INTRODUCTION
Cardiovascular complications are mainly responsible for the high morbidity and mortality in diabetic patients. Diabetes itself is recognized as a strong independent risk factor for cardiovascular disease. According to the Framingham Heart Study, diabetes is associated with an odds ratio of 2-4 for cardiovascular mortality compared with nondiabetic subjects[1]. These results were confirmed in the recent Atherosclerosis Risk in Communities (ARIC) study[2].
There is a convincing evidence from epidemiological and pathophysiological studies that hyperglycemia per se is largely responsible for the harmful effects of diabetes. Today, evidence exists from long-term follow-up population-based studies in patients with type 1 and type 2 diabetes. This evidence clearly suggests that hyperglycemia is a key risk factor not only for diabetes-related disease, but also for cardiovascular and all-cause mortality. On the basis of these long-term observations, one can assume an increase in the risk of cardiovascular disease by 18% for each unit (%) glycated hemoglobin HbA1c[3]. In the Glucose Tolerance in Acute Myocardial Infarction study of patients with acute coronary syndrome, abnormal glucose tolerance was the strongest independent predictor of subsequent cardiovascular complications and death[4]. In the Asian Pacific Study, fasting plasma glucose was shown to be an independent predictor of cardiovascular events up to a level of 5.2 mmol/L[5].
Glucose level fluctuations and hyperglycemia are triggers for inflammatory responses via increased mitochondrial superoxide production and endoplasmic reticulum stress[6]. Inflammation leads to insulin resistance and β-cell dysfunction, which further aggravates hyperglycemia[7]. The molecular pathways that integrate hyperglycemia, oxidative stress, and diabetic vascular complications have been most clearly described in the pathogenesis of endothelial dysfunction[8], which is considered as the first step in atherogenesis according to the response to injury hypothesis[9].
Mitochondria play a pivotal role in ATP production through oxidative phosphorylation (OXPHOS), a metabolic mechanism that utilizes up to 90% of pyruvate generated from glucose in the cytoplasm[10]. Indeed, mitochondria are directly involved in aerobic glucose metabolism. Accordingly, high blood glucose, through deleterious effects of glucotoxicity and hyperglycemia-induced oxidative stress, may result in the rapid progress of mitochondrial dysfunction[11]. Alterations in mitochondrial DNA (mtDNA), known as homoplasmic and heteroplasmic mutations, may influence mitochondrial OXPHOS capacity, and in turn contribute to the magnitude of oxidative stress in micro- and macrovascular networks in diabetic patients. In this review, we critically consider the impact of mtDNA mutations on the pathogenesis of cardiovascular diabetic complications.
HETEROPLASMY VS HOMOPLASMY
Compared with nuclear DNA repair, mtDNA repair mechanisms are significantly less efficient[12]. This results in increased vulnerability of mtDNA to oxidative stress, and significantly higher mutation rates in the mitochondrial genome compared with the nuclear genome. Indeed, mitochondrial genomes have a higher load of deleterious mutations than nuclear genomes[13].
When damage to mtDNA is not repaired, it can result in a cascade of events ultimately leading to a number of diseases. A mitochondrial disorder can result from the substitution, deletion, and duplication of mtDNA bases. To add to the complexity of mitochondrial disorders, they can arise from one mtDNA mutation or from a number of independent mutations that in turn can lead to more than one disease type.
The naturally occurring circle of mtDNA is also referred to as wild-type. The number of mutations can increase in a particular tissue, while not being reflected in other parts of the body. The mixtures of wild-type and mutant mtDNA coexisting in the same mitochondria are referred to as heteroplasmic mutations. Mitochondrial mutations can also be homoplasmic in nature when cellular mitochondria contain all of the same mutant mtDNA.
Repeated cell division leads to the separation of heteroplasmic and homoplasmic cell lines in a phenomenon of random segregation. Similar process can be observed in the atherosclerotic plaque due to the clonal proliferation of smooth muscle cells (SMCs)[14]. Mutant mtDNA increases with aging, and the cellular energy capacity can decrease. This decrease in turn affects the threshold of minimal cell function[15].
