Copyright
©The Author(s) 2021.
World J Biol Chem. Mar 27, 2021; 12(2): 15-37
Published online Mar 27, 2021. doi: 10.4331/wjbc.v12.i2.15
Published online Mar 27, 2021. doi: 10.4331/wjbc.v12.i2.15
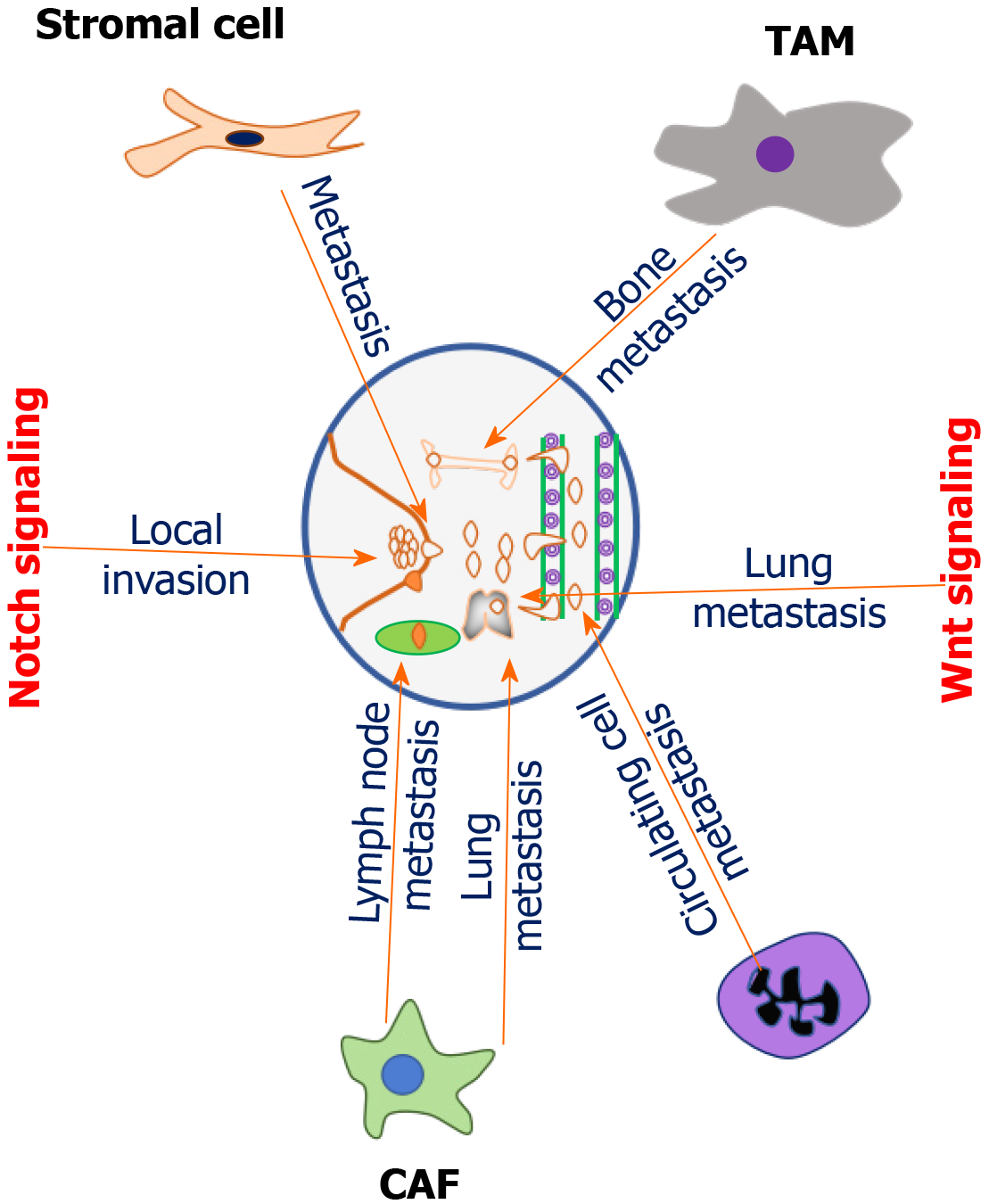
Figure 1 Role of tumor microenvironment components in promoting breast cancer metastasis.
