Copyright
©2012 Baishideng Publishing Group Co.
World J Cardiol. Apr 26, 2012; 4(4): 90-102
Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.90
Published online Apr 26, 2012. doi: 10.4330/wjc.v4.i4.90
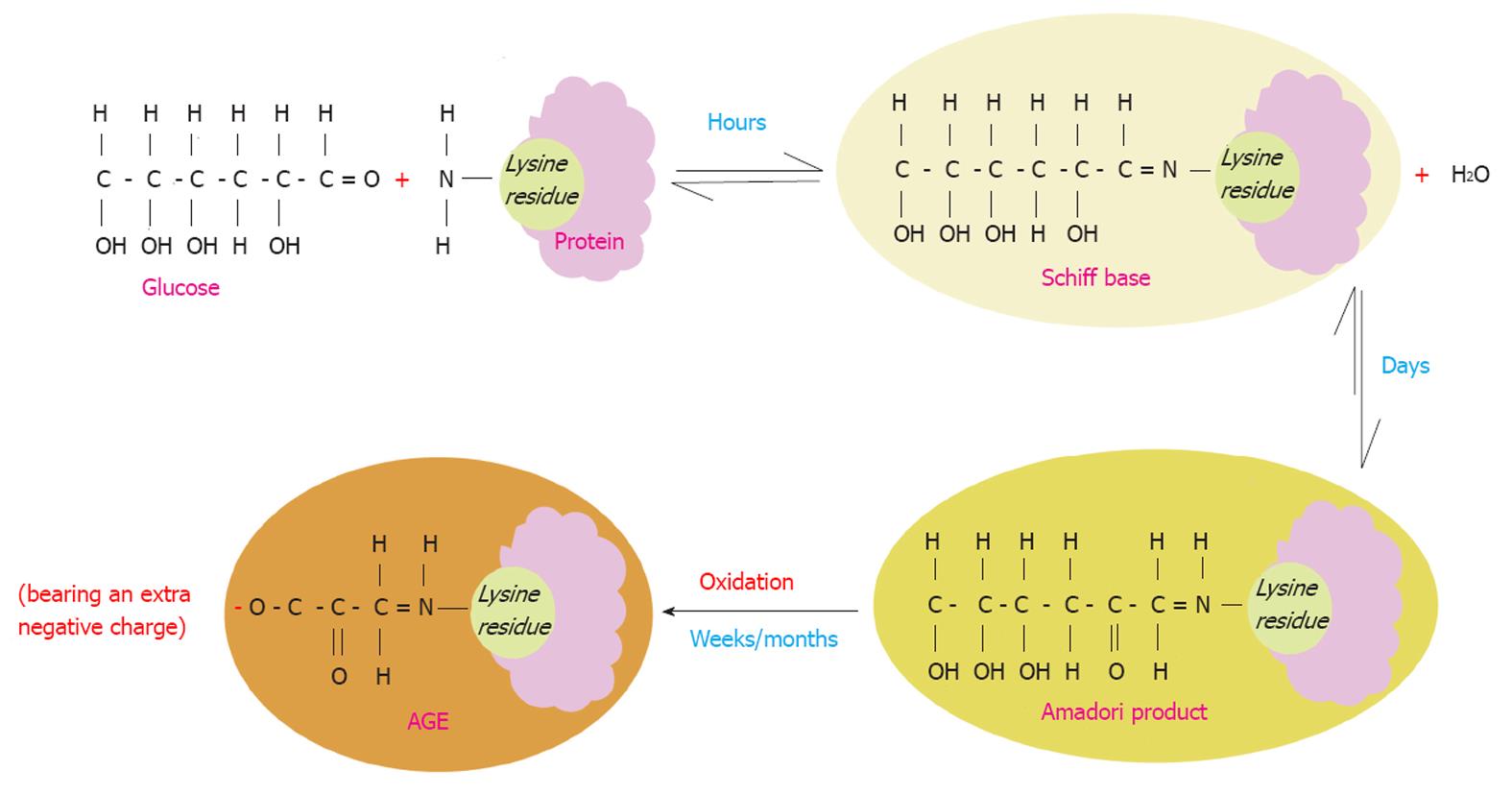
Figure 1 Maillard reaction.