MUTATION THRESHOLD
Although cells may harbor mutant mtDNA, the expression of disease is dependent on the percent of alleles bearing mutations. Modeling confirms that an upper threshold level might exist for mutations beyond which the mitochondrial population collapses, with a subsequent decrease in ATP[16]. This decrease in ATP results in the phenotypic expression of disease[17]. It is estimated that in many patients with clinical manifestations of mitochondrial disorders, the proportion of mutant DNA exceeds 50%[18].
For the MELAS (mitochondrial encephalopathy, lactic acidosis and stroke-like syndrome)-causing mutation m.3243 A>G in the mitochondrial gene encoding tRNALeu, which is also associated with diabetes plus deafness[19], a strong correlation between the level of mutational heteroplasmy and documented disease has been found. Increased percentages of mutant mtDNA in muscle cells (up to 71%) can lead to mitochondrial myopathy[20]. Levels of heteroplasmy of over 80% may lead to recurrent stroke[21] and mutation levels of 95% have been associated with MELAS[18].
Regardless of the type of mutation or the level of heteroplasmy in affected mitochondria, unrepaired damage leads to a decrease in ATP, which in turn causes the phenotypic manifestation of disease. The manifestation of disease not only depends on the ATP level but also on the tissue affected. Various tissues have differing levels of demand on OXPHOS capacity. To evaluate a tissue threshold, Leber’s hereditary optic neuropathy can be used as a model for mitochondrial neurodegenerative disease. For neural and skeletal muscle tissues, the tissue threshold should be as high as or higher than 90% of damaged (mutated) mtDNA[22]. To induce mitochondrial malfunctions, the tissue threshold of the cardiac muscle is estimated to be significantly lower (approximately 64%-67%)[23].
MUTATION THRESHOLD IN VASCULAR WALL TISSUE
For the vascular wall, the mutation threshold is thought to be high enough to induce acute mitochondrial dysfunction. For example, a heteroplasmy level of 95% of the MELAS mutation m.3243 A>G, was found in cytochrome C oxidase-deficient brain microvessels of patients who died from congestive heart failure[24]. Such a high level of mutated allele in brain microvessels is considered to be responsible for stroke-like episodes in the MELAS syndrome. Similarly, in a girl with severe mitochondrial encephalopathy, the vasculature of the affected left cerebral artery was almost homoplasmic for the m.3243A>T mutation in tRNALeu, but with a lower mutant load (about 50% heteroplasmy) in blood and skin fibroblasts[25].
However, in chronic vascular disease such as atherosclerosis, a mutation threshold in the affected vessel wall (e.g., in the postmortem aortic atherosclerotic plaques) was observed to be significantly lower. For example, for mutations m.3256 C>T, m.12315 G>A, m.15059 G>A, and m.15315 G>A, the heteroplasmy range of 18%-66% in the atherosclerotic lesions was 2-3.5-fold that in normal vascular tissue[26]. What are the mechanisms leading to mitochondrial dysfunction in diabetic vascular pathology at mtDNA heteroplasmy levels that are considerably lower than those required, for example, to induce mitochondrial myopathy and/or neuropathy?
MITOCHONDRIAL STRESS AND INSULIN RESISTANCE
As noted above, due to its proximity to reactive oxygen species (ROS), the mitochondrial genome may be particularly susceptible to oxidative damage because of its lack of histones and a deficient mismatch repair system. This combination could be responsible for heteroplasmy levels, e.g., the ratio of both normal and mutated mtDNA in tissues[27].
Mitochondrial dysfunction may be involved in insulin resistance in diabetic skeletal muscle. Expression of genes critical for mitochondrial function, such as the gene encoding peroxisome proliferator-activated receptor-γ coactivator 1α, is decreased in humans with insulin resistance[28]. Energy production is impaired in the muscle of insulin-resistant subjects[29]. Recent findings also implicate mitochondrial dysfunction in atherosclerosis[30].
