|
1
|
Yang Z, Guan F, Bronk L, Zhao L. Multi-omics approaches for biomarker discovery in predicting the response of esophageal cancer to neoadjuvant therapy: A multidimensional perspective. Pharmacol Ther 2024; 254:108591. [PMID: 38286161 DOI: 10.1016/j.pharmthera.2024.108591] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/23/2023] [Revised: 12/02/2023] [Accepted: 01/04/2024] [Indexed: 01/31/2024]
Abstract
Neoadjuvant chemoradiotherapy (NCRT) followed by surgery has been established as the standard treatment strategy for operable locally advanced esophageal cancer (EC). However, achieving pathologic complete response (pCR) or near pCR to NCRT is significantly associated with a considerable improvement in survival outcomes, while pCR patients may help organ preservation for patients by active surveillance to avoid planned surgery. Thus, there is an urgent need for improved biomarkers to predict EC chemoradiation response in research and clinical settings. Advances in multiple high-throughput technologies such as next-generation sequencing have facilitated the discovery of novel predictive biomarkers, specifically based on multi-omics data, including genomic/transcriptomic sequencings and proteomic/metabolomic mass spectra. The application of multi-omics data has shown the benefits in improving the understanding of underlying mechanisms of NCRT sensitivity/resistance in EC. Particularly, the prominent development of artificial intelligence (AI) has introduced a new direction in cancer research. The integration of multi-omics data has significantly advanced our knowledge of the disease and enabled the identification of valuable biomarkers for predicting treatment response from diverse dimension levels, especially with rapid advances in biotechnological and AI methodologies. Herein, we summarize the current status of research on the use of multi-omics technologies in predicting NCRT response for EC patients. Current limitations, challenges, and future perspectives of these multi-omics platforms will be addressed to assist in experimental designs and clinical use for further integrated analysis.
Collapse
Affiliation(s)
- Zhi Yang
- Department of Radiation Oncology, Xijing Hospital, Fourth Military Medical University, 15 West Changle Road, Xi'an, China
| | - Fada Guan
- Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, CT 06510, United States of America
| | - Lawrence Bronk
- Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, United States of America
| | - Lina Zhao
- Department of Radiation Oncology, Xijing Hospital, Fourth Military Medical University, 15 West Changle Road, Xi'an, China.
| |
Collapse
|
|
2
|
Zhong C, Yang D, Zhong L, Xie W, Sun G, Jin D, Li Y. Single-cell and bulk RNA sequencing reveals Anoikis related genes to guide prognosis and immunotherapy in osteosarcoma. Sci Rep 2023; 13:20203. [PMID: 37980450 PMCID: PMC10657454 DOI: 10.1038/s41598-023-47367-3] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/18/2023] [Accepted: 11/13/2023] [Indexed: 11/20/2023] Open
Abstract
Anoikis resistance, a notable factor in osteosarcoma, plays a significant role in tumor invasion and metastasis. This study seeks to identify a distinct gene signature that is specifically associated with the anoikis subcluster in osteosarcoma. Clinical, single-cell, and transcriptional data from TARGET and GEO datasets were used to develop a gene signature for osteosarcoma based on the anoikis subcluster. Univariate Cox and LASSO regression analyses were employed. The signature's predictive value was evaluated using time-dependent ROC and Kaplan-Meier analyses. Functional enrichment analyses and drug sensitivity analyses were conducted. Validation of three modular genes was performed using RT-qPCR and Western blotting. Signature (ZNF583, CGNL1, CXCL13) was developed to predict overall survival in osteosarcoma patients, targeting the anoikis subcluster. The signature demonstrated good performance in external validation. Stratification based on the signature revealed significantly different prognoses. The signature was an independent prognostic factor. The low-risk group showed enhanced immune cell infiltration and improved immune function. Drug sensitivity analysis indicated efficacy of chemotherapy agents. Prognostic nomograms incorporating the signature provided greater predictive accuracy and clinical utility. Signatures related to the anoikis subcluster play a significant role in osteosarcoma progression. Incorporating these findings into clinical decision-making can improve osteosarcoma treatment and patient outcomes.
Collapse
Affiliation(s)
- Cheng Zhong
- Department of Orthopedics, The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, 515000, China
- Department of Orthopedics, Jiangmen Hospital of Traditional Chinese Medicine Affiliated to Jinan University, Jiangmen, 529000, China
| | - Dongliang Yang
- Department of Orthopedics, Tai Shan Hospital of Traditional Chinese Medicine Affiliated to Guangzhou University of Chinese Medicine, Jiangmen, 529000, China
| | - Liping Zhong
- Department of Cardiothoracic Surgery, Jiangmen Hospital of Traditional Chinese Medicine Affiliated to Jinan University, Jiangmen, 529000, China
| | - Weixing Xie
- Department of Orthopedics, The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, 515000, China
| | - Guodong Sun
- Department of Orthopedics, The First Affiliated Hospital of Jinan University, Guangzhou, 510630, China
| | - Daxiang Jin
- Department of Orthopedics, The First Clinical Medical College of Guangzhou University of Chinese Medicine, Guangzhou, 515000, China.
| | - Yuming Li
- Department of Orthopedics, Jiangmen Hospital of Traditional Chinese Medicine Affiliated to Jinan University, Jiangmen, 529000, China.
| |
Collapse
|
|
3
|
Wang XY, Beeraka NM, Xue NN, Yu HM, Yang Y, Liu MX, Nikolenko VN, Liu JQ, Zhao D. Identification of a three-gene prognostic signature for radioresistant esophageal squamous cell carcinoma. World J Clin Oncol 2023; 14:13-26. [PMID: 36699628 PMCID: PMC9850665 DOI: 10.5306/wjco.v14.i1.13] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 07/25/2022] [Revised: 10/25/2022] [Accepted: 12/06/2022] [Indexed: 01/10/2023] Open
Abstract
BACKGROUND Esophageal squamous cell carcinoma (ESCC) is causing a high mortality rate due to the lack of efficient early prognosis markers and suitable therapeutic regimens. The prognostic role of genes responsible for the acquisition of radioresistance in ESCC has not been fully elucidated.
AIM To establish a prognostic model by studying gene expression patterns pertinent to radioresistance in ESCC patients.
METHODS Datasets were obtained from the Gene Expression Omnibus and The Cancer Genome Atlas databases. The edgeR, a Bioconductor package, was used to analyze mRNA expression between different groups. We screened genes specifically responsible for radioresistance to estimate overall survival. Pearson correlation analysis was performed to confirm whether the expression of those genes correlated with each other. Genes contributing to radioresistance and overall survival were assessed by the multivariate Cox regression model through the calculation of βi and risk score using the following formula: 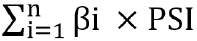 . .
RESULTS We identified three prognostic mRNAs (cathepsin S [CTSS], cluster of differentiation 180 [CD180], and SLP adapter and CSK-interacting membrane protein [SCIMP]) indicative of radioresistance. The expression of the three identified mRNAs was related to each other (r > 0.70 and P < 0.05). As to 1-year and 3-year overall survival prediction, the area under the time-dependent receiver operating characteristic curve of the signature consisting of the three mRNAs was 0.716 and 0.841, respectively. When stratifying patients based on the risk score derived from the signature, the high-risk group exhibited a higher death risk and shorter survival time than the low-risk group (P < 0.0001). Overall survival of the low-risk patients was significantly better than that of the high-risk patients (P = 0.018).
CONCLUSION We have developed a novel three-gene prognostic signature consisting of CTSS, CD180, and SCIMO for ESCC, which may facilitate the prediction of early prognosis of this malignancy.
Collapse
Affiliation(s)
- Xiao-Yan Wang
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Narasimha M Beeraka
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- Department of Pharmaceutical Chemistry, JSS College of Pharmacy, Mysuru 570015, India
| | - Nan-Nan Xue
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Hui-Ming Yu
- Department of Radiation Oncology, Peking University Cancer Hospital & Institute, Beijing 065005, China
| | - Ya Yang
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Mao-Xing Liu
- Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Department of Gastrointestinal Surgery IV, Peking University Cancer Hospital & Institute, Beijing, China
| | - Vladimir N Nikolenko
- Department of Human Anatomy, I. M. Sechenov First Moscow State Medical University, Moscow 119991, Russia
- M.V. Lomonosov Moscow State University, Moscow 119991, Russia
| | - Jun-Qi Liu
- Department of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| | - Di Zhao
- Department of Endocrinology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China
| |
Collapse
|
|
4
|
Wu P, Zhang Z, Yuan Y, Zhang C, Zhang G, Xue L, Yang H, Wang L, Zheng X, Zhang Y, Yuan Y, Lei R, Yang Z, Zheng B, Xue Q, Sun N, He J. A tumor immune microenvironment-related integrated signature can predict the pathological response and prognosis of esophageal squamous cell carcinoma following neoadjuvant chemoradiotherapy: A multicenter study in China. Int J Surg 2022; 107:106960. [PMID: 36257585 DOI: 10.1016/j.ijsu.2022.106960] [Citation(s) in RCA: 3] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/25/2022] [Revised: 09/25/2022] [Accepted: 10/11/2022] [Indexed: 12/24/2022]
Abstract
BACKGROUND Currently, there are insufficient indicators for the reliable assessment of treatment response following neoadjuvant chemoradiotherapy (nCRT) in patients with esophageal squamous cell carcinoma (ESCC). Considering the essential role of protein-coding and non-coding RNAs in gene regulation and cellular processes, we systematically explored the molecular features and clinical significance of mRNA and lncRNA in 249 pretreatment biopsies from four hospitals in three districts with a high incidence of ESCC patients in China. METHODS During the discovery phrase, 13 differentially expressed genes were identified and validated between samples with a complete pathological response (pCR) and those with an incomplete pathological response (<pCR). Subsequently, we constructed a predictive mRNA and lncRNA signature (SERPINE1, LINC00592, and PRKAG2-AS1) using Fisher's linear discriminant analysis (FLDA) with stepwise variant-selection, followed by validation of its predictive ability in both internal and external cohorts. RESULTS Our signature showed great value in predicting the response to nCRT among ESCC samples and acted as an independent predictive indicator, in addition to demonstrating great potential in estimating patient prognosis. Interestingly, we found that patients with a high signature score had lower PD-L1 and IDO1 expression levels but higher CD8+ T cells infiltration, suggesting that PD-L1 and IDO1 are negatively correlated with a high signature score and further associated with pCR and a better prognosis. CONCLUSION The present study identified a promising three-gene-based predictive signature that has powerful clinical implications for the identification of pCR and a good prognosis among patients with ESCC. Further immune-related exploration may provide an opportunity for future therapeutic combination.
Collapse
Affiliation(s)
- Peng Wu
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China Department of Pharmacology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, China Department of Pathology, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China Department of Pathology, Anyang Cancer Hospital, The Fourth Affiliated Hospital of Henan University of Science and Technology, Anyang, Henan, 455000, China Department of Otology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, 450052, China Department of Radiotherapy, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, 450008, China Department of General Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, Henan, 450008, China
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Collapse
|
|
5
|
Epithelial-Mesenchymal Transition Gene Signature Is Associated with Neoadjuvant Chemoradiotherapy Resistance and Prognosis of Esophageal Squamous Cell Carcinoma. DISEASE MARKERS 2022; 2022:3534433. [PMID: 36072903 PMCID: PMC9442501 DOI: 10.1155/2022/3534433] [Citation(s) in RCA: 6] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 05/28/2022] [Accepted: 08/02/2022] [Indexed: 11/28/2022]
Abstract
Background Neoadjuvant chemoradiotherapy (neo-CRT) in combination with surgery increases survival compared to surgery alone, as indicated by the esophageal squamous cell carcinoma (ESCC) treatment recommendations. However, the benefits of neo-CRT are diverse among patients. Consequently, the development of new biomarkers that correlate with neo-CRT might be important for the treatment of ESCC. Methods The differentially expressed genes (DEG) between responsive and resistant samples from the GSE45670 dataset were obtained. On the TCGA dataset, survival analysis was performed to identify prognosis-related-EMT-genes. For EMT score model construction, lasso regression analysis in the TCGA cohort was used to identify the genes. In the TCGA-ESCC cohort, age, stage, and EMT score were used to construct a nomogram. Results In total, 10 prognosis-related-EMT-genes were obtained. These 10 genes consisted of 6 risky genes and 4 protective genes. Based on the lasso analysis and univariate Cox regression, an EMT score model consisting of 7 genes (CLEC18A, PIR, KCNN4, MST1R, CAPG, ALDH5A1, and COX7B) was identified. ESCC patients with a high EMT score have a worse prognosis. These genes were differentially expressed between responsive and resistant patients and had a high accuracy for distinguishing resistant and responsive patients. Conclusions The identified genes have the potential to function as molecular biomarkers for predicting ESCC patients' resistance to neo-CRT. This research may aid in the elucidation of the molecular processes driving resistance and the identification of targets for improving the prognosis for ESCC.
Collapse
|
|
6
|
Li Y, Liu J, Cai XW, Li HX, Cheng Y, Dong XH, Yu W, Fu XL. Biomarkers for the prediction of esophageal cancer neoadjuvant chemoradiotherapy response: A systemic review. Crit Rev Oncol Hematol 2021; 167:103466. [PMID: 34508841 DOI: 10.1016/j.critrevonc.2021.103466] [Citation(s) in RCA: 6] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/10/2021] [Revised: 08/04/2021] [Accepted: 08/29/2021] [Indexed: 11/18/2022] Open
Abstract
Neoadjuvant chemoradiotherapy followed by surgery has been established as the standard treatment for locally advanced esophageal cancer. For patients with complete regression after neoadjuvant chemotherapy, active surveillance rather than planned surgery has been proposed as an organ preservation strategy. Reliable biomarkers to predict chemoradiation response is needed. We first summarized the previous reports of biomarkers with the potential to predict the treatment response of esophageal cancer neoadjuvant chemoradiotherapy. These traditional biomarkers are classified into three groups: genetic biomarkers, RNA biomarkers, and protein biomarkers. We then summarized some special types of biomarkers, including metabolites biomarkers, immune and tumor microenvironment biomarkers, and microbiome biomarkers.