Breast tumor microenvironment is typified with different types of components with typical cellular and molecular functions. Tumor associated stromal cells promote metastasis through the induction of cytokine (CXCL8) production by activating the expression of notch1 via TNFα-induced p65 activation. Cancer-associated fibroblasts (CAFs) accelerate lymph node metastasis of triple-negative breast cancer (TNBC) cells by increasing the intrusion of polarized macrophages in TNBC patients. Tumor associated macrophages promote bone metastasis by secreting interleukin 12. CAFs promoted lung metastasis of TNBC cells in the homograft tumor model by activating transforming growth factor β. Cancer associated neutrophils mediates metastasis by leukotrienes. Neutrophils enhance metastatic ability of circulating breast cancer (BC) cells. Notch signaling promotes local invasion of BC cells. Wnt signaling modulates metastasis of BC cells to the lung. TAM: Tumor-associated macrophage; CAF: Cancer-associated fibroblast.
Figure 2 Modulation of angiogenesis by innate immune cells and other communicators in breast tumor microenvironment.
In breast tumor microenvironment, innate immune cells and other communicators modulate angiogenesis. Tumor-associated macrophages promote angiogenesis by increasing the expression of CCL18, CD34, and microvascular density and assisting breast cancer cell modeling into stem cells for recruitment of immune-suppressive cells. Cancer-associated fibroblasts promote tumor angiogenesis by enhancing the expression of interleukin (IL) 6, IL-8, IL-11 and IL-15 as well as by changing the balance between pro-and anti-angiogenic factors via hypoxia-induced angiogenesis regulator. Tumor-derived exosomal Annexin II induce angiogenesis by recruiting macrophages to secrete IL-6 and tumor necrosis factor-α by activating p38MAPK, nuclear factor-κappa beta, and STAT3 signaling pathways. Exosomal miRNAs contribute to the development of tumor angiogenesis by enhancing the vasculature remodeling genes, Ephrin A3 and PTP1B. TAM: Tumor-associated macrophage; CAF: Cancer-associated fibroblast; HAIR: Hypoxia-induced angiogenesis regulator; NF-κβ: Nuclear factor-κappa beta; TNF: Tumor necrosis factor; IL: Interleukin; BC: Breast cancer.
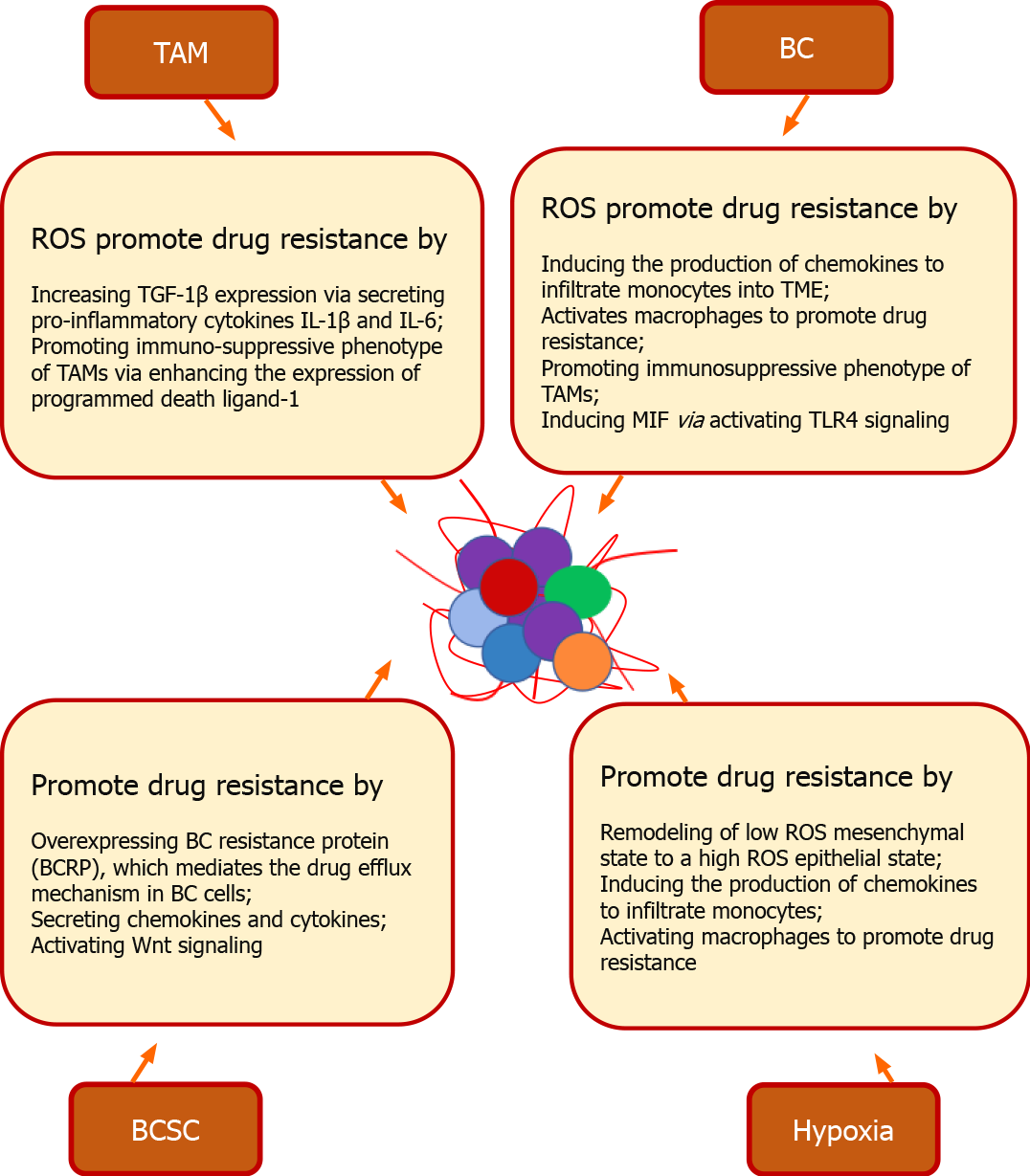
Figure 3 Cancer stem cell and redox mediated drug resistance in breast cancer.
