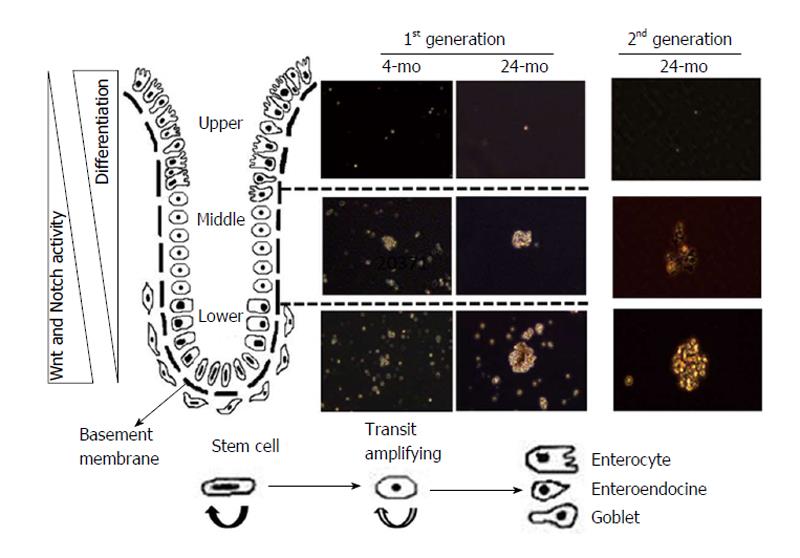
Figure 1 Changes in colonosphere forming potential of mucosal cells isolated from different regions of colonic crypt: Young (4-5 mo) and aged (22-24 mo) Fischer 344 rats were euthanatized by CO2 asphyxiation following an overnight fast.
The colon was removed, rinsed with cold PBS, everted, filled with a 5-10 mL protease solution [1 mg/mL collagenase 1 and 20 μg/mL hyaluronidase 1 in 0.05% Trypsin-EDTA (1X) with 2% BSA] and ligated at both ends. The colon was placed in 0.05% Trypsin-EDTA (1X) and incubated for 30 min at room temperature. To obtain the cells from the upper part of the colonic crypt, the colonic bag was transferred into 50 mL DMEM/F12 and incubated for 60 min at room temperature. For cells from the middle region of the crypt, the colonic bag was transferred into fresh 50 mL DMEM/F-12 and incubated at room temperature for another 45 min. Finally, the colonic bag was incubated further for 45 min at room temperature to obtain the cells from the lower part of the crypt. The dispersed mucosal cells were collected by centrifugation at 500 g for 5 min, washed with DMEM/F12, immediately suspended and cultured in serum-free stem cell medium containing DMEM/F12 (1:1) supplemented with B27, 20 ng/mL epidermal growth factor, 10 ng/mL fibroblast growth factor, 50 μg/mL gentamicin and antibiotic-anti-mycotic. First generation colonospheres were observed after 7 and 14 d. The colonospheres were collected, trypsinized and re-suspended in stem cell medium for formation of second generation colonospheres. PBS: Phosphate buffer saline; BSA: Bovine serum albumin.
- Citation: Nangia-Makker P, Yu Y, Majumdar AP. Role of cancer stem cells in age-related rise in colorectal cancer. World J Gastrointest Pathophysiol 2015; 6(4): 86-89
- URL: https://www.wjgnet.com/2150-5330/full/v6/i4/86.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v6.i4.86