Collapse
Affiliation(s)
- Yue Li
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China; Shanghai Jiao Tong University School of Medicine, Shanghai, China.
| | - Jun Liu
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
| | - Xu-Wei Cai
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
| | - Hong-Xuan Li
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
| | - Yan Cheng
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
| | - Xiao-Huan Dong
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China
| | - Wen Yu
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
| | - Xiao-Long Fu
- Department of Radiation Oncology, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai, China.
| |
Collapse
|
|
7
|
Klein S, Duda DG. Machine Learning for Future Subtyping of the Tumor Microenvironment of Gastro-Esophageal Adenocarcinomas. Cancers (Basel) 2021; 13:4919. [PMID: 34638408 PMCID: PMC8507866 DOI: 10.3390/cancers13194919] [Citation(s) in RCA: 4] [Impact Index Per Article: 1.0] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/22/2021] [Revised: 09/27/2021] [Accepted: 09/28/2021] [Indexed: 12/11/2022] Open
Abstract
Tumor progression involves an intricate interplay between malignant cells and their surrounding tumor microenvironment (TME) at specific sites. The TME is dynamic and is composed of stromal, parenchymal, and immune cells, which mediate cancer progression and therapy resistance. Evidence from preclinical and clinical studies revealed that TME targeting and reprogramming can be a promising approach to achieve anti-tumor effects in several cancers, including in GEA. Thus, it is of great interest to use modern technology to understand the relevant components of programming the TME. Here, we discuss the approach of machine learning, which recently gained increasing interest recently because of its ability to measure tumor parameters at the cellular level, reveal global features of relevance, and generate prognostic models. In this review, we discuss the relevant stromal composition of the TME in GEAs and discuss how they could be integrated. We also review the current progress in the application of machine learning in different medical disciplines that are relevant for the management and study of GEA.
Collapse
Affiliation(s)
- Sebastian Klein
- Gerhard-Domagk-Institute for Pathology, University Hospital Münster, 48149 Münster, Germany
- Institute for Pathology, Faculty of Medicine, University Hospital Cologne, University of Cologne, 50931 Cologne, Germany
| | - Dan G. Duda
- Edwin L. Steele Laboratories for Tumor Biology, Department of Radiation Oncology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02478, USA
| |
Collapse
|
|
8
|
Wang J, Yu P, Luo J, Sun Z, Yu J, Wang J. Transcriptomic and microRNA Expression Profiles Identify Biomarkers for Predicting Neo-Chemoradiotherapy Response in Esophageal Squamous Cell Carcinomas (ESCC). Front Pharmacol 2021; 12:626972. [PMID: 33935718 PMCID: PMC8082678 DOI: 10.3389/fphar.2021.626972] [Citation(s) in RCA: 8] [Impact Index Per Article: 2.0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/10/2020] [Accepted: 02/10/2021] [Indexed: 01/01/2023] Open
Abstract
Neo-chemoradiotherapy (nCRT) before surgery is a standard treatment for locally advanced esophageal cancers. However, the treatment outcome of nCRT varied with different patients. This study aimed to identify potential biomarkers for prediction of nCRT-response in patients with esophageal squamous cell carcinoma (ESCC). Microarray datasets of nCRT responder and non-responder samples (access number GSE45670 and GSE59974) of patients with ESCC were downloaded from Gene Expression Omnibus (GEO) database. The mRNA expression profiles of cancer biopsies from four ESCC patients were analyzed before and after nCRT. Differentially expressed genes (DEGs) and miRNAs were screened between nCRT responder and non-responder ESCC samples. Functional enrichment analysis was conducted for these DEGs followed by construction of protein-protein interaction (PPI) network and miRNA-mRNA regulatory network. Finally, univariate survival analysis was performed to identify candidate biomarkers with prognostic values in ESCC. We identified numerous DEGs and differentially expressed miRNAs from nCRT responder group. GO and KEGG analysis showed that the dysregulated genes were mainly involved in biological processes and pathways, including "response to stimulus", "cellular response to organic substance", "regulation of signal transduction", "AGE-RAGE signaling pathway in diabetic complications", and "steroid hormone biosynthesis". After integration of PPI network and miRNA-mRNA network analysis, we found eight genes, TNF, AKR1C1, AKR1C2, ICAM1, GPR68, GNB4, SERPINE1 and MMP12, could be candidate genes associated with disease progression. Univariate cox regression analysis showed that there was no significant correlation between dysregulated miRNAs (such as hsa-miR-34b-3p, hsa-miR-127-5p, hsa-miR-144-3p, and hsa-miR-486-5p, et al.) and overall survival of ESCC patients. Moreover, abnormal expression of MMP12 was significantly correlated with pathological degree, TNM stage, lymph nodes metastasis, and overall survival of ESCC patients (p < 0.05). Taken together, our study identified that MMP12 might be a useful tumor biomarker and therapeutic target for ESCC.
Collapse
Affiliation(s)
- Jian Wang
- Department of Radiotherapy, Jiangyin People's Hospital, Jiangyin, China
| | - Pengyi Yu
- Department of Cardiothoracic Surgery, The Third Affiliated Hospital of Soochow University, Jiangsu, China
| | - Judong Luo
- Department of Radiotherapy, The Affiliated Changzhou Second People's Hospital of Nanjing Medical University, Jiangsu, China
| | - Zhiqiang Sun
- Department of Radiotherapy, The Affiliated Changzhou Second People's Hospital of Nanjing Medical University, Jiangsu, China
| | - Jingping Yu
- Department of Radiotherapy, The Affiliated Changzhou Second People's Hospital of Nanjing Medical University, Jiangsu, China
| | - Jianlin Wang
- Department of Radiotherapy, The Affiliated Changzhou Second People's Hospital of Nanjing Medical University, Jiangsu, China
| |
Collapse
|
|
9
|
Edge SD, Renard I, Pyne E, Li C, Moody H, Roy R, Beavis AW, Archibald SJ, Cawthorne CJ, Maher SG, Pires IM. PI3K inhibition as a novel therapeutic strategy for neoadjuvant chemoradiotherapy resistant oesophageal adenocarcinoma. Br J Radiol 2021; 94:20201191. [PMID: 33434085 DOI: 10.1259/bjr.20201191] [Citation(s) in RCA: 1] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 02/06/2023] Open
Abstract
OBJECTIVE Neoadjuvant chemoradiotherapy (neo-CRT) prior to surgery is the standard of care for oesophageal adenocarcinoma (OAC) patients. Unfortunately, most patients fail to respond to treatment. MiR-187 was previously shown to be downregulated in neo-CRT non-responders, whist in vitro miR-187 overexpression enhanced radiosensitivity and upregulated PTEN. This study evaluates the role of miR-187 and downstream PI3K signalling in radiation response in OAC. METHODS The effect of miR-187 overexpression on downstream PI3K signalling was evaluated in OAC cell lines by qPCR and Western blotting. PTEN expression was analysed in OAC pre-treatment biopsies of neo-CRT responders and non-responders. Pharmacological inhibition of PI3K using GDC-0941 was evaluated in combination with radiotherapy in two-dimensional and three-dimensional OAC models in vitro and as a single agent in vivo. Radiation response in vitro was assessed via clonogenic assay. RESULTS PTEN expression was significantly decreased in neo-CRT non-responders. MiR-187 overexpression significantly upregulated PTEN expression and inhibited downstream PI3K signalling in vitro. GDC-0941 significantly reduced viability and enhanced radiation response in vitro and led to tumour growth inhibition as a single agent in vivo. CONCLUSION Targeting of PI3K signalling is a promising therapeutic strategy for OAC patients who have repressed miR-187 expression and do not respond to conventional neo-CRT. ADVANCES IN KNOWLEDGE This is the first study evaluating the effect of PI3K inhibition on radiosensitivity in OAC, with a particular focus on patients that do not respond to neo-CRT. We have shown for the first time that targeting of PI3K signalling is a promising alternative therapeutic strategy for OAC patients who do not respond to conventional neo-CRT.
Collapse
Affiliation(s)
- Sarah D Edge
- Hypoxia and Tumour Microenvironment Lab, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Hull, UK
| | - Isaline Renard
- Positron Emission Tomography Centre, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, UK, Hull, UK
| | - Emily Pyne
- Hypoxia and Tumour Microenvironment Lab, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Hull, UK
| | - Chun Li
- Hypoxia and Tumour Microenvironment Lab, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Hull, UK
| | - Hannah Moody
- Hypoxia and Tumour Microenvironment Lab, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Hull, UK.,Institute of Cancer Therapeutics, School of Medicine and Medical Sciences, University of Bradford, Bradford, United Kingdom
| | - Rajarshi Roy
- Queen's Centre for Oncology and Haematology, Castle Hill Hospital, Cottingham, UK
| | - Andrew W Beavis
- Faculty of Health and Well Being, Sheffield-Hallam University, Sheffield, UK.,Department of Medical Physics, Queen's Centre for Oncology, Hull University Teaching Hospitals NHS Trust, Cottingham, UK.,Faculty of Health Sciences, University of Hull, Hull, UK
| | - Stephen J Archibald
- Positron Emission Tomography Centre, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, UK, Hull, UK
| | - Christopher J Cawthorne
- Positron Emission Tomography Centre, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, UK, Hull, UK.,Nuclear Medicine and Molecular Imaging, Department of Imaging and Pathology, KU Leuven, Leuven, Belgium
| | - Stephen G Maher
- Department of Surgery, Trinity Translational Medicine Institute, Trinity College Dublin, St. James's Hospital, Dublin, Ireland
| | - Isabel M Pires
- Hypoxia and Tumour Microenvironment Lab, Department of Biomedical Sciences, Faculty of Health Sciences, University of Hull, Hull, UK
| |
Collapse
|
|
10
|
Lavery A, Turkington RC. Transcriptomic biomarkers for predicting response to neoadjuvant treatment in oesophageal cancer. Gastroenterol Rep (Oxf) 2020; 8:411-424. [PMID: 33442473 PMCID: PMC7793050 DOI: 10.1093/gastro/goaa065] [Citation(s) in RCA: 2] [Impact Index Per Article: 0.4] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 12/20/2019] [Revised: 04/21/2020] [Accepted: 07/15/2020] [Indexed: 02/07/2023] Open
Abstract
Oesophageal cancer is a devastating disease with poor outcomes and is the sixth leading cause of cancer death worldwide. In the setting of resectable disease, there is clear evidence that neoadjuvant chemotherapy and chemoradiotherapy result in improved survival. Disappointingly, only 15%-30% of patients obtain a histopathological response to neoadjuvant therapy, often at the expense of significant toxicity. There are no predictive biomarkers in routine clinical use in this setting and the ability to stratify patients for treatment could dramatically improve outcomes. In this review, we aim to outline current progress in evaluating predictive transcriptomic biomarkers for neoadjuvant therapy in oesophageal cancer and discuss the challenges facing biomarker development in this setting. We place these issues in the wider context of recommendations for biomarker development and reporting. The majority of studies focus on messenger RNA (mRNA) and microRNA (miRNA) biomarkers. These studies report a range of different genes involved in a wide variety of pathways and biological processes, and this is explained to a large extent by the different platforms and analysis methods used. Many studies are also vastly underpowered so are not suitable for identifying a candidate biomarker. Multiple molecular subtypes of oesophageal cancer have been proposed, although little is known about how these relate to clinical outcomes. We anticipate that the accumulating wealth of genomic and transcriptomic data and clinical trial collaborations in the coming years will provide unique opportunities to stratify patients in this poor-prognosis disease and recommend that future biomarker development incorporates well-designed retrospective and prospective analyses.
Collapse
Affiliation(s)
- Anita Lavery
- Patrick G Johnston Centre for Cancer Research, Queen’s University Belfast, Belfast, UK
| | - Richard C Turkington
- Patrick G Johnston Centre for Cancer Research, Queen’s University Belfast, Belfast, UK
| |
Collapse
|
|
11
|
Zhang C, Zhang G, Sun N, Zhang Z, Xue L, Zhang Z, Yang H, Luo Y, Zheng X, Zhang Y, Yuan Y, Lei R, Yang Z, Zheng B, Wang L, Che Y, Wang F, Wang S, Gao S, Xue Q, Zhang Y, He J. An individualized immune signature of pretreatment biopsies predicts pathological complete response to neoadjuvant chemoradiotherapy and outcomes in patients with esophageal squamous cell carcinoma. Signal Transduct Target Ther 2020; 5:182. [PMID: 32883946 PMCID: PMC7471268 DOI: 10.1038/s41392-020-00221-8] [Citation(s) in RCA: 21] [Impact Index Per Article: 4.2] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 03/30/2020] [Revised: 05/20/2020] [Accepted: 06/04/2020] [Indexed: 12/24/2022] Open
Abstract
No clinically available biomarkers can predict pathological complete response (pCR) for esophageal squamous cell carcinomas (ESCCs) with neoadjuvant chemoradiotherapy (nCRT). Considering that antitumor immunity status is an important determinant for nCRT, we performed an integrative analysis of immune-related gene profiles from pretreatment biopsies and constructed the first individualized immune signature for pCR and outcome prediction of ESCCs through a multicenter analysis. During the discovery phase, 14 differentially expressed immune-related genes (DEIGs) with greater than a twofold change between pCRs and less than pCRs (
Collapse
Affiliation(s)
- Chaoqi Zhang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Guochao Zhang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Nan Sun
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China.
| | - Zhen Zhang
- Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, Henan, China
| | - Liyan Xue
- Department of Pathology, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Zhihui Zhang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Haijun Yang
- Department of Pathology, Anyang Cancer Hospital, The Fourth Affiliated Hospital of Henan University of Science and Technology, Anyang, 455000, Henan, China
| | - Yuejun Luo
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Xiaoli Zheng
- Department of Radiotherapy, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, 450008, Henan, China
| | - Yonglei Zhang
- Department of General Surgery, The Affiliated Cancer Hospital of Zhengzhou University, Zhengzhou, 450008, Henan, China
| | - Yufen Yuan
- Department of Pathology, Anyang Cancer Hospital, The Fourth Affiliated Hospital of Henan University of Science and Technology, Anyang, 455000, Henan, China
| | - Ruixue Lei
- Department of Pathology, Anyang Cancer Hospital, The Fourth Affiliated Hospital of Henan University of Science and Technology, Anyang, 455000, Henan, China
| | - Zhaoyang Yang
- Department of Pathology, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Bo Zheng
- Department of Pathology, National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Le Wang
- Department of Otology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, Henan, China
| | - Yun Che
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Feng Wang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Sihui Wang
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Shugeng Gao
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Qi Xue
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China
| | - Yi Zhang
- Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, 450052, Henan, China.
