Published online Jul 26, 2021. doi: 10.4330/wjc.v13.i7.223
Peer-review started: January 17, 2021
First decision: February 14, 2021
Revised: February 26, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: July 26, 2021
Fractional flow reserve (FFR) measurement is commonly used in the cardiac catheterization laboratory to assess the functional significance of coronary arterial plaques. Robust real-world data on complications and modes of failure of FFR guidewires are limited.
To characterize these outcomes by analyzing the post-marketing surveillance data from the United States Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE) database for commonly used FFR guidewi
The MAUDE database was queried from January 2010 through April 2020 for 3 FFR guidewires [PressureWireTM X (Abbott), CometTM (Boston Scientific), and VerrataTM (Philips)] by searching for the following events: “Injury”, “malfunction”, “death”, and “other”. This yielded 544 reports. After excluding incomplete reports, 486 reports were analyzed.
Guidewire tip fracture was the most commonly reported mode of failure, in 174 (35.8%) cases followed by guidewire kinking (n = 152, 31.3%), communication failure (n = 141, 29.0%), and shaft fracture (n = 67, 13.8%). In total, 133 (27.4%) device failures resulted in patient adverse events. The most common adverse event was retained guidewire tip, in 71 (53.4%) cases, followed by freshly de
FFR guidewire failures can occur because of various mechanisms and cause patient adverse events. The MAUDE database serves as an important platform for improved collaboration among clinicians, device manufacturers, and regulators to improve device performance and optimize patient outcomes. Our analysis provides mechanistic insights of FFR guidewire failure and associated adverse events but cannot verify causality or provide a comparison among different guidewires.
Core Tip: We analyzed post-marketing surveillance data from the Food and Drug Administration Manufacturer and User Facility Device Experience database to outline the most common adverse events and modes of failure encountered with Fractional Flow Reserve (FFR) coronary guidewires. Guidewire tip fracture was the most com
- Citation: Khalid N, Pandey Y, Khalid U, Kamran H, Wermers JP, Chhabra L, Alam M, Jneid H, Kayani WT. Modes of failure with fractional flow reserve guidewires: Insights from the manufacturer and user facility device experience database. World J Cardiol 2021; 13(7): 223-229
- URL: https://www.wjgnet.com/1949-8462/full/v13/i7/223.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i7.223
Fractional flow reserve (FFR) is an essential measurement in the cardiac catheterization laboratory to assess intracoronary physiology. It is obtained by using a pressure sensing guidewire to calculate flow in the epicardial coronary arteries and determine the functional significance of stenosis. The benefits of an FFR-based revascularization strategy in coronary artery disease are well-established. Landmark clinical trials[1-5] have demonstrated that an FFR-guided decision to perform per
Performing FFR requires the insertion of an additional guidewire into the patient’s arterial system, which increases the risk of procedural complications. However, robust real-world data on the complications and modes of failure of commonly used FFR guidewires are limited. We aim to characterize these outcomes by analyzing the post-marketing surveillance data from the United States Food and Drug Administration (FDA) Manufacturer and User Facility Device Experience (MAUDE) database for commonly used FFR guidewires.
The MAUDE database is an electronic repository created by the FDA to capture major adverse events involving medical devices[7]. Reporting is either mandatory (manufacturers and device-user facilities) or voluntary (medical personnel, patients, and consumers). Developed in the 1990s, the database is updated monthly, with each report containing information on the device, event date, and event description by the provider and the manufacturer. The MAUDE database was queried from January 1, 2010, through April 1, 2020, for three commonly utilized FFR guidewires [Pressure
Tables 1 and 2 show a complete list of reported modes of failure and adverse patient events, respectively, categorized by each FFR coronary guidewire. Percentages repre
| Device mode of failure | VerrataTM (Philips), n = 199 | CometTM (Boston Scientific), n = 180 | PressureWireTM X (Abbott), n = 107 | Total, n = 486 |
