Copyright
©2014 Baishideng Publishing Group Co.
World J Gastrointest Pathophysiol. Feb 15, 2014; 5(1): 18-32
Published online Feb 15, 2014. doi: 10.4291/wjgp.v5.i1.18
Published online Feb 15, 2014. doi: 10.4291/wjgp.v5.i1.18
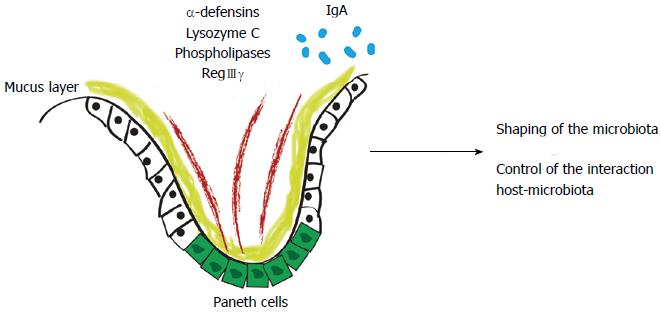
Figure 1 First line of defense of the intestinal barrier shapes the gut microbiota.
Antimicrobial peptides are produced by Paneth cells, such as alpha-defensins, lysozyme C, phospholipases and C-type lectin, primarily regenerating islet-derived 3-gamma (RegIIIγ) or by enterocytes (RegIIIγ). In the adaptive immunity scenario, system effectors are secreted into the intestinal lumen, restricting bacterial penetration into the host mucus and mucosal tissue.
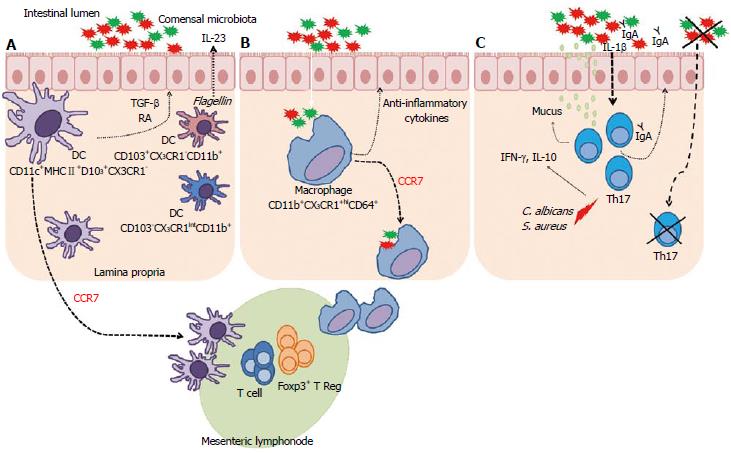
Figure 2 Complex interaction between microbiota, dendritic cells and macrophages.
A: CD11c+MHCII+D103+CX3CR1 cells migrate to the mesenteric lymph nodes (MLN) through a C-C chemokine receptor type 7 (CCR7) dependent mechanism. These dendritic cells (DCs) can generate and activate CD8+ T cells and Treg Foxp3+ cells. These DCs also have the ability to produce transforming growth factor-β (TGF-β) and retinoic acid (RA). CD103+CX3CR1-CD11b+ DCs can produce interleukin (IL)-23 in response to flagellin. Other DCs in lamina propria are CD103-CX3CR1intCD11b+; B: CD11b+CX3CR1hiCD64+ macrophages in the lamina propria contribute to the intestinal homeostasis through the production of anti-inflammatory cytokines and are able to recognize commensal microbiota beyond the epithelial barrier. These macrophages had previously been described as non-migratory were able to migrate into the MLNs, in a CCR7-dependent manner, carrying non-invasive bacteria captured in the intestinal lumen induces T lymphocyte responses; C: Under steady state, Th17 cells are usually found in the lamina propria of the small intestine, where its development depends on the presence of dietary antigens and commensal microbiota. These cells have important effects on the intestinal epithelium, improving the barrier function by stimulating mucin production, function of tight junction proteins, and increasing the transport of IgA to lumen. Candida albicans and Staphylococcus aureus induce Th17 cells, which produce interferon (IFN)-γ and IL-10. In the absence of microbiota, Th17 cells are not found. IL-1β, induced by commensal bacteria, is critical for differentiation of Th17 cells in the intestine.
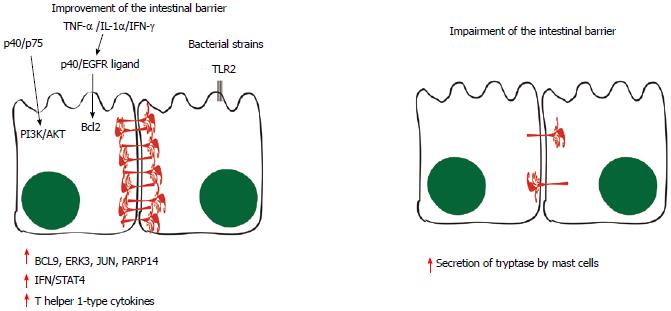
Figure 3 Pathways involved in the improvement and in the impairment of the intestinal barrier.
Some probiotic molecules seem to modulate changes in host cell signaling, such as the p40 and p75 proteins, which comodulate phosphoinositide 3-kinase (PI3K)/Akt signaling. When tumor necrosis factor-α (TNF-α), interleukin (IL)-1α and interferon (IFN)-γ are secreted, the p40 protein and unidentified epidermal growth factor receptor (EGFR) ligands stimulate the production of Bcl2, stabilizing tight junctions and promoting epithelial barrier function and cell survival. Toll-like receptor (TLR) and NOD-like receptor (NLR) signaling triggered by microbe-associated molecular patterns (MAMPs) are likely to have roles in the production of physical and chemical defenses in the small intestine, limiting numbers of mucosa-associated bacteria and preventing bacterial penetration of host tissues. Moreover, BCL-9, ERK3, JUN and poly(ADP-ribose) polymerase (PARP)14 have also been implicated in the signaling events induced by probiotics, leading to induction of IFN/STAT4 pathway activation and to the production of T helper 1-type cytokines. On the other hand, evidences suggest that the mast cell tryptase is involved in the degradation of the tight-junction proteins and increased permeability, since the infiltration and activation of these cells are increased in inflammatory bowel syndrom patients in association with higher output of tryptase from their mucosal biopsies. BCL9: B-cell lymphoma-9; JNK: c-Jun N-Terminal Protein Kinase; ERK: Extracellular signal-regulated kinase.
- Citation: Caricilli AM, Castoldi A, Câmara NOS. Intestinal barrier: A gentlemen’s agreement between microbiota and immunity. World J Gastrointest Pathophysiol 2014; 5(1): 18-32
- URL: https://www.wjgnet.com/2150-5330/full/v5/i1/18.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i1.18