| | - Jie He
- Department of Thoracic Surgery, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100021, China.
| |
Collapse
|
|
12
|
Zhang C, Zhang Z, Zhang G, Xue L, Yang H, Luo Y, Zheng X, Zhang Y, Yuan Y, Lei R, Yang Z, Zheng B, Zhang Z, Wang L, Che Y, Wang S, Wang F, Fang L, Zeng Q, Li J, Gao S, Xue Q, Sun N, He J. A three-lncRNA signature of pretreatment biopsies predicts pathological response and outcome in esophageal squamous cell carcinoma with neoadjuvant chemoradiotherapy. Clin Transl Med 2020; 10:e156. [PMID: 32898328 PMCID: PMC7448795 DOI: 10.1002/ctm2.156] [Citation(s) in RCA: 18] [Impact Index Per Article: 3.6] [Reference Citation Analysis] [Abstract] [Key Words] [Grants] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/02/2020] [Revised: 08/04/2020] [Accepted: 08/10/2020] [Indexed: 12/12/2022] Open
Abstract
BACKGROUND Current strategies are insufficient to predict pathologically complete response (pCR) for esophageal squamous cell carcinomas (ESCCs) before treatment. Here, we aim to develop a novel long noncoding RNA (lncRNA) signature for pCR and outcome prediction of ESCCs through a multicenter analysis for a Chinese population. METHODS Differentially expressed lncRNAs (DELs) between pCRs and less than pCR ( RESULTS Twelve DELs were identified from Guangzhou cohort and six lncRNAs were verified. Then, a classifier of three lncRNAs (SCAT1, PRKAG2-AS1, and FLG-AS1) was established and achieved a high accuracy with an area under the receiver operating characteristic curve (AUC) of 0.952 in the training cohort, which was well validated in the internal validation cohort and external cohort with the AUCs of 0.856 and 0.817, respectively. Furthermore, the predictive score was identified as the only independent predictor for pCR. Patients with high discriminant score showed a significantly longer overall and relapse-free survival (P < .05). CONCLUSIONS We developed the first and applicable three-lncRNA signature of pCR and outcome prediction, which is robust and reproducible in multicenter cohorts for ESCCs with nCRT.
Collapse
Affiliation(s)
- Chaoqi Zhang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Zhihui Zhang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Guochao Zhang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Liyan Xue
- Department of PathologyNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Haijun Yang
- Department of PathologyAnyang Cancer HospitalThe Fourth Affiliated Hospital of Henan University of Science and TechnologyAnyangHenanChina
| | - Yuejun Luo
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Xiaoli Zheng
- Department of radiotherapyThe Affiliated Cancer hospital of Zhengzhou UniversityZhengzhouHenanChina
| | - Yonglei Zhang
- Department of General SurgeryThe Affiliated Cancer Hospital of Zhengzhou UniversityZhengzhouHenanChina
| | - Yufen Yuan
- Department of PathologyAnyang Cancer HospitalThe Fourth Affiliated Hospital of Henan University of Science and TechnologyAnyangHenanChina
| | - Ruixue Lei
- Department of PathologyAnyang Cancer HospitalThe Fourth Affiliated Hospital of Henan University of Science and TechnologyAnyangHenanChina
| | - Zhaoyang Yang
- Department of PathologyNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Bo Zheng
- Department of PathologyNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Zhen Zhang
- Biotherapy CenterThe First Affiliated Hospital of Zhengzhou UniversityZhengzhouHenanChina
| | - Le Wang
- Department of OtologyThe First Affiliated Hospital of Zhengzhou UniversityZhengzhouHenanChina
| | - Yun Che
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Sihui Wang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Feng Wang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Lingling Fang
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Qingpeng Zeng
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Jiagen Li
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Shugeng Gao
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Qi Xue
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Nan Sun
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| | - Jie He
- Department of Thoracic SurgeryNational Cancer CenterNational Clinical Research Center for CancerCancer HospitalChinese Academy of Medical Sciences and Peking Union Medical CollegeBeijingChina
| |
Collapse
|
|
13
|
Chevrollier GS, Giugliano DN, Palazzo F, Keith SW, Rosato EL, Evans Iii NR, Berger AC. Patients with Non-response to Neoadjuvant Chemoradiation for Esophageal Cancer Have No Survival Advantage over Patients Undergoing Primary Esophagectomy. J Gastrointest Surg 2020; 24:288-298. [PMID: 30809782 DOI: 10.1007/s11605-019-04161-9] [Citation(s) in RCA: 9] [Impact Index Per Article: 1.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 08/15/2018] [Accepted: 02/05/2019] [Indexed: 01/31/2023]
Abstract
BACKGROUND Survival for patients with locally advanced esophageal cancer remains dismal. Non-response to neoadjuvant chemoradiation (nCRT) portends worse survival. We hypothesized that patients undergoing up-front esophagectomy may have better survival than those who do not respond to nCRT. METHODS We identified all patients undergoing esophagectomy with a pathologic stage of II or greater at our institution between 1994 and 2015 and separated them into two groups: those who received nCRT and those undergoing up-front esophagectomy. The neoadjuvant group was further separated into patients downstaged to pathologic stage 0 or I (responders) and patients with either the same or higher pathologic stage after nCRT, or with pathologic stage II disease or greater (non-responders). Overall survival was compared between groups using Kaplan-Meier statistics. Covariate-adjusted Cox modeling was used to estimate hazard ratios (HR) for mortality associated with non-response. RESULTS Overall, 287 patients met inclusion criteria. Fifty-nine percent of the responders had pathologic complete response (pCR). The majority of non-responders and primary esophagectomy patients had stage II or III disease (94%). Median survival was 58.3 months in responders, 23.9 months in non-responders, and 29.1 months in primary esophagectomy patients (p < 0.01). The HR for mortality associated with non-response was 1.82 compared to response to nCRT (p < 0.01) and 1.09 compared to primary esophagectomy (p = 0.71). CONCLUSIONS In patients with esophageal cancer who do not respond to nCRT, neoadjuvant therapy may represent a toxic and costly treatment modality that does not improve survival and may delay potentially curative resection. Further research is needed to identify potential non-responders with advanced resectable disease and allow individual tailoring of pre-surgical decision-making.
Collapse
Affiliation(s)
- Guillaume S Chevrollier
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA
| | - Danica N Giugliano
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA
| | - Francesco Palazzo
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA
| | - Scott W Keith
- Department of Pharmacology and Experimental Therapeutics, Sidney Kimmel Medical College of Thomas Jefferson University, 1015 Chestnut Street, Suite 520, Philadelphia, PA, 19107, USA
| | - Ernest L Rosato
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA
| | - Nathaniel R Evans Iii
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA
| | - Adam C Berger
- Department of Surgery, Sidney Kimmel Medical College of Thomas Jefferson University, 1100 Walnut Street, Suite 500, Philadelphia, PA, 19107, USA.
| |
Collapse
|
|
14
|
Yuan X, Piao L, Wang L, Han X, Zhuang M, Liu Z. Pivotal roles of protein 4.1B/DAL‑1, a FERM‑domain containing protein, in tumor progression (Review). Int J Oncol 2019; 55:979-987. [PMID: 31545421 DOI: 10.3892/ijo.2019.4877] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 05/09/2019] [Accepted: 09/04/2019] [Indexed: 11/06/2022] Open
Abstract
Protein 4.1B/DAL‑1, encoded by erythrocyte membrane protein band 4.1‑like 3 (EPB41L3), belongs to the protein 4.1 superfamily, a group of proteins that share a conserved four.one‑ezrin‑radixin‑moesin (FERM) domain. Protein 4.1B/DAL‑1 serves a crucial role in cytoskeletal organization and a number of processes through multiple interactions with membrane proteins via its FERM, spectrin‑actin‑binding and C‑terminal domains. A number of studies have indicated that a loss of EPB41L3 expression is commonly observed in lung cancer, breast cancer, esophageal squamous cell carcinoma and meningiomas. DNA methylation and a loss of heterozygosity have been reported to contribute to the downregulation of EPB41L3. To date, the biological functions of protein 4.1B/DAL‑1 in carcinogenesis remain unknown. The present review summarizes the current understanding of the role of protein 4.1B/DAL‑1 in cancer and highlights its potential as a cancer diagnostic and prognostic biomarker in cancer therapeutics.
Collapse
Affiliation(s)
- Xiaofeng Yuan
- Department of Orthopaedics, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, P.R. China
| | - Lianhua Piao
- Institute of Bioinformatics and Medical Engineering, Jiangsu University of Technology, Changzhou, Jiangsu 213001, P.R. China
| | - Luhui Wang
- Department of Orthopaedics, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, P.R. China
| | - Xu Han
- Department of Urology, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, P.R. China
| | - Ming Zhuang
- Department of Orthopaedics, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, P.R. China
| | - Zhiwei Liu
- Department of Orthopaedics, The Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu 213000, P.R. China
| |
Collapse
|
|
15
|
Kosovec JE, Zaidi AH, Pounardjian TS, Jobe BA. The Potential Clinical Utility of Circulating Tumor DNA in Esophageal Adenocarcinoma: From Early Detection to Therapy. Front Oncol 2018; 8:610. [PMID: 30619750 PMCID: PMC6297385 DOI: 10.3389/fonc.2018.00610] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Subscribe] [Scholar Register] [Received: 09/04/2018] [Accepted: 11/28/2018] [Indexed: 12/11/2022] Open
Abstract
Esophageal adenocarcinoma (EAC) is a lethal cancer requiring improved screening strategies and treatment options due to poor detection methods, aggressive progression, and therapeutic resistance. Emerging circulating tumor DNA (ctDNA) technologies may offer a unique non-invasive strategy to better characterize the highly heterogeneous cancer and more clearly establish the genetic modulations leading to disease progression. The presented review describes the potential advantages of ctDNA methodologies as compared to current clinical strategies to improve clinical detection, enhance disease surveillance, evaluate prognosis, and personalize treatment. Specifically, we describe the ctDNA-targetable genetic markers of prognostic significance to stratify patients into risk of progression from benign to malignant disease and potentially offer cost-effective screening of established cancer. We also describe the application of ctDNA to more effectively characterize the heterogeneity and particular mutagenic resistance mechanisms in real-time to improve prognosis and therapeutic monitoring strategies. Lastly, we discuss the inconsistent clinical responses to currently approved therapies for EAC and the role of ctDNA to explore the dynamic regulation of novel targeted and immunotherapies to personalize therapy and improve patient outcomes. Although there are clear limitations of ctDNA technologies for immediate clinical deployment, this review presents the prospective role of such applications to potentially overcome many of the notable hurdles to treating EAC patients. A deeper understanding of complex EAC tumor biology may result in the progress toward improved clinical outcomes.
Collapse
Affiliation(s)
- Juliann E Kosovec
- Esophageal and Lung Institute, Allegheny Health Network, Pittsburgh, PA, United States
| | - Ali H Zaidi
- Esophageal and Lung Institute, Allegheny Health Network, Pittsburgh, PA, United States
| | - Tamar S Pounardjian
- Esophageal and Lung Institute, Allegheny Health Network, Pittsburgh, PA, United States
| | - Blair A Jobe
- Esophageal and Lung Institute, Allegheny Health Network, Pittsburgh, PA, United States
| |
Collapse
|
|
16
|
Sollinger HW, Al-Qaoud T, Bath N, Redfield RR. The "UW-LPHS Test": A New Test to Predict the Outcome of Renal Autotransplant for Loin Pain Hematuria Syndrome. EXP CLIN TRANSPLANT 2018; 16:651-655. [PMID: 30251941 DOI: 10.6002/ect.2018.0236] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.6] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 01/05/2023]
Abstract
OBJECTIVES The objectives of this pilot study were twofold. First, we aimed to elicit whether the "UW-LPHS test" definitively localizes pain from patients' loin pain hematuria syndrome to the ureter and thus proves our hypothesis. Second, we aimed to understand whether a positive UW-LPHS test predicts a successful outcome after renal autotransplant. MATERIALS AND METHODS The UW-LPHS test is described in detail in this manuscript. Briefly, 0.5% bupivacaine is injected into the ureter of the affected side and kept there using a balloon catheter for 5 minutes. RESULTS All six patients studied had complete pain relief at a mean follow-up of 9.2 months after renal autotransplant. All patients were successfully weaned from opioids and have returned to a normal lifestyle. CONCLUSIONS The UW-LPHS test can be used to predict renal autotransplant outcomes and should be applied to all patients who are being considered for this operation.
Collapse
Affiliation(s)
- Hans W Sollinger
- From the Division of Transplantation, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA
| | | | | | | |
Collapse
|
|
17
|
Li L, Yue GGL, Lee JKM, Wong ECW, Fung KP, Yu J, Lau CBS, Chiu PWY. Gene expression profiling reveals the plausible mechanisms underlying the antitumor and antimetastasis effects of Andrographis paniculata in esophageal cancer. Phytother Res 2018; 32:1388-1396. [PMID: 29577460 DOI: 10.1002/ptr.6074] [Citation(s) in RCA: 13] [Impact Index Per Article: 1.9] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/11/2017] [Revised: 02/01/2018] [Accepted: 02/12/2018] [Indexed: 12/28/2022]
Abstract
Esophageal cancer (EC) is a seriously invasive malignancy with high mortality and poor prognosis. Metastasis of EC is the major cause of mortality. Our studies previously demonstrated that a herbal medicine Andrographis paniculata (AP) significantly suppressed EC growth and metastasis in vitro and in vivo. However, the underlying mechanisms responsible for these effects have not yet been systematically elucidated. In this context, gene expression profiling of AP-treated squamous EC cells (EC-109) was performed to reveal the regulatory mechanisms of AP in antitumor and antimetastasis signaling pathways using gene expression microarray analysis. Differentially expressed genes were identified by Affymetrix Gene Chip, followed by the real-time polymerase chain reaction validation. The results showed that the canonical pathways were significantly regulated by AP treatment, including multiple genes related to proliferation, apoptosis, intercellular adhesion, metastatic processes, and drug resistance, such as WNT, TGF-β, MAPK and ErbB signaling pathways, and ATP-binding cassette transporter subfamily members. This genomic study emerges candidate molecular targets and pathways to reveal the mechanisms involved in AP's effects, which provides scientific evidence to support the clinical application of AP in EC treatment.