| Guidewire distal tip fracture | 55 (27.6) | 68 (37.8) | 51 (47.7) | 174 (35.8) |
| Guidewire kinking | 37 (18.6) | 103 (57.2) | 12 (11.2) | 152 (31.3) |
| Communication failure | 64 (32.2) | 59 (32.8) | 18 (16.8) | 141 (29.0) |
| Failure to advance guidewire | 62 (31.2) | 36 (20.0) | 14 (13.1) | 112 (23.0) |
| Peeled guidewire coating | 6 (3.0) | 83 (46.1) | 0 (0) | 89 (18.3) |
| Guidewire shaft fracture | 25 (12.6) | 22 (12.2) | 10 (9.3) | 67 (13.8) |
| Adverse patient events | VerrataTM (Philips), n = 58 | CometTM (Boston Scientific), n = 29 | PressureWireTM X (Abbott), n = 46 | Total, n = 133 |
| Retained guidewire tip | 27 (46.6) | 22 (75.9) | 22 (47.8) | 71 (53.4) |
| Stent dislodgement | 18 (31.0) | 0 (0) | 8 (17.4) | 26 (19.6) |
| Vessel dissection | 8 (13.0) | 4 (13.8) | 11 (23.9) | 23 (17.3) |
| Death | 2 (3.4) | 2 (6.9) | 3 (6.5) | 7 (5.3) |
| Vessel perforation | 3 (5.2) | 1 (3.5) | 2 (4.4) | 6 (4.5) |
This study demonstrates the modes of failure of commonly used FFR guidewires and highlights the potential for adverse patient outcomes. Two general categories of guidewire failure can occur: structural failure and errors in signal communication. The most common structural failures reported in this study were distal tip fractures (35.8%), kinking (31.3%), peeled coating (18.3%), and shaft fractures (13.8%). A majority of devices in this study failed from more than one of the above mechanisms. Most structural failures were attributed to operator handling issues, defined as having occurred at any point after the device was removed from packaging. Structural failures add complexity for the operator, as demonstrated by 23% of reports noting the inability to advance the guidewire. Structural failures are also directly associated with patient harm: 53% of adverse patient events were related to fractured guidewire tips that remained in the coronary arteries. Retained guidewire fragments increase the risk of dissection, embolization, and thrombus formation and necessitate further interven
The other category of guidewire failure is an error in signal communication, which contributed to 29% of guidewire failures in this study. Pressure sensors at the tip of FFR guidewires transmit data to an external hub that processes information for clinician interpretation. In all three FFR guidewires assessed in this study, commu
FFR guidewires can cause patient adverse events. This study demonstrates that vessel dissection, vessel perforation, and stent dislodgement can occur with FFR guidewire use. These are clinically significant complications that must be recognized early and managed carefully to avoid further patient harm. The age of dislodged stents was not documented in the reports; however, operators should exercise caution when advancing or withdrawing an FFR guidewire across any freshly deployed stents. Seven deaths were reported among the cases reviewed. While this is noteworthy, there is not enough information to link the use of FFR guidewires with these deaths.
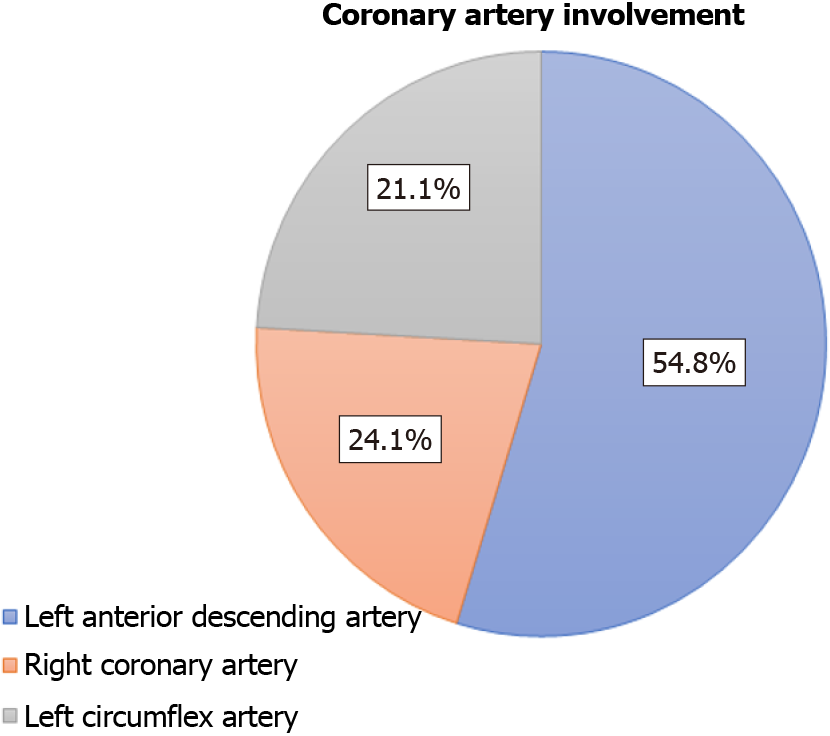
A majority of all reported events (54.8%) occurred in the LAD, followed by 24.1% in the right coronary artery and 21.1% in the left circumflex artery. A higher rate of reported failures in the LAD may be attributed to generally higher rates of FFR procedures performed in this vessel; however, this information was not captured in our data. Additionally, coronary characteristics such as tortuosity, calcification, and disease severity can also impact individual coronary outcomes. Without this in
The MAUDE database has several inherent limitations that impact the interpretation of our study. Notably, only cases with adverse events are reported in the database, and reporting is partially voluntary. Successful cases are not reported, and there is no information on the overall frequency of the device use. Without this information, we cannot derive the incidence of failure rates associated with the use of these guidewires or compare outcomes among different devices. Adverse events may be reported both by users and manufacturers, leading to duplicate reports and difficulty in discriminating. Another notable limitation of the database is that data are provided in a non-standardized narrative form. Not all failed devices were returned to the manufacturer for standard inspections. Without standardized information on the clinical context of failure events, these data cannot be used to imply causality or comparison between procedures or devices.