Insulin resistance causes circulating fatty acids to increase. Increased oxidation of fatty acids by aortic endothelial cells was recently reported to accelerate production of superoxide by the mitochondrial electron transport chain[31]. This effect was associated with proatherogenic vascular effects, and prevented by either blocking of release of fatty acids from adipose tissue or inhibition of mitochondrial fatty acid oxidation, consistent with a role for increased mitochondrial metabolism in vascular disease. In macrophage foam cells, oxidized fatty acids may be then widely esterified and form a pool of oxidized ethers containing cholesterol and 7-ketocholesterol, which exhibit high resistance to lysosomal esterases, and indeed accumulate within the lysosomes of foam cells[32]. The accumulation of oxidized lipids in foam cells subsequently aggravates atherogenesis through the recruitment of new monocytes and macrophages to the lesion site, inducing a local proinflammatory reaction and further proatherogenic events[33]. Elevated blood glucose was shown to significantly increase the amount of foam cells and accumulation of oxidized low density lipoproteins in cultivated arterial wall cells[34].
Human atherosclerotic samples obtained during vascular surgery show greater mtDNA damage than non-atherosclerotic samples obtained from age-matched transplant donors[35]. Mitochondrial damage precedes the development of atherosclerosis and tracks the extent of the lesion in apoE-null mice, and mitochondrial dysfunction caused by heterozygous deficiency of a superoxide dismutase increases atherosclerosis and vascular mitochondrial damage in the same model[35].
Blood vessels destined to develop atherosclerosis may be characterized by inefficient ATP production due to the uncoupling of respiration and OXPHOS. Blood vessels have regions of hypoxia[2], which lower the ratio of state 3 (phosphorylating) to state 4 (nonphosphorylating) respiration[36]. Human atherosclerotic lesions have been known for decades to be deficient in essential fatty acids[37], a condition that causes respiratory uncoupling[38] and atherosclerosis[39].
Vascular inducible expression of uncoupling protein 1 (UCP1), the prototypical inner mitochondrial membrane anion transporter found in brown fat, increases atherosclerosis and several markers of oxidative damage in apoE-null mice[40]. Mitochondrial dysfunction resulting from UCP1 expression in blood vessels causes renin-dependent hypertension[40], and a mitochondrial mutation m.4291 T>C associated with hypertension has been described in humans[41]. Uncoupling increases respiration, which might account for evidence of increased oxidative modifications.
Takagi et al[42] reported the anti-atherosclerotic effect of the presence of the minor allele A of the m.5178 C>A polymorphism (in fact, a homoplasmic mutation) of the MT-ND2 gene in a Japanese population. Matsunaga et al[43] observed significantly less frequent plaque formation in the bilateral carotid arteries of type 2 diabetic carriers of the allele m.5178A thereby suggesting an anti-atherogenic effect of this mtDNA variant. Kokaze et al[44] then showed significantly higher serum levels of high-density lipoprotein in males carrying 5178A compared with those having 5178C, and reduced concentrations of serum triglycerides in female carriers of 5178A than in those with 5178C. Therefore, the finding by Kokaze et al[44] helps to explain, at least in part, the anti-atherogenic effect of the allele m. 5178A due to its relation with the favorable lipid profile[45]. The nucleotide change causes leucine-to-methionine substitution at codon 237 (Leu237Met) of the NADH dehydrogenase subunit 2 located in the loop between 7th and 8th transmembrane domains of the mitochondrial protein[42]. Given that this methionine residue is exposed at the surface of respiratory ComplexI, this residue may be available as an efficient oxidant scavenger. ComplexIaccepts electrons from NADH, transfers them to ubiquinone, and uses the energy released to pump protons across the mitochondrial inner membrane[46]. Thus, the Leu237Met replacement in the ND2 subunit might have a protective effect against oxidative damage to mitochondria.
In a pilot study performed in 192 unrelated participants taken randomly from a Russian population, 24 of whom (12.5%) were affected with type 2 diabetes, we observed that the level of m.14846 G>A heteroplasmy in circulating peripheral mononuclear blood cells was 1.3-fold higher in diabetic patients (34.5% vs 26.5%, P = 0.022) compared with non-diabetic subjects (unpublished data). This mutation occurs within the MT-CYB gene encoding cytochrome b and results in glycine-to-serine substitution at position 34, thus affecting the intermediate transfer of electrons in mitochondrial respiratory chains, reducing the enzymatic function of cytochrome B, and is thought to be associated with mitochondrial myopathies.