In breast tumor microenvironment (TME), a fine orchestration between breast cancer (BC) stem cells (BCSC) and TME cells promotes drug resistance. BCSC promote drug resistance by overexpressing BC resistance protein, which mediates the drug efflux mechanism in BC cells. Stem cells derived from bone marrow, adipose tissue, and fibroblast enhances the drug resistance by activating developmental pathways via secreting chemokines and cytokines. Wnt signaling of BCSC along with glutathione overexpressing genes mediates resistance. Hypoxic TME promotes drug resistance by remodeling of cells from a low reactive oxygen species (ROS) mesenchymal state to a high ROS epithelial state. High ROS from cancer cells induces the production of chemokines to infiltrate monocytes into TME and activates macrophages to promote drug resistance. ROS also induces drug resistance by promoting the immunosuppressive phenotype of tumor-associated macrophages via enhancing the expression of programmed death ligand-1 via NF-κB (nuclear factor-κappa beta) signaling. Multinucleated cells promote drug resistance by increasing the secretion of vascular endothelial growth factor and macrophage migration inhibitory factor (MIF) via RAS/MAPK pathway-dependent hypoxia-inducible factor-1α. Macrophage derived ROS induces drug resistance by increasing transforming growth factor 1β expression via secreting pro-inflammatory cytokines interleukin (IL)-1β and IL-6. ROS also facilitates drug resistance by inducing MIF via promoting phosphorylation of ERK. via activating TLR4 (toll-like receptor 4) signaling. TAM: Tumor-associated macrophage; BC: Breast cancer; ROS: Reactive oxygen species; TGF: Transforming growth factor; IL: Interleukin; TME: Tumor microenvironment; MIF: Macrophage migration inhibitory factor; TLR: Toll-like receptor; BCSC: Breast cancer stem cell.
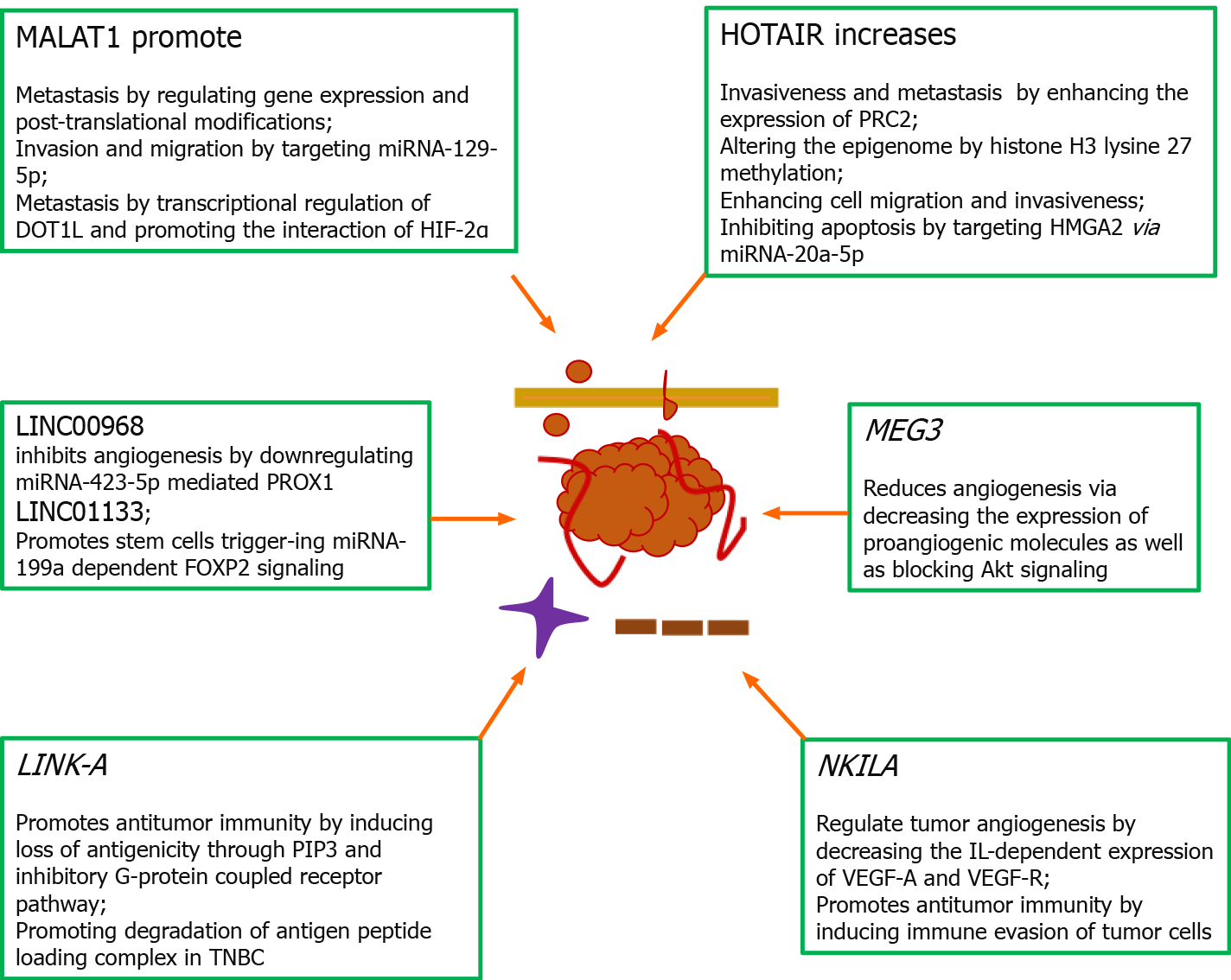
Figure 4 Role of long non-coding RNAs in the pathophysiology of breast cancer.
Long non-coding RNAs are reported to mediate pathophysiological developments such as metastasis as well as immune suppression within the tumor microenvironment. Metastasis-associated lung adenocarcinoma transcript 1 promotes growth and metastasis by controlling gene expression and post-translational modifications, invasion and migration by targeting