The landmark clinical trials that support the routine frontline use of FFR did not specifically address adverse events related to the use of FFR guidewires. Conse
Fractional flow reserve (FFR) measurement is an essential tool in the cardiac catheterization laboratory to assess the functional significance of coronary artery lesions. Robust real-world data on the commonly reported complications and modes of failure associated with the FFR guidewires are scarce.
The landmark clinical trials that support routine physiologic lesion assessment with FFR did not specifically address adverse events associated with the use of FFR guidewires. Accordingly, this provided us the impetus to explore common short
The objective of our study was to investigate the most commonly reported adverse events and failure modes associated with commonly used FFR guidewires by analy
We queried the MAUDE database from January 2010 through April 2020 for 3 FFR guidewires [PressureWireTM X (Abbott), CometTM (Boston Scientific), and VerrataTM (Philips)] by searching for the following events: “Injury”, “malfunction”, “death”, and “other”. The search yielded 544 reports. After excluding incomplete and duplicate reports, 486 reports were included in the final analysis.
The most commonly reported mode of failure was guidewire tip fracture described in 174 (35.8%) cases followed by guidewire kinking (n = 152, 31.3%), communication failure (n = 141, 29.0%), and shaft fracture (n = 67, 13.8%). One hundred thirty-three (27.4%) device failures caused patient adverse events. The most commonly reported adverse event was retained guidewire tip described in 71 (53.4%) cases, followed by freshly deployed stent dislodgment (n = 26, 19.6%) and coronary artery dissection (n = 23, 17.3%). Seven deaths were reported.
FFR guidewire failures can occur because of myriad mechanisms and cause patient adverse events. Understanding the methods of FFR guidewire failure is critical for interventionalists to develop operational awareness, forebode challenging situations, evaluate common complications, and assuage adverse patient events. The MAUDE database serves as an important pulpit for improved collaboration among physicians, device manufacturers, and regulators to improve device performance and optimize patient outcomes.
Intermediate coronary lesions are commonly encountered during cardiac catheterization and present a diagnostic dilemma. Physiologic testing using a pressure wire-based system is appropriate for these lesions. The introduction of newer nonhy
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Leowattana W S-Editor: Fan JR L-Editor: A P-Editor: Yuan YY
| 1. | Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van' t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2974] [Cited by in F6Publishing: 2851] [Article Influence: 190.1] [Reference Citation Analysis (0)] |
| 2. | De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P, Fearon WF; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1837] [Cited by in F6Publishing: 1831] [Article Influence: 152.6] [Reference Citation Analysis (0)] |
| 3. | Li J, Elrashidi MY, Flammer AJ, Lennon RJ, Bell MR, Holmes DR, Bresnahan JF, Rihal CS, Lerman LO, Lerman A. Long-term outcomes of fractional flow reserve-guided vs. angiography-guided percutaneous coronary intervention in contemporary practice. Eur Heart J. 2013;34:1375-1383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Smits PC, Abdel-Wahab M, Neumann FJ, Boxma-de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E; Compare-Acute Investigators. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234-1244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 444] [Cited by in F6Publishing: 494] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 5. | Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, Kim JH, Chae IH, Yoon JH, Her SH, Seung KB, Chung WY, Yoo SY, Lee JB, Choi SW, Park K, Hong TJ, Lee SY, Han M, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park SJ; IRIS-FFR Investigators†. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017;135:2241-2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, Wijns W. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928-2934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 573] [Cited by in F6Publishing: 587] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 7. | FDA. MAUDE database. [cited 10 January 2021]. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm. [Cited in This Article: ] |
| 8. | Khalid N, Javed H, Rogers T, Hashim H, Shlofmitz E, Wermers JP, Chen Y, Musallam A, Khan JM, Torguson R, Bernardo NL, Waksman R. Adverse events with orbital atherectomy: an analytic review of the MAUDE database. EuroIntervention. 2020;16:e325-e327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Al-Moghairi AM, Al-Amri HS. Management of retained intervention guide-wire: a literature review. Curr Cardiol Rev. 2013;9:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Kern MJ. Comparing FFR tools: New wires and a pressure microcatheter. Cath Lab Digest. 2016;24:5 [cited 10 January 2021]. Available from: https://www.cathlabdigest.com/article/Comparing-FFR-Tools-New-Wires-Pressure-Microcatheter. [Cited in This Article: ] |