For reasons that are unclear, brown fat, the tissue defined by respiratory uncoupling, encases chest and neck blood vessels in humans[47]. Most fatty acid oxidation, which is promoted by peroxisome proliferator-activated receptor α (PPARα) activation, occurs in the mitochondria. Mitochondrial effects could explain why PPARα-deficient mice are protected from diet-induced insulin resistance and atherosclerosis[48] as well as glucocorticoid-induced insulin resistance and hypertension[49]. Caloric restriction, which improves features of insulin resistance, increases mitochondrial biogenesis and, surprisingly, enhances the efficiency of ATP production[50]. Dysfunctional mitochondria in cultured cells can be rescued by transfer of mitochondria from adult stem cells[51], raising the possibility of restoration of normal bioenergetics in the vasculature to treat atherosclerosis associated with insulin resistance.
GLUCOTOXICITY IN DIABETES AND MITOCHONDRIAL DYSFUNCTION
There are several reports suggesting a role for various mitochondrial mutations in diabetic complications. In a Japanese population, a total of eight mtDNA mutations, including m.3243 A>G, were found to be associated with reduced insulin secretion and increased risk of vascular diabetic complications[52]. Regarding the diabetes-associated mutation m.3243 A>G, a 4-fold higher accumulation of this mutation was observed in diabetic patients compared with non-diabetic subjects[53]. Furthermore, this mutation was associated with diabetes duration[54].
The mutant tRNALeu(UUR) can recognize all four nucleotides at the third position of the codon, giving rise to the translation of not only the usual UUR (R = A or G) leucine codons but also UUY (Y = C or U) phenylalanine codons, which could eventually lead to the incorporation of leucine into phenylalanine sites at a certain rate[55]. The resulting synthesis of premature proteins due to this mistranslation could affect cells considerably, even if there is only a minor amount (usually, 0.02%-0.03%) of the 3243 A>G mutant mtDNA. Of interest, m.3243 A>G itself increases the intracellular production of ROS[56], which could in turn cause secondary somatic mutations[57]. An increased frequency of somatic transversion mutations in two segments of mtDNA (control region and gene encoding tRNALeu(UUR)) was found in diabetic patients compared to sex- and age-matched healthy subjects, and the mutation incidence correlated with hyperglycemia, e.g., level of glycated hemoglobin HbA1c[58].
In diabetic hyperglycemia, harmful effects on the vascular endothelial cells could be mainly realized through glucotoxicity and uncontrolled ROS production. Under hyperglycemic conditions such as diabetes, free radical production may arise from glucose self-oxidation[59], oxidative degradation of Amadori products[60], interaction of advanced glycation end-products (AGEs) and the AGE receptor[61], and an increase in the redox potential[62]. Glucose, in its aldehyde form, reacts with the amino groups of proteins to form a Schiff base which rearranges to a stable ketoamine adduct[63]. This process, called non-enzymatic glycation of protein, is particularly enhanced in diabetes and causes enzyme inactivation through oxidation, formation of AGEs, and protein-protein cross-linking[6]. Accumulation of AGEs in the vascular wall is accompanied by infiltration of the vasculature with macrophages, which engulf AGEs and actively contribute to inducing a local inflammation at the injury site[64].
In the diabetic vascular wall, oxidative stress causes numerous deleterious effects on mitochondrial function, including enhanced mtDNA damage (as reflected by increased levels of 7,8-dihydro-8-oxoguanine, a potential mutagen[65], in blood and urine of diabetic subjects), irreversible modification and inactivation of mitochondrial enzymes, uncoupling of the respiratory chain, reduced OXPHOS capacity, and mitochondrial superoxide overproduction by endothelial cells[66]. Indeed, due to the extensive oxidative stress and gluxotoxicity, even lower levels of heteroplasmy mtDNA mutations may be enough to induce atherogenic and atherosclerotic changes in diabetic vessels.