tumor suppressor miRNA-129-5p, metastasis by transcriptionally regulating disruptor of telomeric silencing 1-like and promoting the interaction of hypoxia-inducible factor-2α. HOTAIR (HOX antisense intergenic RNA) increases invasiveness and metastasis by enhancing the expression of polycomb repressive complex 2 and altering the epigenome by histone H3 lysine 27 methylation, enhancing cell migration and invasiveness and inhibiting apoptosis through targeting high mobility AT-hook 2 via miRNA-20a-5p. MEG3 reduced angiogenesis by decreasing the expression of proangiogenic molecules as well as blocking Akt signaling. NKILA [nuclear factor-κappa beta (NF-κB) interacting lncRNA] negatively regulates tumor angiogenesis by decreasing the interleukin-dependent expression of vascular endothelial growth factor (VEGF)-A and VEGF-R through inhibiting NF-κB signaling and promotes antitumor immunity by inducing immune evasion of tumor cells via sensitizing T-cells through activation-induced cell death mechanism[57]. LINC00968 inhibits capillary formation by downregulating miRNA-423-5p mediated PROX1. LINC01133 promotes stem cell phenotypes by triggering miRNA-199a dependent FOXP2 signaling via modulation of Kruppel-like factor 4. Silencing of lncRNA-21 in tumor-associated macrophages induced apoptosis and reduced cell migration and invasion. LINK-A promotes antitumor immunity by inducing loss of antigenicity through PIP3 and inhibitory G-protein coupled receptor pathway as well as attenuating protein kinase A-dependent phosphorylation of E3 ubiquitin ligase TRIM71 and degradation of antigen peptide loading complex in triple-negative breast cancer. MALAT1: Metastasis-associated lung adenocarcinoma transcript 1; DOT1L: Disruptor of telomeric silencing 1-like; HIF: Hypoxia-inducible factor; HOTAIR: HOX antisense intergenic RNA; PRC2: Polycomb repressive complex 2; HMGA2: High mobility AT-hook 2; TNBC: Triple-negative breast cancer; IL: Interleukin; VEGF: Vascular endothelial growth factor.
- Citation: Malla RR, Farran B, Nagaraju GP. Understanding the function of the tumor microenvironment, and compounds from marine organisms for breast cancer therapy. World J Biol Chem 2021; 12(2): 15-37
- URL: https://www.wjgnet.com/1949-8454/full/v12/i2/15.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v12.i2.15