Collapse
Affiliation(s)
- Lin Li
- Department of Surgery, Prince of Wales Hospital, Shatin, New Territories, Hong Kong
| | - Grace Gar-Lee Yue
- Institute of Chinese Medicine and State Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK), Shatin, New Territories, Hong Kong
| | - Julia Kin-Ming Lee
- Institute of Chinese Medicine and State Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK), Shatin, New Territories, Hong Kong
| | - Eric Chun-Wai Wong
- Institute of Chinese Medicine and State Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK), Shatin, New Territories, Hong Kong
| | - Kwok-Pui Fung
- Institute of Chinese Medicine and State Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK), Shatin, New Territories, Hong Kong.,School of Biomedical Sciences, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
| | - Jun Yu
- Department of Medicine and Therapeutics and State Key Laboratory of Digestive Disease, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong
| | - Clara Bik-San Lau
- Institute of Chinese Medicine and State Key Laboratory of Phytochemistry and Plant Resources in West China (CUHK), Shatin, New Territories, Hong Kong
| | - Philip Wai-Yan Chiu
- Department of Surgery, Prince of Wales Hospital, Shatin, New Territories, Hong Kong
| |
Collapse
|
|
18
|
Visser E, Franken IA, Brosens LAA, Ruurda JP, van Hillegersberg R. Prognostic gene expression profiling in esophageal cancer: a systematic review. Oncotarget 2018; 8:5566-5577. [PMID: 27852047 PMCID: PMC5354930 DOI: 10.18632/oncotarget.13328] [Citation(s) in RCA: 33] [Impact Index Per Article: 4.7] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/06/2016] [Accepted: 10/13/2016] [Indexed: 12/18/2022] Open
Abstract
Background Individual variability in prognosis of esophageal cancer highlights the need for advances in personalized therapy. This systematic review aimed at elucidating the prognostic role of gene expression profiles and at identifying gene signatures to predict clinical outcome. Methods A systematic search of the Medline, Embase and the Cochrane library databases (2000-2015) was performed. Articles associating gene expression profiles in patients with esophageal adenocarcinoma or squamous cell carcinoma to survival, response to chemo(radio)therapy and/or lymph node metastasis were identified. Differentially expressed genes and gene signatures were extracted from each study and combined to construct a list of prognostic genes per outcome and histological tumor type. Results This review includes a total of 22 studies. Gene expression profiles were related to survival in 9 studies, to response to chemo(radio)therapy in 7 studies, and to lymph node metastasis in 9 studies. The studies proposed many differentially expressed genes. However, the findings were heterogeneous and only 12 (ALDH1A3, ATR, BIN1, CSPG2, DOK1, IFIT1, IFIT3, MAL, PCP4, PHB, SPP1) of the 1.112 reported genes were identified in more than 1 study. Overall, 16 studies reported a prognostic gene signature, which was externally validated in 10 studies. Conclusion This systematic review shows heterogeneous findings in associating gene expression with clinical outcome in esophageal cancer. Larger validated studies employing RNA next-generation sequencing are required to establish gene expression profiles to predict clinical outcome and to select optimal personalized therapy.
Collapse
Affiliation(s)
- Els Visser
- Department of Surgery, University Medical Center Utrecht, The Netherlands
| | - Ingrid A Franken
- Department of Surgery, University Medical Center Utrecht, The Netherlands
| | | | - Jelle P Ruurda
- Department of Surgery, University Medical Center Utrecht, The Netherlands
| | | |
Collapse
|
|
19
|
Lynam-Lennon N, Heavey S, Sommerville G, Bibby BAS, Ffrench B, Quinn J, Gasch C, O'Leary JJ, Gallagher MF, Reynolds JV, Maher SG. MicroRNA-17 is downregulated in esophageal adenocarcinoma cancer stem-like cells and promotes a radioresistant phenotype. Oncotarget 2017; 8:11400-11413. [PMID: 28002789 PMCID: PMC5355274 DOI: 10.18632/oncotarget.13940] [Citation(s) in RCA: 33] [Impact Index Per Article: 4.1] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/28/2016] [Accepted: 11/21/2016] [Indexed: 02/06/2023] Open
Abstract
Resistance to neoadjuvant chemoradiation therapy (CRT) remains a critical barrier to the effective treatment of esophageal adenocarcinoma (EAC). Cancer stem-like cells (CSCs) are a distinct subpopulation of cells implicated in the resistance of tumors to anti-cancer therapy. However, their role in the resistance of EAC to CRT is largely unknown. In this study, using a novel in vitro isogenic model of radioresistant EAC, we demonstrate that radioresistant EAC cells have enhanced tumorigenicity in vivo, increased expression of CSC-associated markers and enhanced holoclone forming ability. Further investigation identified a subpopulation of cells that are characterised by high aldehyde dehydrogenase (ALDH) activity, enhanced radioresistance and decreased expression of miR-17-5p. In vitro, miR-17-5p was demonstrated to significantly sensitise radioresistant cells to X-ray radiation and promoted the repression of genes with miR-17-5p binding sites, such as C6orf120. In vivo, miR-17-5p was significantly decreased, whilst C6orf120 was significantly increased, in pre-treatment EAC tumour samples from patients who demonstrated a poor response to neoadjuvant CRT. This study sheds novel insights into the role of CSCs in the resistance of EAC to CRT and highlights miR-17-5p as a potential biomarker of CRT sensitivity and novel therapeutic target in treatment resistant EAC.
Collapse
Affiliation(s)
- Niamh Lynam-Lennon
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland
| | - Susan Heavey
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland
| | - Gary Sommerville
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland
| | - Becky A S Bibby
- Cancer Biology and Therapeutics Lab, School of Life Sciences, University of Hull, Hull, United Kingdom
| | - Brendan Ffrench
- Department of Histopathology, Trinity College Dublin, Sir Patrick Dun Laboratory, Central Pathology Laboratory, St James's Hospital, Dublin 8, Ireland.,Molecular Pathology Laboratory, Coombe Women and Infant's University Hospital, Dublin 8, Ireland
| | - Jennifer Quinn
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland
| | - Claudia Gasch
- Department of Histopathology, Trinity College Dublin, Sir Patrick Dun Laboratory, Central Pathology Laboratory, St James's Hospital, Dublin 8, Ireland.,Molecular Pathology Laboratory, Coombe Women and Infant's University Hospital, Dublin 8, Ireland
| | - John J O'Leary
- Department of Histopathology, Trinity College Dublin, Sir Patrick Dun Laboratory, Central Pathology Laboratory, St James's Hospital, Dublin 8, Ireland.,Molecular Pathology Laboratory, Coombe Women and Infant's University Hospital, Dublin 8, Ireland
| | - Michael F Gallagher
- Department of Histopathology, Trinity College Dublin, Sir Patrick Dun Laboratory, Central Pathology Laboratory, St James's Hospital, Dublin 8, Ireland.,Molecular Pathology Laboratory, Coombe Women and Infant's University Hospital, Dublin 8, Ireland
| | - John V Reynolds
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland
| | - Stephen G Maher
- Trinity Translational Medicine Institute, Department of Surgery, Trinity College Dublin, St James's Hospital, Dublin 8, Ireland.,Cancer Biology and Therapeutics Lab, School of Life Sciences, University of Hull, Hull, United Kingdom
| |
Collapse
|
|
20
|
Testa U, Castelli G, Pelosi E. Esophageal Cancer: Genomic and Molecular Characterization, Stem Cell Compartment and Clonal Evolution. MEDICINES (BASEL, SWITZERLAND) 2017; 4:E67. [PMID: 28930282 PMCID: PMC5622402 DOI: 10.3390/medicines4030067] [Citation(s) in RCA: 61] [Impact Index Per Article: 7.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Received: 08/23/2017] [Revised: 09/05/2017] [Accepted: 09/07/2017] [Indexed: 12/20/2022]
Abstract
Esophageal cancer (EC) is the eighth most common cancer and is the sixth leading cause of death worldwide. The incidence of histologic subtypes of EC, esophageal adenocarcinoma (EAC) and esophageal squamous carcinoma (ESCC), display considerable geographic variation. EAC arises from metaplastic Barrett's esophagus (BE) in the context of chronic inflammation secondary to exposure to acid and bile. The main risk factors for developing ESCC are cigarette smoking and alcohol consumption. The main somatic genetic abnormalities showed a different genetic landscape in EAC compared to ESCC. EAC is a heterogeneous cancer dominated by copy number alterations, a high mutational burden, co-amplification of receptor tyrosine kinase, frequent TP53 mutations. The cellular origins of BE and EAC are still not understood: animal models supported a cellular origin either from stem cells located in the basal layer of esophageal epithelium or from progenitors present in the cardia region. Many studies support the existence of cancer stem cells (CSCs) able to initiate and maintain EAC or ESCC. The exact identification of these CSCs, as well as their role in the pathogenesis of EAC and ESCC remain still to be demonstrated. The reviewed studies suggest that current molecular and cellular characterization of EAC and ESCC should serve as background for development of new treatment strategies.
Collapse
Affiliation(s)
- Ugo Testa
- Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, 00141 Rome, Italy.
| | - Germana Castelli
- Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, 00141 Rome, Italy.
| | - Elvira Pelosi
- Department of Hematology, Oncology and Molecular Medicine, Istituto Superiore di Sanità, 00141 Rome, Italy.
| |
Collapse
|
|
21
|
Gusella M, Pezzolo E, Modena Y, Barile C, Menon D, Crepaldi G, La Russa F, Fraccon AP, Pasini F. Predictive genetic markers in neoadjuvant chemoradiotherapy for locally advanced esophageal cancer: a long way to go. Review of the literature. THE PHARMACOGENOMICS JOURNAL 2017; 18:14-22. [PMID: 28607505 DOI: 10.1038/tpj.2017.25] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Track Full Text] [Subscribe] [Scholar Register] [Received: 12/02/2016] [Revised: 03/29/2017] [Accepted: 04/14/2017] [Indexed: 12/31/2022]
Abstract
The role of genetic molecular markers in neoadjuvant treatment for locally advanced esophageal cancer has been reviewed, focusing strictly on concurrent chemoradiation protocols followed by surgery. Eleven studies evaluated the role of mRNA expression profile; the end point was overall survival (OS) in two studies and different definitions of histological response in nine. Genes reported as significant were involved in cell cycle control (30), apoptosis (7), structural molecules (9), cell metabolism (6) and DNA repair (1). Seven studies reported about 15 microRNA (miRNA) molecules associated with OS (2) or histological response (13), however, defined with different classifications. Their target genes were prevalently involved in cell cycle control (4), apoptosis (1), cell adhesion (1), migration (1) and angiogenesis (1). Gene polymorphisms (single-nucleotide polymorphisms (SNPs)) have been evaluated in 8 studies reporting 10 variants associated with survival or pathological response. OS was the end point in six of these studies. SNPs reported as significant were involved in DNA repair system (4), detoxification (2), folate metabolism (6), drug efflux (2) and others (2). In a study, a panel including histology, pathological response and five SNPs discriminated two subsets of patients with 5-year survival rates of 79.3% and 26.3% (hazard ratio 6.25, P<0.0001). In another study, combination of stage, grade and 4 miRNAs improved prediction of pathological response (P=10-30). At present, given the great inconsistency of the data and the variability of the end points, definite conclusions are extremely difficult, if not impossible. More consistent data can derive only from analyses obtained from patients included in prospective randomized trials while panels combining genetic and clinical factors may improve prediction.
Collapse
Affiliation(s)
- M Gusella
- Laboratory of Pharmacology and Molecular Biology, Department of Oncology, San Luca Hospital, Rovigo, Italy
| | - E Pezzolo
- Laboratory of Pharmacology and Molecular Biology, Department of Oncology, San Luca Hospital, Rovigo, Italy
| | - Y Modena
- Medical Oncology Unit, Department of Oncology, S. Maria della Misericordia Hospital, Rovigo, Italy
| | - C Barile
- Medical Oncology Unit, Department of Oncology, S. Maria della Misericordia Hospital, Rovigo, Italy
| | - D Menon
- Medical Oncology Unit, Department of Oncology, S. Maria della Misericordia Hospital, Rovigo, Italy
| | - G Crepaldi
- Medical Oncology Unit, Department of Oncology, S. Maria della Misericordia Hospital, Rovigo, Italy
| | - F La Russa
- Medical Oncology Unit, Department of Oncology, S. Maria della Misericordia Hospital, Rovigo, Italy
| | - A P Fraccon
- Medical Oncology Unit, Pederzoli Hospital, Peschiera del Garda (Verona), Italy
| | - F Pasini
- Medical Oncology Unit, Pederzoli Hospital, Peschiera del Garda (Verona), Italy
| |
Collapse
|
|
22
|
Specific gene expression profiles are associated with a pathologic complete response to neoadjuvant therapy in esophageal adenocarcinoma. Am J Surg 2017; 213:915-920. [PMID: 28385379 DOI: 10.1016/j.amjsurg.2017.03.024] [Citation(s) in RCA: 13] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/09/2017] [Revised: 01/18/2017] [Accepted: 03/21/2017] [Indexed: 01/22/2023]
Abstract
BACKGROUND Predicting treatment response to chemo-radiotherapy (CRT) in esophageal cancer remains an unrealized goal despite studies linking constellations of genes to prognosis. We aimed to determine if specific expression profiles are associated with pathologic complete response (pCR) after neoadjuvant CRT. METHODS Eleven genes previously associated with esophageal cancer prognosis were identified. Esophageal adenocarcinoma (EAC) patients treated with neoadjuvant CRT and esophagectomy were included. Patients were classified into two groups: pCR and no-or-incomplete response (NR). Polymerase chain reaction was used to evaluate gene expression. Omnibus testing was applied to overall gene expression differences between groups, and log-rank tests compared individual genes. RESULTS Eleven pCR and eighteen NR patients were analyzed. Combined expression profiles were significantly different between pCR and NR groups (p < 0.01). The gene CCL28 was over-expressed in pCR patients (Log-HR: 1.53, 95%CI: 0.46-2.59, p = 0.005), and DKK3 was under-expressed in pCR (Log-HR: -1.03 95%CI: -1.97, -0.10, p = 0.031). CONCLUSION EAC tumors that demonstrated a pCR have genetic profiles that are significantly different from typical NR profiles. The genes CCL28 and DKK3 are potential predictors of treatment response.