The 4977bp “common deletion”, present in the mtDNA of patients with a variety of degenerative diseases, has been detected at low levels (an average of 0.35% of total mtDNA) in the SMCs in atherosclerotic lesions of the aorta of patients undergoing surgery for aneurysm, as well as in diseased aortas at autopsy[67]. There were no correlations observed between the amount of mtDNA4977 deletion in atherosclerotic lesions and the levels of adducts and oxidative damage to nuclear DNA for each patient, except for a significant increase in mtDNA4977 in subjects older than 72 years vs younger than 72 years[68]. The true physiological significance of age-dependent accumulation of this mitochondrial mutation is still unclear. However, the mtDNA4977 deletion was shown to accumulate much faster in brain and heart tissue due to greater metabolic activity and minimal cell turnover compared to other tissues[69,70]. Indeed, it is reasonable to suppose that cardiac tissue of ischemic patients may be more susceptible to the accumulation of mtDNA damage that, in turn, can be an important cause of additional mitochondrial dysfunction and cardiac function decline. Indeed, these mutations could alter cellular energy capacity, increasing mitochondrial oxidative damage, which additionally can exacerbate the defects of electron transport in cardiac tissue and produce deleterious ROS. The reason that the mtDNA4977 deletion is not substantially elevated in arterial wall cells is thought to be the nature of the cells themselves. These cells did not have the energy demands/proliferation rate of cells such as cardiac myocytes, in which tissue the “common deletion” has been historically detected at higher frequency[67].
STRUCTURAL ALTERATIONS OF MITOCHONDRIA IN VASCULAR PATHOLOGY
It is impossible to exclude that mtDNA mutations in vascular diseases might be accompanied by structural changes in the mitochondria. Even though there are a large number of electron-microscopic studies that described structural changes of vascular cells in different vascular diseases[71-74], there is a paucity (if any) of ultrastructural studies devoted solely to examining morphological changes of mitochondria in vascular diseases.