Schematic illustration of the Maillard process that starts by a non-enzymatic reaction of a protein lysine residue (as an example of the most common residues that get glycated) and glucose with consequent loss of a water molecule. This is a reversible reaction that takes place over a few hours leading to the formation of an unstable compound (Schiff base). The latter undergoes molecular rearrangements over a period of days yielding a more stable compound known as (the Amadori product). Over months/years, the Amadori product turns to a very stable compound known as advanced glycation end product (AGE) through a series of reactions such as oxidation. Therefore AGE molecules acquire more negative charges. AGEs are irreversible once they are formed. The Maillard reaction leads to denaturation and browning of the modified proteins.
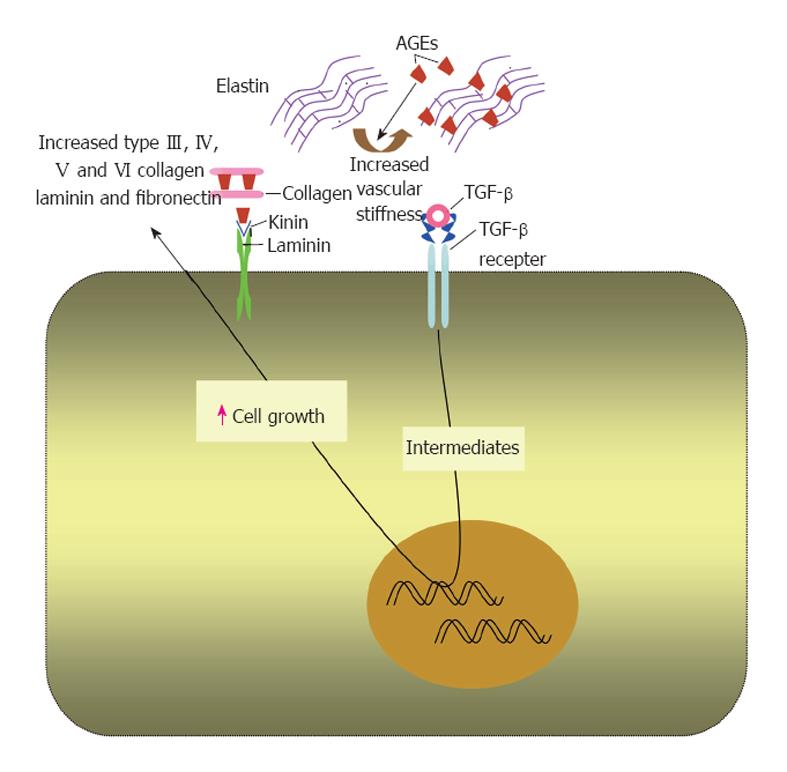
Figure 2 Effects of advanced glycation end products on extracellular matrix proteins.
In extracellular matrix, advanced glycation end products (AGEs) form on different molecules as collagen, laminin and elastin. This alters the physiological properties of the matrix and increases its stiffness. AGEs upregulate transforming growth factor (TGF)-β that increases the production of extracellular matrix components by binding to its receptor.
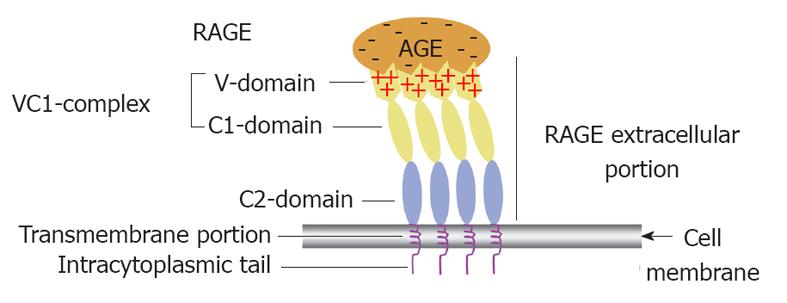
Figure 3 Receptor for advanced glycation end product (i.
e., colon) receptor structure and its functional implication in binding different advanced glycation end products. A diagrammatic illustration of the structure of the receptor for advanced glycation end product (RAGE) showing that it is composed of an extracelluar portion, a transmembrane portion and an intracytoplasmic tail. The extracelluar portion comprises three domain V, C1 and C2. The first two are believed to work together as a single functional complex (VC1) whereas the C2 domain remains attached to the VC1 complex but works independently from it. The diagram also illustrates how multiple RAGE receptors polymerise within the cell membrane to facilitate high affinity binding of the positively charged V domain with the negatively charged advanced glycation end products (AGEs) independent of their chemical structure. That is why RAGE is considered one of the pattern recognition receptors.