Collapse
|
|
23
|
Chen GZ, Zhu HC, Dai WS, Zeng XN, Luo JH, Sun XC. The mechanisms of radioresistance in esophageal squamous cell carcinoma and current strategies in radiosensitivity. J Thorac Dis 2017; 9:849-859. [PMID: 28449496 PMCID: PMC5394057 DOI: 10.21037/jtd.2017.03.23] [Citation(s) in RCA: 61] [Impact Index Per Article: 7.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/07/2016] [Accepted: 01/19/2017] [Indexed: 12/21/2022]
Abstract
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related death worldwide. Surgery is the primary form of treatment, but the survival is poor, especially for patients with locally advanced esophageal cancer. Radiotherapy has been a critical treatment option that may be combined with chemotherapy in patients with unresectable esophageal cancer. However, resistance to chemoradiotherapy might result in treatment failures and cancer relapse. This review will mainly focus on the possible cellular mechanisms and tumor-associated microenvironmental (TAM) factors that result in radioresistance in patients with esophageal cancer. In addition, current strategies to increase radiosensitivity, including targeted therapy and the use of radiosensitive biomarkers in clinical treatment, are discussed in this review.
Collapse
Affiliation(s)
- Guang-Zong Chen
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Hong-Cheng Zhu
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Wang-Shu Dai
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xiao-Ning Zeng
- Department of Respiratory Medicine, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Jin-Hua Luo
- Department of Thoracic Surgery, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| | - Xin-Chen Sun
- Department of Radiation Oncology, The First Affiliated Hospital, Nanjing Medical University, Nanjing 210029, China
| |
Collapse
|
|
24
|
Martens-de Kemp SR, Brink A, van der Meulen IH, de Menezes RX, te Beest DE, Leemans CR, van Beusechem VW, Braakhuis BJ, Brakenhoff RH. The FA/BRCA Pathway Identified as the Major Predictor of Cisplatin Response in Head and Neck Cancer by Functional Genomics. Mol Cancer Ther 2016; 16:540-550. [DOI: 10.1158/1535-7163.mct-16-0457] [Citation(s) in RCA: 21] [Impact Index Per Article: 2.3] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 07/18/2016] [Revised: 11/18/2016] [Accepted: 12/06/2016] [Indexed: 11/16/2022]
|
|
25
|
Can CT-PET and Endoscopic Assessment Post-Neoadjuvant Chemoradiotherapy Predict Residual Disease in Esophageal Cancer? Ann Surg 2016; 264:831-838. [DOI: 10.1097/sla.0000000000001902] [Citation(s) in RCA: 44] [Impact Index Per Article: 4.9] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/24/2022]
|
|
26
|
Stiekema J, Cats A, Boot H, Langers AMJ, Balague Ponz O, van Velthuysen MLF, Braaf LM, Nieuwland M, van Sandick JW. Biobanking of fresh-frozen endoscopic biopsy specimens from esophageal adenocarcinoma. Dis Esophagus 2016; 29:1100-1106. [PMID: 26541751 DOI: 10.1111/dote.12430] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Indexed: 12/11/2022]
Abstract
The process of preparing endoscopic esophageal adenocarcinoma samples for next-generation DNA/RNA sequencing is poorly described. Therefore, we assessed the feasibility and pitfalls of preparing esophageal adenocarcinoma endoscopic biopsies toward DNA/RNA samples suitable for next-generation sequencing. In this prospective study, four tumor biopsy samples were collected from consecutive esophageal cancer patients during esophagogastroduodenoscopy and fresh-frozen in liquid nitrogen. DNA and RNA were isolated from samples with a tumor percentage of at least 50%. For next-generation sequencing, double-stranded DNA (dsDNA) is required and high-quality RNA preferred. The quantity dsDNA and RNA quantity and quality were assessed with the Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). Biopsy samples of 69 consecutive patients with esophageal adenocarcinoma were included. In five patients (7%), the tumor percentage was less than 50% in all four biopsies. Using a protocol allowing simultaneous DNA and RNA isolation, the median dsDNA yield was 2.4 μg (range 0.1-12.0 μg) and the median RNA yield was 0.5 μg (range 0.01-2.05 μg). The median RNA integrity number of samples that were fresh-frozen within 30 minutes after sampling was 6.7 (range 4.2-8.9) compared with 2.5 (1.8-4.5) for samples that were fresh-frozen after 2 hours. The results from this study show that obtaining dsDNA and RNA for next-generation sequencing from endoscopic esophageal adenocarcinoma samples is feasible. Tumor percentage and dsDNA/RNA yield and quality emphasize the need for sampling multiple biopsies and minimizing the delay before fresh-freezing.
Collapse
Affiliation(s)
- J Stiekema
- Department of Surgery, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - A Cats
- Department of Gastroenterology and Hepatology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - H Boot
- Department of Gastroenterology and Hepatology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - A M J Langers
- Department of Gastroenterology and Hepatology, Leiden University Medical Center, Leiden, The Netherlands
| | - O Balague Ponz
- Department of Pathology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - M L F van Velthuysen
- Department of Pathology, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - L M Braaf
- Core Facility Molecular Pathology and Biobanking, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - M Nieuwland
- Deep Sequencing Facility, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| | - J W van Sandick
- Department of Surgery, The Netherlands Cancer Institute, Amsterdam, The Netherlands
| |
Collapse
|
|
27
|
Possible prediction of the response of esophageal squamous cell carcinoma to neoadjuvant chemotherapy based on gene expression profiling. Oncotarget 2016; 7:4531-41. [PMID: 26673820 PMCID: PMC4826224 DOI: 10.18632/oncotarget.6554] [Citation(s) in RCA: 19] [Impact Index Per Article: 2.1] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 08/27/2015] [Accepted: 11/21/2015] [Indexed: 12/03/2022] Open
Abstract
Background Heterogeneous efficacy of neoadjuvant chemotherapy has led to controversies that have limited its application in clinical practice. Thus, we aimed to identify potential biomarkers predicting esophageal squamous cell carcinoma (ESCC) chemo-responsiveness by gene expression profiling. Methods CCK8 assay was used to evaluate the growth inhibitory effect of different concentrations of cisplatin and paclitaxel on the ESCC cell lines EC109, KYSE450, KYSE410, KYSE510, and KYSE150 to differentiate between chemosensitive and chemoresistant cell lines. Gene expression profiling and Real-time PCR were applied to analyze and validate the gene expression differences between chemosensitive and chemoresistant cell lines. IHC was conducted to examine the expression of selected target markers MUC4, MUC13, and MUC20 in 186 ESCC resection samples and the relationships between their expression and tumor regression grade was analyzed. Moreover, RNAi was conducted to instantly block the expression of MUC4, MUC13, and MUC20 to observe their influences on chemo-responsiveness. Results EC109 was found to be relatively sensitive to both cisplatin and paclitaxel, while KYSE410 was relatively resistant to cisplatin, KYSE510 was relatively resistant to paclitaxel. Gene expression profiling analysis showed that 2018 genes were differentially expressed in sensitive cell line compared to resistant cell lines. The expression patterns of MUC4, MUC13, MUC20 were validated. Low expression of MUC4 and MUC20 in resection samples was significantly correlated with better TRG. Blockage of MUC20 and MUC13 decreased the drug-resistance capacity and chemosensitivity, respectively. Conclusions MUC4 and MUC20 were identified as potential biomarkers for predicting the efficacy of neoadjuvant chemotherapy in ESCC patients.
Collapse
|
|
28
|
Lynam-Lennon N, Bibby BA, Mongan AM, Marignol L, Paxton CN, Geiersbach K, Bronner MP, O'Sullivan J, Reynolds J, Maher SG. Low miR-187 expression promotes resistance to chemoradiation therapy in vitro and correlates with treatment failure in patients with esophageal adenocarcinoma. Mol Med 2016; 22:molmed.2016.00020. [PMID: 27254108 DOI: 10.2119/molmed.2016.00020] [Citation(s) in RCA: 26] [Impact Index Per Article: 2.9] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/20/2016] [Accepted: 05/11/2016] [Indexed: 12/13/2022] Open
Abstract
Esophageal adenocarcinoma (EAC) has a poor prognosis and is increasing in incidence in many western populations. Neoadjuvant chemoradiation therapy (CRT) followed by surgery is increasingly the standard of care for locally advanced EAC; however, resistance to treatment is a significant clinical problem. The identification of both novel biomarkers predicting response to treatment and novel therapeutic targets to enhance the efficacy of CRT are key to improving survival rates in EAC. In this study we performed global microRNA (miRNA) profiling of pre-treatment EAC biopsies and identified 67 miRNA significantly altered in patients who are resistant to CRT. One of these miRNA, miR-187, was significantly decreased in pre-treatment EAC tumors from patients having a poor response to neoadjuvant CRT, highlighting downregulation of miR-187 as a potential mechanism of treatment resistance in EAC. In vitro, miR-187 was demonstrated to play a functional role in modulating sensitivity to X-ray radiation and cisplatin in EAC and its dysregulation was demonstrated to be due to chromosomal alterations. In vitro, miR-187 altered expression of a diverse array of pathways, including the immune regulator complement component 3 (C3), serum levels of which we have previously demonstrated to predict patient response to CRT. In vivo, expression of C3 was significantly increased in tumors from patients having a poor response to CRT. This study highlights for the first time a role for miR-187 as a novel biomarker of response to CRT and a potential therapeutic target for enhancing the efficacy of CRT in EAC.
Collapse
Affiliation(s)
| | - Becky A Bibby
- University of Hull, Hull, Kingston upon Hull, United Kingdom of Great Britain and Northern Ireland
| | | | | | | | - Katherine Geiersbach
- University of Utah and ARUP Laboratories, United States.,Arup Institute for Clinical and Experimental Pathology, United States
| | - Mary P Bronner
- University of Utah and ARUP Laboratories, United States.,Arup Institute for Clinical and Experimental Pathology, United States
| | | | - John Reynolds
- University of Dublin Trinity College, Dublin, Ireland
| | - Stephen G Maher
- University of Dublin Trinity College, Dublin, Ireland.,University of Hull, Hull, Kingston upon Hull, United Kingdom of Great Britain and Northern Ireland
| |
Collapse
|
|
29
|
Wen J, Luo K, Liu H, Liu S, Lin G, Hu Y, Zhang X, Wang G, Chen Y, Chen Z, Li Y, Lin T, Xie X, Liu M, Wang H, Yang H, Fu J. MiRNA Expression Analysis of Pretreatment Biopsies Predicts the Pathological Response of Esophageal Squamous Cell Carcinomas to Neoadjuvant Chemoradiotherapy. Ann Surg 2016; 263:942-8. [PMID: 26445467 DOI: 10.1097/sla.0000000000001489] [Citation(s) in RCA: 36] [Impact Index Per Article: 4.0] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 02/05/2023]
Abstract
OBJECTIVE To identify miRNA markers useful for esophageal squamous cell carcinoma (ESCC) neoadjuvant chemoradiotherapy (neo-CRT) response prediction. SUMMARY Neo-CRT followed by surgery improves ESCC patients' survival compared with surgery alone. However, CRT outcomes are heterogeneous, and no current methods can predict CRT responses. METHODS Differentially expressed miRNAs between ESCC pathological responders and nonresponders after neo-CRT were identified by miRNA profiling and verified by real-time quantitative polymerase chain reaction (qPCR) of 27 ESCCs in the training set. Several class prediction algorithms were used to build the response-classifying models with the qPCR data. Predictive powers of the models were further assessed with a second set of 79 ESCCs. RESULTS Ten miRNAs with greater than a 1.5-fold change between pathological responders and nonresponders were identified and verified, respectively. A support vector machine (SVM) prediction model, composed of 4 miRNAs (miR-145-5p, miR-152, miR-193b-3p, and miR-376a-3p), were developed. It provided overall accuracies of 100% and 87.3% for discriminating pathological responders and nonresponders in the training and external validation sets, respectively. In multivariate analysis, the subgroup determined by the SVM model was the only independent factor significantly associated with neo-CRT response in the external validation sets. CONCLUSIONS Combined qPCR of the 4 miRNAs provides the possibility of ESCC neo-CRT response prediction, which may facilitate individualized ESCC treatment. Further prospective validation in larger independent cohorts is necessary to fully assess its predictive power.
Collapse
Affiliation(s)
- Jing Wen
- *State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, China †Guangdong Esophageal Cancer Institute Guangzhou, China ‡Department of Thoracic Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China §Department of Radiotherapy, Sun Yat-sen University Cancer Center, Guangzhou, China ¶Guangzhou Haige Communications Group Incorporated Company, Guangzhou, China ||School of Electronic & Information Engineering, South China University of Technology, Guangzhou, China **Department of Thoracic Surgery, Cancer Hospital of Shantou University Medical College, Shantou, China ††Department of Radiotherapy, Cancer Hospital of Shantou University Medical College, Shantou, China ‡‡Department of Anesthesiology, Sun Yat-sen University Cancer Center, Guangzhou, China
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Collapse
|
|
30
|
Anvari K, Aledavood SA, Toussi MS, Forghani MN, Mohtashami S, Rajabi MT, Shandiz FH, Nosrati F, Nowferesti G, Salek R. A clinical trial of neoadjuvant concurrent chemoradiotherapy followed by resection for esophageal carcinoma. JOURNAL OF RESEARCH IN MEDICAL SCIENCES 2015; 20:751-6. [PMID: 26664422 PMCID: PMC4652308 DOI: 10.4103/1735-1995.168377] [Citation(s) in RCA: 8] [Impact Index Per Article: 0.8] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Figures] [Subscribe] [Scholar Register] [Indexed: 01/26/2023]
Abstract
Background: Esophageal carcinoma is a common malignancy in the North East of Iran. Combined modality treatments have been adopted to improve survival in patients with esophageal carcinoma. In this trial, we evaluated the efficacy and toxicity of a preoperative concurrent chemoradiotherapy protocol in the patients with locally advanced esophageal carcinoma. Materials and Methods: Between 2006 and 2011, eligible patients with locally advanced esophageal carcinoma underwent concurrent radiotherapy and chemotherapy and 3-4 weeks later, esophagectomy. Pathologic response, overall survival rate, toxicity, and feasibility were evaluated. Results: One hundred ninety-seven patients with a median age of 59 (range: 27-70) entered the protocol. One hundred ninety-four cases (98.5%) had esophageal squamous cell carcinoma. Grades 3-4 of toxicity in patients undergoing neoadjuvant chemoradotherapy were as follows: Neutropenia in 21% and esophagitis in 2.5% of cases. There were 11 (5.6%) early death probably due to the treatment-related toxicities. One hundred twenty-seven patients underwent surgery with postsurgical mortality of 11%. In these cases, the complete pathological response was shown in 38 cases (29.9%) with a 5-year overall survival rates of 48.2% and median overall survival of 44 months (95% confidence interval, 24.46-63.54). Conclusion: The pathological response rate and the overall survival rate are promising in patients who completed the protocol as receiving at least one cycle of chemotherapy. However, the treatment toxicities were relatively high.
Collapse
Affiliation(s)
- Kazem Anvari
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Seyed Amir Aledavood
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Mehdi Seilanian Toussi
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Mohammad Naser Forghani
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Samira Mohtashami
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Mohammad Taghi Rajabi
- Endoscopic and Minimally Invasive Surgery Research Center, Ghaem Hospital, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Fatemeh Homaee Shandiz
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Fatemeh Nosrati
- Omid Hospital, Radiation Oncology Specialist, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Gholamhossein Nowferesti
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| | - Roham Salek
- Cancer Research Center, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
| |
Collapse
|
|
31
|
Bowen JM, White I, Smith L, Tsykin A, Kristaly K, Thompson SK, Karapetis CS, Tan H, Game PA, Irvine T, Hussey DJ, Watson DI, Keefe DMK. Pre-therapy mRNA expression of TNF is associated with regimen-related gastrointestinal toxicity in patients with esophageal cancer: a pilot study. Support Care Cancer 2015; 23:3165-3172. [PMID: 25814442 DOI: 10.1007/s00520-015-2696-7] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.4] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/11/2014] [Accepted: 03/09/2015] [Indexed: 12/16/2022]
Abstract
PURPOSE Esophageal cancer has a high mortality rate, and its multimodality treatment is often associated with significant rates of severe toxicity. Effort is needed to uncover ways to maximize effectiveness of therapy through identification of predictive markers of response and toxicity. As such, the aim of this study was to identify genes predictive of chemoradiotherapy-induced gastrointestinal toxicity using an immune pathway-targeted approach. METHODS Adults with esophageal cancer treated with chemotherapy consisting of 5-fluorouracil and cisplatin and 45-50 Gy radiation were recruited to the study. Pre-therapy-collected whole blood was analyzed for relative expression of immune genes using real-time polymerase chain reaction (RT-PCR). Gene expression was compared between patients who experienced severe regimen-related gastrointestinal toxicity vs. those experiencing mild to moderate toxicity. RESULTS Blood from 31 patients were analyzed by RT-PCR. Out of 84 immune genes investigated, TNF was significantly elevated (2.05-fold, p = 0.025) in the toxic group (n = 12) compared to the non-toxic group (n = 19). Nausea and vomiting was the most commonly documented severe toxicity. No associations between toxicity and response, age, sex, histology, or treatment were evident. CONCLUSIONS This study supports evidence of TNF as a predictive biomarker in regimen-related gastrointestinal toxicity. Confirming these findings in a larger cohort is warranted.
Collapse
Affiliation(s)
- J M Bowen
- School of Medical Sciences, University of Adelaide, Adelaide, Australia.
| | - I White
- School of Medicine, University of Adelaide, Adelaide, Australia
| | - L Smith
- Discipline of Surgery, University of Adelaide, Adelaide, Australia
| | - A Tsykin
- Centre for Cancer Biology, SA Pathology, Adelaide, South Australia, Australia
| | - K Kristaly
- School of Medicine, University of Adelaide, Adelaide, Australia
| | - S K Thompson
- Discipline of Surgery, University of Adelaide, Adelaide, Australia
| | - C S Karapetis
- School of Medicine, Flinders University, Bedford Park, Australia
| | - H Tan
- RAH Cancer Centre, Royal Adelaide Hospital, Adelaide, Australia
| | - P A Game
- Discipline of Surgery, University of Adelaide, Adelaide, Australia
| | - T Irvine
- Department of Surgery, Flinders University, Adelaide, South Australia, Australia
| | - D J Hussey
- Department of Surgery, Flinders University, Adelaide, South Australia, Australia
| | - D I Watson
- Department of Surgery, Flinders University, Adelaide, South Australia, Australia
| | - D M K Keefe
- School of Medicine, University of Adelaide, Adelaide, Australia
| |
Collapse
|
|
32
|
Tao CJ, Lin G, Xu YP, Mao WM. Predicting the Response of Neoadjuvant Therapy for Patients with Esophageal Carcinoma: an In-depth Literature Review. J Cancer 2015; 6:1179-86. [PMID: 26516367 PMCID: PMC4615355 DOI: 10.7150/jca.12346] [Citation(s) in RCA: 15] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Subscribe] [Scholar Register] [Received: 04/08/2015] [Accepted: 06/12/2015] [Indexed: 12/24/2022] Open
Abstract
Currently, the most promising strategy to improve the prognosis of advanced esophageal cancer is neoadjuvant chemoradiation (CRT) followed by surgery. However, patients who achieved pathological complete response can experience more survival benefit. Therefore, it is critical to identify the responders early in the course of treatment. Published data demonstrate that clinic-histopathological factors, molecular biomarkers, and functional imaging are predictive of neoadjuvant therapy. The existing biomarkers, including epidermal growth factor receptors, angiogenetic factors, transcription factors, tumor suppressor genes, cell cycle regulators, nucleotide excision repair pathway, cytokines, and chemotherapy associated genes, need to be validated and novel biomarkers warrant further exploration. Positron emission tomography (PET) is useful for differentiating the responders of neoadjuvant CRT. The most valuable parameters and the time point of performing PET in the course of treatment remains to be elucidated. Furthermore, predictive models incorporating the multiple categories of factors need to be established with a large, prospective, and homogeneous patient cohort in the future. Standardization of staging, biomarker detection method, and image acquisition protocol will be critical for the generalization of this model. Prospective, multi-center controlled trials, which stratified patients according to these predictive factors, will help guide individualized treatment strategies for patients with esophageal cancer.
Collapse
Affiliation(s)
- Chang-Juan Tao
- 1. Department of Radiation Oncology, Zhejiang Cancer Hospital, No. 38 Guangji Rd., Hangzhou, Zhejiang, 310022, China
| | - Gang Lin
- 1. Department of Radiation Oncology, Zhejiang Cancer Hospital, No. 38 Guangji Rd., Hangzhou, Zhejiang, 310022, China
| | - Ya-Ping Xu
- 1. Department of Radiation Oncology, Zhejiang Cancer Hospital, No. 38 Guangji Rd., Hangzhou, Zhejiang, 310022, China
| | - Wei-Min Mao
- 2. Department of Thoracic Surgery, Zhejiang Cancer Hospital, No. 38 Guangji Rd., Hangzhou, Zhejiang, 310022, China
| |
Collapse
|
|
33
|
Hemoglobin level influences tumor response and survival after neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma. World J Surg 2015; 38:2046-51. [PMID: 24615604 DOI: 10.1007/s00268-014-2486-2] [Citation(s) in RCA: 17] [Impact Index Per Article: 1.7] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 02/06/2023]
Abstract
BACKGROUND Neoadjuvant chemoradiotherapy (nCRT) followed by esophagectomy confers a survival benefit on patients with esophageal cancer. However, nCRT might be less meaningful for poor responders. Thus, being able to predict responses would help ensure the selection of optimal therapy. METHODS We reviewed data from 123 patients with esophageal squamous cell carcinoma (ESCC) who underwent nCRT that comprised concurrent radiation (40 Gy) and chemotherapy followed by esophagectomy. We assessed associations between clinical and blood data obtained before starting nCRT and the pathologic response. RESULTS We compared good (Japan Esophageal Society response evaluation criteria grades 3/2; n = 89, 72.4%) and poor (grades 1/0; n = 34, 27.6%) responders. Performance status (p = 0.02), hemoglobin level (p = 0.005), and platelet counts (p = 0.03) were statistically significant pretherapeutic factors for a response to nCRT. Multivariable analysis subsequently selected the hemoglobin level (odds ratio 1.52; 95% confidence interval 1.08-2.15; p = 0.02) as the sole independent predictor. Receiver operating characteristic curves showed that the optimal cutoff for pretherapeutic hemoglobin was 13 g/dl for predicting a response. We found that 48.8 and 17.1% of patients with hemoglobin level ≤13 and >13 g/dl, respectively, were poor responders (p = 0.0002), with 5-year overall survival rates of 40.9 and 58.9%, respectively (p = 0.048). CONCLUSIONS Pretherapeutic hemoglobin levels can influence responses and survival after nCRT for ESCC. Thus, hemoglobin levels can serve as a useful marker for tailoring optimal therapies for individual patients with advanced ESCC.
Collapse
|
|
34
|
Bibby BAS, Reynolds JV, Maher SG. MicroRNA-330-5p as a Putative Modulator of Neoadjuvant Chemoradiotherapy Sensitivity in Oesophageal Adenocarcinoma. PLoS One 2015. [PMID: 26221725 PMCID: PMC4519309 DOI: 10.1371/journal.pone.0134180] [Citation(s) in RCA: 31] [Impact Index Per Article: 3.1] [Reference Citation Analysis] [Abstract] [MESH Headings] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/18/2022] Open
Abstract
Oesophageal adenocarcinoma (OAC) is the sixth most common cause of cancer deaths worldwide, and the 5-year survival rate for patients diagnosed with the disease is approximately 17%. The standard of care for locally advanced disease is neoadjuvant chemotherapy or, more commonly, combined neoadjuvant chemoradiation therapy (neo-CRT) prior to surgery. Unfortunately, ~60-70% of patients will fail to respond to neo-CRT. Therefore, the identification of biomarkers indicative of patient response to treatment has significant clinical implications in the stratification of patient treatment. Furthermore, understanding the molecular mechanisms underpinning tumour response and resistance to neo-CRT will contribute towards the identification of novel therapeutic targets for enhancing OAC sensitivity to CRT. MicroRNAs (miRNA/miR) function to regulate gene and protein expression and play a causal role in cancer development and progression. MiRNAs have also been identified as modulators of key cellular pathways associated with resistance to CRT. Here, to identify miRNAs associated with resistance to CRT, pre-treatment diagnostic biopsy specimens from patients with OAC were analysed using miRNA-profiling arrays. In pre-treatment biopsies miR-330-5p was the most downregulated miRNA in patients who subsequently failed to respond to neo-CRT. The role of miR-330 as a potential modulator of tumour response and sensitivity to CRT in OAC was further investigated in vitro. Through vector-based overexpression the E2F1/p-AKT survival pathway, as previously described, was confirmed as a target of miR-330 regulation. However, miR-330-mediated alterations to the E2F1/p-AKT pathway were insufficient to significantly alter cellular sensitivity to chemotherapy (cisplatin and 5-flurouracil). In contrast, silencing of miR-330-5p enhanced, albeit subtly, cellular resistance to clinically relevant doses of radiation. This study highlights the need for further investigation into the potential of miR-330-5p as a predictive biomarker of patient sensitivity to neo-CRT and as a novel therapeutic target for manipulating cellular sensitivity to neo-CRT in patients with OAC.
Collapse
Affiliation(s)
- Becky A. S. Bibby
- School of Biological, Biomedical and Environmental Sciences, University of Hull, Hull, United States of America
| | - John V. Reynolds
- Department of Surgery, Institute of Molecular Medicine, Trinity College Dublin, St. James Hospital, Dublin, Ireland
| | - Stephen G. Maher
- School of Biological, Biomedical and Environmental Sciences, University of Hull, Hull, United States of America
- Department of Surgery, Institute of Molecular Medicine, Trinity College Dublin, St. James Hospital, Dublin, Ireland
- * E-mail:
| |
Collapse
|
|
35
|
Circulating mRNA Profiling in Esophageal Squamous Cell Carcinoma Identifies FAM84B As A Biomarker In Predicting Pathological Response to Neoadjuvant Chemoradiation. Sci Rep 2015; 5:10291. [PMID: 25980316 PMCID: PMC4434848 DOI: 10.1038/srep10291] [Citation(s) in RCA: 25] [Impact Index Per Article: 2.5] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 11/25/2014] [Accepted: 04/09/2015] [Indexed: 12/12/2022] Open
Abstract
Esophageal cancer patients with pathological complete response (pCR) to neoadjuvant chemoradiation (CRT) have favorable outcomes. Currently, there was no reliable biomarker predicting the response to CRT. Perioperative circulating mRNA may be associated with prognosis, but its application for predicting treatment response is unclear. We prospectively assessed the value of circulating messenger RNA (mRNA) profiling in predicting pCR for esophageal squamous cell carcinoma (ESCC). Patients with ESCC completing CRT followed by surgery were enrolled for analysis. Venous peripheral blood was obtained before and after CRT, and total RNA was extracted for hybridization-based whole genome expression analysis and quantitative RT-PCR. We found circulating expression profiling was significantly altered after CRT. Altered FAM84B expression was significantly predictive of pCR. The decrease of serum FAM84B protein level after CRT was also associated with pCR. Immunohistochemistry and western blot confirmed that FAM84B protein was overexpressed in the majority of patients and ESCC cell lines. Furthermore, knockdown of FAM84B delayed tumor growth in ectopic xenografts. We demonstrated the decreased of circulating FAM84B mRNA and protein after neoadjuvant CRT may predict pCR, and FAM84B protein is overexpressed in ESCC. The potential of FAM84B as a novel predictive biomarker, and its biological functions deserve further investigation.