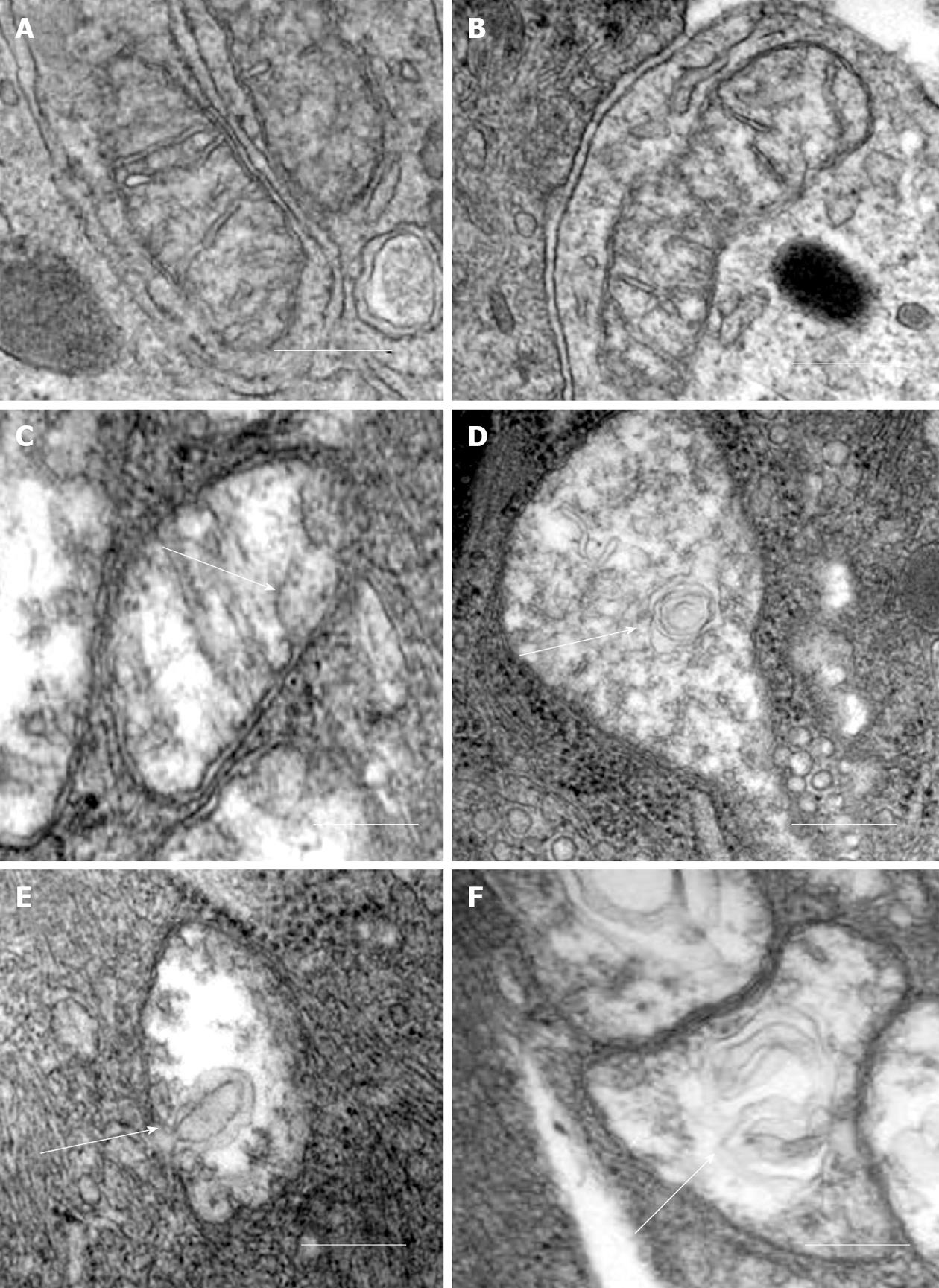
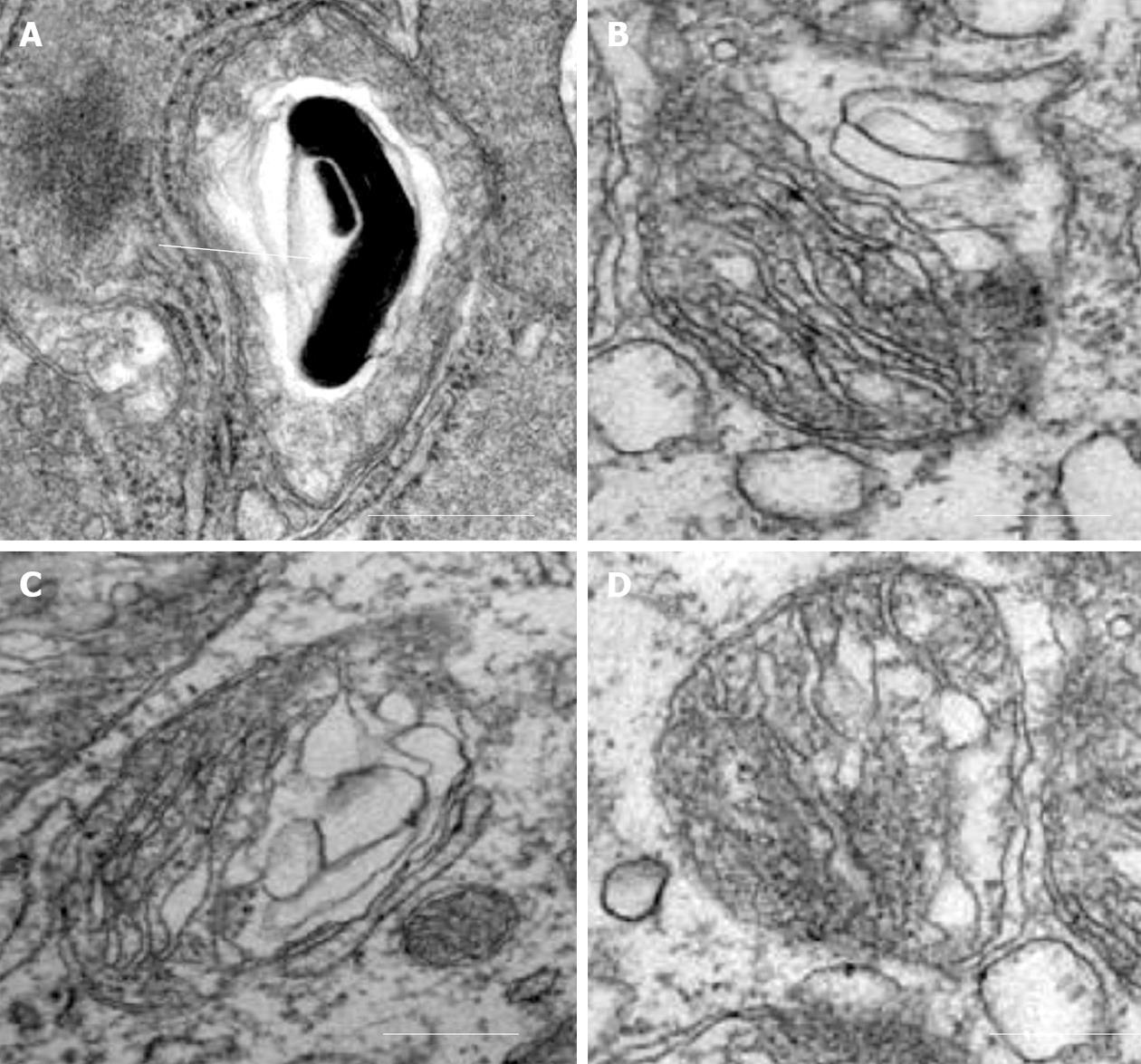
Our recent electron microscopic study of human atherosclerotic lesions revealed marked structural alterations of intimal cell mitochondria (unpublished data). The study showed that in atherosclerotic lesions, mitochondria exhibited different appearances of their internal organization (Figures 1 and 2). While in some mitochondria, cristae were distinct and the surrounding membranes were well preserved (Figure 1A and B), others were characterized by disruption of cristae structures (Figure 1A-F). In mitochondria with disrupted cristae, the formation of vacuole-like structures was evident (Figure 1C-F). The zones where the formation of vacuole-like structures occurred were characterized by edema of the mitochondrial matrix (Figure 1C-F). In some intimal cells located in atherosclerotic lesions, the presence of myelin-like structures was observed in edematous mitochondria (Figure 2A). The fact that in the same arterial specimens, only some mitochondria were found to be swollen or showed destructive alteration while other were “intact” indicates that the structural alterations of mitochondria, observed in the present study, were not a reflection of autolysis degeneration which could occur in autopsy material, but rather represented in situ morphological heterogeneity of mitochondrial appearances in the arterial intima.
Figure 1 Different ultrastructural appearances of mitochondria in the arterial intima.
A, B: “Intact” appearances of mitochondria with well-defined cristae and well-preserved surrounding membranes; C-F: Destructive alterations of cristae and the formation of vacuole-like structures (shown by arrows) in zones of edematous matrix of mitochondria. Electron microscopy, scale = 200 nm.
Figure 2 Alterations of mitochondria in intimal cells in atherosclerotic lesions.
A: Myelin-like structure within a swollen mitochondrion (shown by arrow); B-D: Destruction of cristae and surrounding membranes of mitochondria. Electron microscopy, scale = 200 nm.