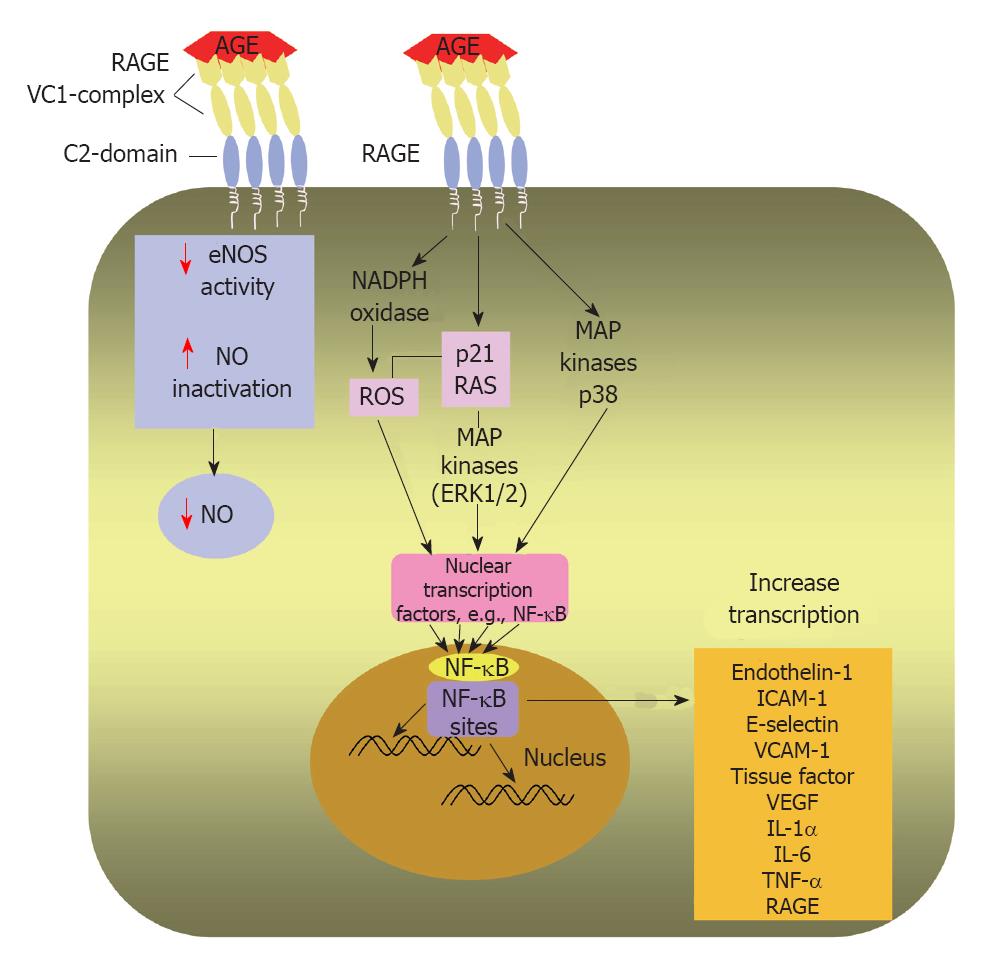
Figure 4 Intracellular effects of AGEs after AGE/RAGE binding.
Diagrammatic representation of AGE/RAGE interaction on the surface of an endothelial cell leads to transduction of a signalling cascade; activates nicotinamide adenine dinucleotide phosphate oxidase and enhances ROS production and phosphorylate p21 RAS and MAPKs. Moreover, the AGE/RAGE interaction induces signalling through activation of p38 MAPK. A main step in AGE/RAGE signalling is activation of NF-κB and its translocation to the nucleus, where it enhances transcription of target genes as endothelin-1, ICAM-1, E-selectin and tissue factor. Hence, AGE/RAGE binding triggers an inflammatory cascade. AGE may decrease NO availability by reducing eNOS activity and by inactivating NO. AGE: Advanced glycation end-product; RAGE: Receptor for advanced glycation end products; ROS: Reactive oxygen species; MAPKs: Mitogen-activated protein kinases; ERK1/2: Extracellular signal-regulated kinase 1/2; NF-κB: Nuclear factor kappa B; ICAM-1: Intercellular adhesion molecule-1; VCAM-1: Vascular cell adhesion molecule-1; VEGF: Vascular endothelial growth factor; IL-1α: Interleukin-1α; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-α; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide.
- Citation: Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World J Cardiol 2012; 4(4): 90-102
- URL: https://www.wjgnet.com/1949-8462/full/v4/i4/90.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i4.90