Collapse
|
|
36
|
van Rossum PSN, van Hillegersberg R, Reerink O, Ruurda JP. Neoadjuvant chemoradiotherapy for stage I and II esophageal cancer. J Clin Oncol 2014; 33:287-8. [PMID: 25452449 DOI: 10.1200/jco.2014.58.1272] [Citation(s) in RCA: 4] [Impact Index Per Article: 0.4] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 11/20/2022] Open
Affiliation(s)
| | | | - Onne Reerink
- University Medical Center Utrecht, Utrecht, the Netherlands
| | - Jelle P Ruurda
- University Medical Center Utrecht, Utrecht, the Netherlands
| |
Collapse
|
|
37
|
Duan XF, Tang P, Yu ZT. Neoadjuvant chemoradiotherapy for resectable esophageal cancer: an in-depth study of randomized controlled trials and literature review. Cancer Biol Med 2014; 11:191-201. [PMID: 25364580 PMCID: PMC4197424 DOI: 10.7497/j.issn.2095-3941.2014.03.005] [Citation(s) in RCA: 12] [Impact Index Per Article: 1.1] [Reference Citation Analysis] [Abstract] [Key Words] [Download PDF] [Journal Information] [Subscribe] [Scholar Register] [Received: 02/27/2014] [Accepted: 06/19/2014] [Indexed: 12/15/2022] Open
Abstract
Surgery following neoadjuvant chemoradiotherapy (NCRT) is a common multidisciplinary treatment for resectable esophageal cancer (EC). After analyzing 12 randomized controlled trials (RCTs), we discuss the key issues of surgery in the management of resectable EC. Along with chemoradiotherapy, NCRT is recommended for patients with squamous cell carcinoma (SCC) and adenocarcinoma (AC), and most chemotherapy regimens are based on cisplatin, fluorouracil (FU), or both (CF). However, taxane-based schedules or additional studies, together with newer chemotherapies, are warranted. In nine clinical trials, post-operative complications were similar without significant differences between two treatment groups. In-hospital mortality was significantly different in only 1 out of 10 trials. Half of the randomized trials that compare NCRT with surgery in EC demonstrate an increase in overall survival or disease-free survival. NCRT offers a great opportunity for margin negative resection, decreased disease stage, and improved loco-regional control. However, NCRT does not affect the quality of life when combined with esophagectomy. Future trials should focus on the identification of optimum regimens and selection of patients who are most likely to benefit from specific treatment options.
Collapse
Affiliation(s)
- Xiao-Feng Duan
- Department of Esophageal Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, China
| | - Peng Tang
- Department of Esophageal Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, China
| | - Zhen-Tao Yu
- Department of Esophageal Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, China
| |
Collapse
|
|
38
|
Elliott JA, O'Farrell NJ, King S, Halpenny D, Malik V, Muldoon C, Johnston C, Reynolds JV. Value of CT–PET after neoadjuvant chemoradiation in the prediction of histological tumour regression, nodal status and survival in oesophageal adenocarcinoma. Br J Surg 2014; 101:1702-11. [DOI: 10.1002/bjs.9670] [Citation(s) in RCA: 42] [Impact Index Per Article: 3.8] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/27/2014] [Revised: 08/01/2014] [Accepted: 09/10/2014] [Indexed: 12/22/2022]
Abstract
Abstract
Background
The role of CT–PET after neoadjuvant chemoradiation (nCRT) for prediction of pathological response and oncological outcome in oesophageal and junctional adenocarcinoma (OAC) is unclear. The relationship between complete metabolic response (cMR), pathological complete response (pCR) and nodal status has not been clarified.
Methods
Patients with locally advanced OAC selected to receive nCRT and surgery with curative intent, on the basis of staging that included CT–PET positivity, were included. Repeat scanning (PET2) with an identical protocol was performed 2–4 weeks after completion of nCRT (cisplatin and 5-fluorouracil plus 44 Gy radiation). Changes in [18F]fluorodeoxyglucose uptake, considered as either a maximum standardized uptake value (SUVmax) or a relative reduction (%ΔSUVmax), and PET-predicted nodal status following nCRT were compared with histopathological response, histological node positivity and survival.
Results
One hundred consecutive patients with PET-positive OAC were studied. Following nCRT, PET2 identified M1 disease in 2·0 per cent of patients. There were no significant associations between PET2 SUVmax or %ΔSUVmax with respect to primary tumour stage (ypT) (P = 0.216 and P = 0·975 respectively), tumour regression grade (P = 0·109 and P = 0·232), pCR (P = 0·633 and P = 0·870) or complete resection (R0) (P = 0·440 and P = 0·235). The sensitivity of PET2 for ypN was 10 per cent. %ΔSUVmax was not associated with disease-free or overall survival (P = 0·162 and P = 0·154 respectively). Of 46 patients with a cMR on PET2, 37 (80 per cent) had histological evidence of residual tumour in the resected specimen, and cMR was not associated with overall survival benefit (P = 0·478).
Conclusion
CT–PET following nCRT for OAC has poor prognostic and discriminatory value for clinical application.
Collapse
Affiliation(s)
- J A Elliott
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| | - N J O'Farrell
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| | - S King
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| | - D Halpenny
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| | - V Malik
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| | - C Muldoon
- Department of Pathology, St James's Hospital, Dublin, Ireland
| | - C Johnston
- Department of Radiology, St James's Hospital, Dublin, Ireland
| | - J V Reynolds
- Department of Surgery, Trinity Centre for Health Sciences, Trinity College Dublin and St James's Hospital, Dublin, Ireland
| |
Collapse
|
|
39
|
Wen J, Yang H, Liu MZ, Luo KJ, Liu H, Hu Y, Zhang X, Lai RC, Lin T, Wang HY, Fu JH. Gene expression analysis of pretreatment biopsies predicts the pathological response of esophageal squamous cell carcinomas to neo-chemoradiotherapy. Ann Oncol 2014; 25:1769-1774. [PMID: 24907633 DOI: 10.1093/annonc/mdu201] [Citation(s) in RCA: 75] [Impact Index Per Article: 6.8] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/23/2022] Open
Abstract
BACKGROUND Neoadjuvant chemoradiotherapy (neo-CRT) followed by surgery has been shown to improve esophageal squamous cell carcinoma (ESCC) patients' survival compared with surgery alone. However, the outcomes of CRT are heterogeneous, and no clinical or pathological method can currently predict CRT response. In this study, we aim to identify mRNA markers useful for ESCC CRT-response prediction. PATIENTS AND METHODS Gene expression analyses were carried out on pretreated cancer biopsies from 28 ESCCs who received neo-CRT and surgery. Surgical specimens were assessed for pathological response to CRT. The differentially expressed genes identified by expression profiling were validated by real-time quantitative polymerase chain reaction (qPCR), and a classifying model was built from qPCR data using Fisher's linear discriminant analysis. The predictive power of this model was further assessed in a second set of 32 ESCCs. RESULTS The profiling of the 28 ESCCs identified 10 differentially expressed genes with more than a twofold change between patients with pathological complete response (pCR) and less than pCR ( CONCLUSION The expression levels of three genes determined by qPCR provide a possible model for ESCC CRT prediction, which will facilitate the individualization of ESCC treatment. Further prospective validation in larger independent cohorts is necessary to fully assess its predictive power.
Collapse
Affiliation(s)
- J Wen
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou
| | - H Yang
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology
| | - M Z Liu
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Radiotherapy
| | - K J Luo
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology
| | - H Liu
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Radiotherapy
| | - Y Hu
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology
| | - X Zhang
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology
| | - R C Lai
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Department of Anesthesiology, Sun Yat-sen University Cancer Center, Guangzhou, People's Republic of China
| | - T Lin
- Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology
| | - H Y Wang
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou
| | - J H Fu
- State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangzhou; Guangdong Esophageal Cancer Institute, Guangzhou; Department of Thoracic Oncology.
| |
Collapse
|
|
40
|
Uemura N, Kondo T. Current status of predictive biomarkers for neoadjuvant therapy in esophageal cancer. World J Gastrointest Pathophysiol 2014; 5:322-334. [PMID: 25133032 PMCID: PMC4133529 DOI: 10.4291/wjgp.v5.i3.322] [Citation(s) in RCA: 18] [Impact Index Per Article: 1.6] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Download PDF] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 12/25/2013] [Revised: 01/27/2014] [Accepted: 04/29/2014] [Indexed: 02/06/2023] Open
Abstract
Neoadjuvant therapy has been proven to be extremely valuable and is widely used for advanced esophageal cancer. However, a significant proportion of treated patients (60%-70%) does not respond well to neoadjuvant treatments and develop severe adverse effects. Therefore, predictive markers for individualization of multimodality treatments are urgently needed in esophageal cancer. Recently, molecular biomarkers that predict the response to neoadjuvant therapy have been explored in multimodal approaches in esophageal cancer and successful examples of biomarker identification have been reported. In this review, promising candidates for predictive molecular biomarkers developed by using multiple molecular approaches are reviewed. Moreover, treatment strategies based on the status of predicted biomarkers are discussed, while considering the international differences in the clinical background. However, in the absence of adequate treatment options related to the results of the biomarker test, the usefulness of these diagnostic tools is limited and new effective therapies for biomarker-identified nonresponders to cancer treatment should be concurrent with the progress of predictive technologies. Further improvement in the prognosis of esophageal cancer patients can be achieved through the introduction of novel therapeutic approaches in clinical practice.
Collapse
|
|
41
|
TPX2 expression is associated with cell proliferation and patient outcome in esophageal squamous cell carcinoma. J Gastroenterol 2014; 49:1231-40. [PMID: 23963785 DOI: 10.1007/s00535-013-0870-6] [Citation(s) in RCA: 42] [Impact Index Per Article: 3.8] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Submit a Manuscript] [Subscribe] [Scholar Register] [Received: 04/17/2013] [Accepted: 08/02/2013] [Indexed: 02/04/2023]
Abstract
BACKGROUND The molecular and genetic changes underlying esophageal squamous cell carcinoma (ESCC) tumor formation and rapid progression are poorly understood. Using high-throughput data analysis, we examined molecular changes involved in ESCC pathogenesis and investigated their clinical relevance. METHODS Five independent microarray datasets were examined for differentially expressed genes and pathways. For validation, mRNA expression in tumor and matched normal tissues from 16 ESCC cases was examined by cDNA microarray, and protein expression in 97 ESCC specimens was investigated using immunohistochemical stains. The association between clinicopathological parameters and the expression of Aurora kinase A (Aurora-A) and TPX2 was analyzed. The impact of TPX2 expression was also assessed in ESCC cancer cells. RESULTS AURKA and TPX2, members of the "Role of Ran in mitotic spindle regulation" pathway, were selected for further investigation. Verification by cDNA microarray showed that both genes were overexpressed in tumor tissues, and immunohistochemical staining showed Aurora-A and TPX2 expression in 88.4 and 90.6 % of ESCC specimens, respectively. High TPX2 expression was a significant prognosticator for overall and disease-free survival in univariate analysis and remained an independent prognostic factor in multivariate analysis (HR 1.802, p = 0.037). TPX2 knockdown clones showed inhibited cellular proliferation in growth curve studies and formed fewer colonies in the clonogenic assay. CONCLUSIONS Using bioinformatics resources, which were validated by microarray analysis and immunohistochemistry stains, and manipulation of TPX2 expression in ESCC cell lines, we demonstrated that TPX2 expression is associated with cell proliferation and poor prognosis among patients with resected ESCC.
Collapse
|
|
42
|
Cheng JCH, Graber MS, Hsu FM, Tsai CL, Castaneda L, Lee JM, Chang DT, Koong AC. High serum levels of vascular endothelial growth factor-A and transforming growth factor-β1 before neoadjuvant chemoradiotherapy predict poor outcomes in patients with esophageal squamous cell carcinoma receiving combined modality therapy. Ann Surg Oncol 2014; 21:2361-2368. [PMID: 24623035 DOI: 10.1245/s10434-014-3611-z] [Citation(s) in RCA: 21] [Impact Index Per Article: 1.9] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 10/24/2013] [Indexed: 01/03/2025]
Abstract
BACKGROUND AND PURPOSE This study was aimed at using proximity ligation assay (PLA) followed by enzyme-linked immunosorbent assay (ELISA) to identify serum biomarkers that predict treatment response and survival for patients with esophageal squamous cell carcinoma (ESCC) undergoing neoadjuvant concurrent chemoradiotherapy (CCRT) followed by esophagectomy. METHODS Seventy-nine patients with ESCC receiving CCRT of taxane-based/5-fluorouracil-based chemotherapy and 40 Gy followed by surgery were enrolled. Serum samples were collected before and <1 month after CCRT. Fifteen biomarkers were analyzed using PLA. Biomarkers significantly correlating with pathological response/survival were verified by ELISA. Associations of the serum level of biomarkers and clinical factors with pathological response, disease-free survival (DFS), and overall survival (OS) were evaluated by analysis of variance and log-rank tests. RESULTS Thirty patients had complete response (38 %), 37 had microscopic residual disease (47 %), and 12 had macroscopic residual disease (15 %). With a median follow-up of 52.8 months, the median DFS was 43 months. Among the 15 biomarkers screened by PLA, vascular endothelial growth factor (VEGF)-A and transforming growth factor (TGF)-β1 were significantly associated with pathological response and/or DFS. These biomarkers were further analyzed by ELISA to confirm initial biomarker findings by PLA. After ELISA of these two markers, only VEGF-A levels were significantly correlated with pathological response. On multivariate analysis, patients with combined high pre-CCRT VEGF-A and TGF-β1 levels (greater than or equal to the median), independent of pathological response, had significantly worse DFS (11 months vs. median not reached; p = 0.007) and OS (16 vs. 46 months; p = 0.07). CONCLUSIONS Pre-CCRT serum VEGF-A and TGF-β1 levels may be used to predict pathological response and survivals for ESCC patients receiving combined-modality therapy.
Collapse
|
|
43
|
Lynam-Lennon N, Maher SG, Maguire A, Phelan J, Muldoon C, Reynolds JV, O’Sullivan J. Altered mitochondrial function and energy metabolism is associated with a radioresistant phenotype in oesophageal adenocarcinoma. PLoS One 2014; 9:e100738. [PMID: 24968221 PMCID: PMC4072695 DOI: 10.1371/journal.pone.0100738] [Citation(s) in RCA: 70] [Impact Index Per Article: 6.4] [Reference Citation Analysis] [Abstract] [MESH Headings] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 12/20/2013] [Accepted: 05/30/2014] [Indexed: 01/12/2023] Open
Abstract
Neoadjuvant chemoradiation therapy (CRT) is increasingly the standard of care for locally advanced oesophageal cancer. A complete pathological response to CRT is associated with a favourable outcome. Radiation therapy is important for local tumour control, however, radioresistance remains a substantial clinical problem. We hypothesise that alterations in mitochondrial function and energy metabolism are involved in the radioresistance of oesophageal adenocarcinoma (OAC). To investigate this, we used an established isogenic cell line model of radioresistant OAC. Radioresistant cells (OE33 R) demonstrated significantly increased levels of random mitochondrial mutations, which were coupled with alterations in mitochondrial function, size, morphology and gene expression, supporting a role for mitochondrial dysfunction in the radioresistance of this model. OE33 R cells also demonstrated altered bioenergetics, demonstrating significantly increased intracellular ATP levels, which was attributed to enhanced mitochondrial respiration. Radioresistant cells also demonstrated metabolic plasticity, efficiently switching between the glycolysis and oxidative phosphorylation energy metabolism pathways, which were accompanied by enhanced clonogenic survival. This data was supported in vivo, in pre-treatment OAC tumour tissue. Tumour ATP5B expression, a marker of oxidative phosphorylation, was significantly increased in patients who subsequently had a poor pathological response to neoadjuvant CRT. This suggests for the first time, a role for specific mitochondrial alterations and metabolic remodelling in the radioresistance of OAC.
Collapse
Affiliation(s)
| | - Stephen G. Maher
- Department of Surgery, Trinity College Dublin, Dublin, Ireland
- Cancer Biology and Therapeutics Lab, School of Biological, Biomedical and Environmental Sciences, University of Hull, Hull, United Kingdom
| | - Aoife Maguire
- Department of Pathology, St James’s Hospital, Dublin, Ireland
| | - James Phelan
- Department of Surgery, Trinity College Dublin, Dublin, Ireland
| | - Cian Muldoon
- Department of Pathology, St James’s Hospital, Dublin, Ireland
| | | | | |
Collapse
|
|
44
|
Huang RW, Chao YK, Wen YW, Chang HK, Tseng CK, Chan SC, Liu YH. Predictors of pathological complete response to neoadjuvant chemoradiotherapy for esophageal squamous cell carcinoma. World J Surg Oncol 2014; 12:170. [PMID: 24885430 PMCID: PMC4050419 DOI: 10.1186/1477-7819-12-170] [Citation(s) in RCA: 36] [Impact Index Per Article: 3.3] [Reference Citation Analysis] [Abstract] [Track Full Text] [Download PDF] [Figures] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/27/2014] [Accepted: 05/06/2014] [Indexed: 11/10/2022] Open
Abstract
BACKGROUNDS In this study, we evaluated the factors associated with a pathologic complete response (pCR) after neoadjuvant chemoradiotherapy (nCRT) for esophageal squamous cell carcinoma (ESCC). METHODS Pre-nCRT parameters in ESCC patients treated between 1999 and 2006 were analyzed to identify predictors of pCR. All patients received 5-fluorouracil/cisplatin-based chemotherapy and external beam radiation followed by scheduled esophagectomy. Variables were analyzed using univariate and multivariate analyses with pCR as the dependent variable. Estimated pCR rate was calculated with a regression model. RESULTS Fifty-nine (20.9%) of 282 patients achieved pCR. Univariate analysis identified four patient factors (age, smoking status, drinking history and hypertension), one pre-nCRT parameter (tumor length) as significant predictors of pCR (all P <0.05). On multivariate analysis, tumor length ≤3 cm (favorable, odds ratio (OR): 4.85, P = 0.001), patient age >55 years (favorable, OR: 1.95, P = 0.035), and being a non-smoker (favorable, OR: 3.6, P = 0.003) were independent predictors of pCR. The estimated pCR rates based on a logistic regression including those three predictors were 71%, 35 to approximately 58%, 19 to approximately 38%, and 12% for patients with 3, 2, 1 and 0 predictors, respectively. CONCLUSION Age, smoking habit and tumor length were important pCR predictors. These factors may be used to predict outcomes for ESCC patients receiving nCRT, to develop risk-adapted treatment strategies, and to select patients who could participate in trials on new therapies.
Collapse
Affiliation(s)
| | - Yin-Kai Chao
- Division of Thoracic and Cardiovascular Surgery, Chang Gung Memorial Hospital, Linkou, College of Medicine, Chang Gung University, Taoyuan, Taiwan.
| | | | | | | | | | | |
Collapse
|
|
45
|
Lahiff C, Schilling C, Cathcart MC, Mulligan N, Doran P, Muldoon C, Murray D, Pidgeon GP, Reynolds JV, MacMathuna P. Prognostic significance of neuroepithelial transforming gene 1 in adenocarcinoma of the oesophagogastric junction. Br J Surg 2013; 101:55-62. [DOI: 10.1002/bjs.9373] [Citation(s) in RCA: 6] [Impact Index Per Article: 0.5] [Reference Citation Analysis] [Abstract] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Accepted: 10/11/2013] [Indexed: 01/24/2023]
Abstract
Abstract
Background
Neuroepithelial transforming gene 1 (NET1) mediates tumour invasion and metastasis in a number of cancers, including gastric adenocarcinoma. It is an indicator of poor prognosis in breast cancer and glioma. This study examined NET1 expression and its prognostic significance in patients with adenocarcinoma of the oesophagogastric junction (AOG).
Methods
NET1 expression was measured by immunohistochemistry in a tissue microarray, constructed from biobanked tissue collected over a 10-year interval, and linked to a prospectively maintained clinical database.
Results
Using the Siewert classification for AOG, type I tumours expressed significantly higher levels of NET1, with lowest expression in type III and intermediate levels in type II (P = 0·001). In patients with AOG type III, NET1-positive patients were more likely to be female (P = 0·043), have advanced stage cancer (P = 0.035), had a higher number of transmural cancers (P = 0·006) and had a significantly higher median number of positive lymph nodes (P = 0·029). In this subgroup, NET1-positive patients had worse median overall (15 versus 23 months; P = 0·025) and disease-free (11 versus 36 per cent; P = 0·025) survival compared with NET1-negative patients.
Conclusion
Although existing data show differences in clinical and prognostic indices across AOG subtypes, there are no studies showing differences in tumour biology. These data suggest NET1, a known mediator of an aggressive tumour phenotype in a number of gastrointestinal cancers, is expressed differentially across AOG subtypes and may be of prognostic significance in the clinical management of this condition.
Collapse
Affiliation(s)
- C Lahiff
- Gastrointestinal Unit, Mater Misericordiae Hospital, Dublin, Ireland
| | - C Schilling
- Department of Pathology, Mater Misericordiae Hospital, Dublin, Ireland
| | - M-C Cathcart
- Department of Surgery, St James's Hospital and Trinity College, Dublin, Ireland
| | - N Mulligan
- Department of Pathology, Mater Misericordiae Hospital, Dublin, Ireland
| | - P Doran
- University College Dublin School of Medicine and Medical Science, Dublin, Ireland
| | - C Muldoon
- Department of Pathology, St James's Hospital and Trinity College, Dublin, Ireland
| | - D Murray
- University College Dublin School of Medicine and Medical Science, Dublin, Ireland
| | - G P Pidgeon
- Department of Surgery, St James's Hospital and Trinity College, Dublin, Ireland
| | - J V Reynolds
- Department of Surgery, St James's Hospital and Trinity College, Dublin, Ireland
| | - P MacMathuna
- Gastrointestinal Unit, Mater Misericordiae Hospital, Dublin, Ireland
| |
Collapse
|
|
46
|
Classification of Pathologic Response to Neoadjuvant Therapy in Esophageal and Junctional Cancer. Ann Surg 2013; 258:784-92; discussion 792. [DOI: 10.1097/sla.0b013e3182a66588] [Citation(s) in RCA: 66] [Impact Index Per Article: 5.5] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 12/19/2022]
|
|
47
|
Hsu PK, Chien LI, Huang CS, Hsieh CC, Wu YC, Hsu WH, Chou TY. Comparison of survival among neoadjuvant chemoradiation responders, non-responders and patients receiving primary resection for locally advanced oesophageal squamous cell carcinoma: does neoadjuvant chemoradiation benefit all? Interact Cardiovasc Thorac Surg 2013; 17:460-6. [PMID: 23728085 PMCID: PMC3745136 DOI: 10.1093/icvts/ivt216] [Citation(s) in RCA: 18] [Impact Index Per Article: 1.5] [Reference Citation Analysis] [Abstract] [Key Words] [MESH Headings] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/02/2013] [Revised: 03/27/2013] [Accepted: 04/16/2013] [Indexed: 12/21/2022] Open
Abstract
OBJECTIVES While neoadjuvant chemoradiation followed by surgery has been shown to improve the survival of patients with locally advanced oesophageal cancer, it is not known whether neoadjuvant chemoradiation has a beneficial or harmful effect on the non-responders. We aimed to compare the outcomes among neoadjuvant chemoradiation responders, non-responders and patients receiving primary oesophagectomies for resectable locally advanced oesophageal squamous cell carcinoma. METHODS Eighty-four non-T1-2N0 oesophageal squamous cell carcinoma patients were included. Thirty-eight patients received primary resection and 46 patients received neoadjuvant chemoradiation. The overall survival of chemoradiation responders (<50% residual tumour), non-responders (>50% residual tumour and those who shifted to definitive chemoradiation instead of surgery due to tumour progression) and patients receiving primary resection were compared. Clinical parameters were also compared between responders and non-responders. RESULTS There was no overall difference in survival between neoadjuvant chemoradiation and primary resection groups (2-year overall survival rates: 45.6 vs 54.3%, P = 0.442). In patients receiving neoadjuvant chemoradiation followed by surgery, pathological responders had significantly higher 2-year overall survival rates than non-responders (64.5 vs 38.9%, P = 0.043). While the pathological responders had the highest survival rate, clinicopathological non-responders (pathological non-responders and patients with tumour progression during the neoadjuvant chemoradiation period) demonstrated significantly worse outcomes than those receiving primary resection (32.0 vs 54.3%, P = 0.036). However, none of the clinical parameters, including blood profiles, images and baseline tumour characteristics, predicted the response to chemoradiation before treatment. CONCLUSIONS Neoadjuvant chemoradiation non-responders demonstrated no benefit and an even worse outcome compared with those receiving primary resection for locally advanced oesophageal squamous cell carcinoma. However, no significant clinical parameters could be implemented in the clinics to predict the response to neoadjuvant chemoradiation before treatment.
Collapse
Affiliation(s)
- Po-Kuei Hsu
- Department of Surgery, Division of Thoracic Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
- Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan
| | - Ling-I Chien
- Department of Nursing, Taipei Veterans General Hospital, Taipei, Taiwan
| | - Chien-Sheng Huang
- Department of Surgery, Division of Thoracic Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
- Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan
| | - Chih-Cheng Hsieh
- Department of Surgery, Division of Thoracic Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
| | - Yu-Chung Wu
- Department of Surgery, Division of Thoracic Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
| | - Wen-Hu Hsu
- Department of Surgery, Division of Thoracic Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
| | - Teh-Ying Chou
- School of Medicine, National Yang-Ming University, Taipei, Taiwan
- Institute of Clinical Medicine, National Yang-Ming University, Taipei, Taiwan
- Department of Pathology, Taipei Veterans General Hospital, Taipei, Taiwan
| |
Collapse
|
|
48
|
Aoyagi K, Tamaoki M, Nishumura T, Sasaki H. Technical considerations for analyzing EMT-MET data from surgical samples. Cancer Lett 2013; 341:105-10. [PMID: 23933174 DOI: 10.1016/j.canlet.2013.08.001] [Citation(s) in RCA: 3] [Impact Index Per Article: 0.3] [Reference Citation Analysis] [Abstract] [Key Words] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Received: 06/28/2012] [Revised: 06/21/2013] [Accepted: 08/02/2013] [Indexed: 11/26/2022]
Abstract
With an increase of neoadjuvant therapy, biopsy samples have become imperative for cancer research; however, what kind of difference between surgical and endoscopic biopsy samples in gene expression profiles was still unclear. Recently, we reported artificially induced epithelial-mesenchymal transition (aiEMT) in the surgical samples by comparison between gene expression profiles of both samples of the esophagus. This was also found in mouse epithelium under an ischemic condition for 4h. This study will evoke underestimation of the prognostic evaluation power of EMT related markers in past cancer research and prevalence of biopsy samples for in vivo expression profiling with low biases.
Collapse
Affiliation(s)
- Kazuhiko Aoyagi
- Division of Genetics, National Cancer Center Research Institute, Chuo-ku, Tokyo 104-0045, Japan
| | | | | | | |
Collapse
|
|
49
|
Valentini V, Bourhis J, Poortmans P, Coffey M. Donal Hollywood obituary. Radiother Oncol 2013. [DOI: 10.1016/j.radonc.2013.07.001] [Citation(s) in RCA: 0] [Impact Index Per Article: 0] [Reference Citation Analysis] [Track Full Text] [Journal Information] [Subscribe] [Scholar Register] [Indexed: 10/26/2022]
|
|
50
|
Luo A, Chen H, Ding F, Zhang Y, Wang M, Xiao Z, Liu Z. Small proline-rich repeat protein 3 enhances the sensitivity of esophageal cancer cells in response to DNA damage-induced apoptosis. Mol Oncol 2013; 7:955-67. [PMID: 23820115 DOI: 10.1016/j.molonc.2013.05.005] [Citation(s) in RCA: 13] [Impact Index Per Article: 1.1] [Reference Citation Analysis] [Abstract] [Key Words] [Journal Information] [Subscribe] [Scholar Register] [Received: 01/12/2013] [Revised: 05/28/2013] [Accepted: 05/28/2013] [Indexed: 12/12/2022] Open
Abstract
Small proline-rich repeat protein 3 (SPRR3) has been linked with the altered chemoradiosensitivity, however the underlying molecular mechanisms remain elusive. Here, we report that ectopic overexpression of SPRR3 enhanced the sensitivity of cells in response to DNA damage-induced apoptosis via loss of mitochondrial membrane potential (MMP), and increasing activation of caspase 3 in human esophageal cancer cell lines. Conversely, siRNA knockdown of SPRR3 reduced apoptosis. We found that SPRR3 was localized in mitochondria and interacted with Bcl-2 in vivo, thus facilitating Bax mitochondrial translocation and the subsequent release of cytochrome c, and thereby enhancing cell sensitivity to DNA damage stimuli. In clinical samples, expression of SPRR3 was associated with the pathologic response (P = 0.007 in radiotherapy group, P = 0.035 in preoperative radiotherapy group) and good survival of patients with locally advanced esophageal squamous cell carcinoma (ESCC, P = 0.008). Taken together, our results implicate that SPRR3 might serve as a radiation-sensitive predictor of ESCC.
Collapse
Affiliation(s)
- Aiping Luo
- State Key Lab of Molecular Oncology, Cancer Institute & Hospital, Chinese Academy of Medical Sciences, Beijing 100021, PR China
| | | | | | | | | | | | | |
Collapse
|