CONCLUSION
Overall, somatic heteroplasmic mtDNA mutations play a non-redundant role in the development of cardiovascular complications of diabetes. The mutation threshold required for phenotypic (pathogenic) expression of a mutation may be significantly reduced by the presence of diabetic hyperglycemia accompanied by uncontrolled oxidative stress, glucotoxicity, ROS production and abnormalities in lipid metabolism. Furthermore, due to the phenomenon of clonal proliferation of SMCs in the atherosclerotic lesion[75], mtDNA mutation whose heteroplasmy exceeds a threshold level may accumulate in the arterial wall and further contribute to mitochondrial dysfunction in the atheroma. To date, our knowledge about the role of somatic heteroplasmic mtDNA mutations in atherogenesis and other pathological changes in diabetic vessels is still in its infancy. Actually, each etiological mtDNA mutation has its own heteroplasmy threshold that needs to be measured. Evaluation of the true mutation threshold is seriously hampered by significant heterogeneity in heteroplasmy of a mutation of interest in neighboring tissues. In addition, little is known about the precise mechanism by which mutations potentially involved in vascular abnormalities in diabetes could contribute to the pathogenesis of diabetic vasculopathy. It is also important to evaluate the functional consequences of each disease-associated mtDNA mutation depending on its heteroplasmy level. Substantial data obtained about the functionality of homoplasmic mtDNA mutations associated with maternally inherited forms of hypertension[76] may be helpful to select an optimal strategy for functional analysis of mutations associated with diabetic vascular complications.
Peer reviewer: Mustafa Yildiz, MD, PhD, Associate Professor, EC, Cardiologist, Internal Medicine Specialist and Physiologist, Department of Cardiology, Kartal Kosuyolu Yuksek Ihtisas Educational and Research Hospital, 81410 Istanbul, Turkey
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM